Abstract
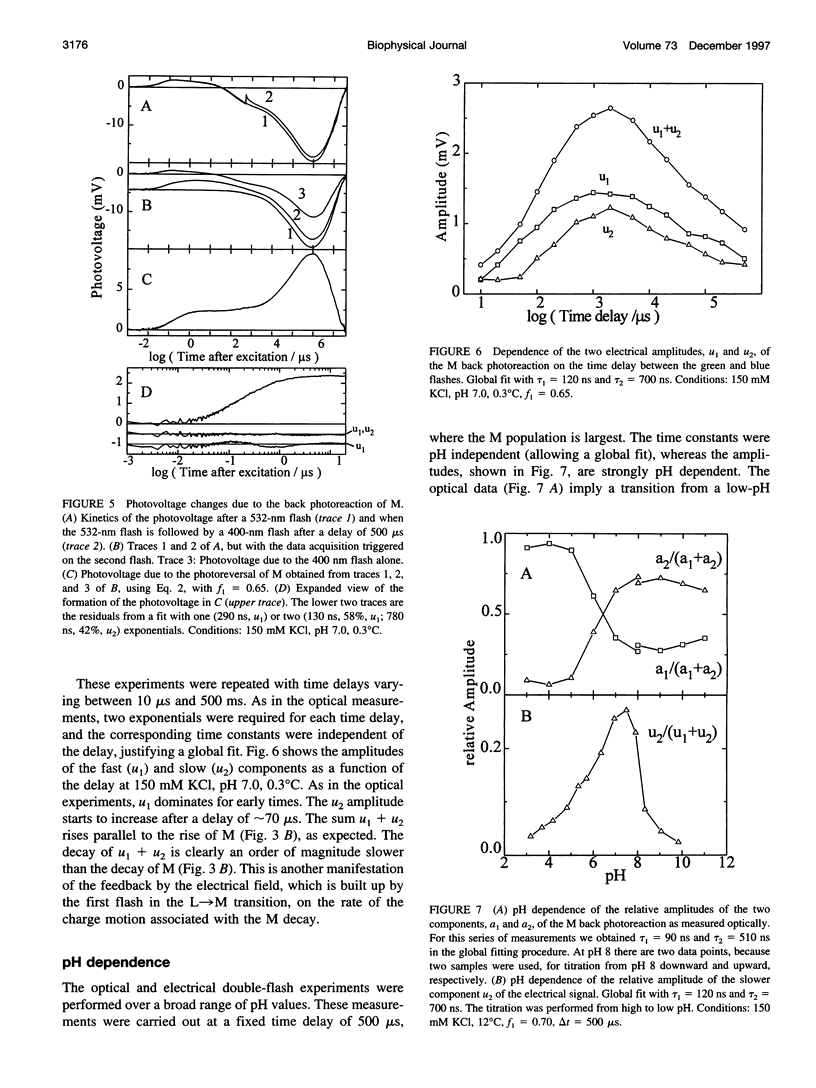
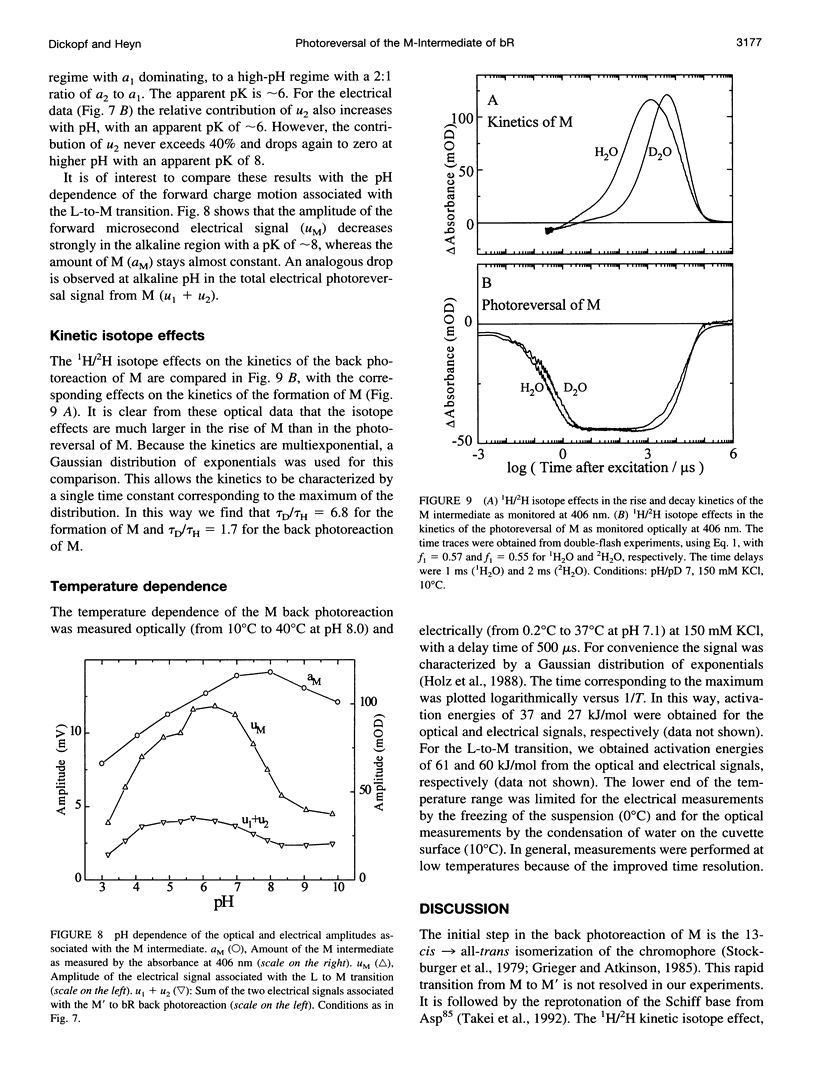
The kinetics of the photoreversal reaction of the M-intermediate of bacteriorhodopsin (bR) was investigated by time-resolved optical absorption spectroscopy and photovoltage measurements using double-flash excitation (a green flash (532 nm) followed by a blue flash (400 nm) after a variable delay). The sign of the photovoltage and the 1H/2H kinetic isotope effect indicate that the Schiff base is reprotonated by a group between the Schiff base and the extracellular surface, probably Asp85. Analysis of the kinetic data shows that the charge movement in 150 mM KCl at 12 degrees C is characterized by two components with time constants of approximately 100 ns and approximately 600 ns, respectively, which are independent of the delay time between the flashes and the pH. The amplitudes of the fast and slow components depend on the delay and the pH. The slower component starts to contribute to the charge movement only after delays longer than 100 micros, is absent at low pH, and increases in amplitude with a pKa of approximately 6. Because the proton release group deprotonates after 70-100 micros and has a transient pKa of 5.8, these results suggest the following assignment: the fast and the combination of fast and slow components represent photoreversal from two M states, with the release group protonated and deprotonated, respectively. The slow phase of the photoreversal starts from a state with the release group deprotonated, and with the pK of Asp85 elevated, and is probably due to the restoration of the pK of Asp85 to its initial low value. This provides further evidence for coupling between the pK's of Asp85 and the release group and suggests that proton release is the first step in the reprotonation switch. At alkaline pH the amplitude of the electrical signal from the back photoreaction decreases with an apparent pK of 8, without a corresponding decrease in the amount of M. At neutral pH the movement of the positively charged guanidinium group of Arg82 from a position near the release group on the surface to Asp85 makes a substantial contribution to the electrical photoreversal amplitude. Above the pK of the release group in the unphotolysed state (approximately 8), Arg82 stays near the surface, leading to a corresponding signal reduction.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexiev U., Mollaaghababa R., Scherrer P., Khorana H. G., Heyn M. P. Rapid long-range proton diffusion along the surface of the purple membrane and delayed proton transfer into the bulk. Proc Natl Acad Sci U S A. 1995 Jan 17;92(2):372–376. doi: 10.1073/pnas.92.2.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balashov S. P., Govindjee R., Imasheva E. S., Misra S., Ebrey T. G., Feng Y., Crouch R. K., Menick D. R. The two pKa's of aspartate-85 and control of thermal isomerization and proton release in the arginine-82 to lysine mutant of bacteriorhodopsin. Biochemistry. 1995 Jul 11;34(27):8820–8834. doi: 10.1021/bi00027a034. [DOI] [PubMed] [Google Scholar]
- Balashov S. P., Govindjee R., Kono M., Imasheva E., Lukashev E., Ebrey T. G., Crouch R. K., Menick D. R., Feng Y. Effect of the arginine-82 to alanine mutation in bacteriorhodopsin on dark adaptation, proton release, and the photochemical cycle. Biochemistry. 1993 Oct 5;32(39):10331–10343. doi: 10.1021/bi00090a008. [DOI] [PubMed] [Google Scholar]
- Balashov S. P., Imasheva E. S., Govindjee R., Ebrey T. G. Titration of aspartate-85 in bacteriorhodopsin: what it says about chromophore isomerization and proton release. Biophys J. 1996 Jan;70(1):473–481. doi: 10.1016/S0006-3495(96)79591-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braiman M. S., Dioumaev A. K., Lewis J. R. A large photolysis-induced pKa increase of the chromophore counterion in bacteriorhodopsin: implications for ion transport mechanisms of retinal proteins. Biophys J. 1996 Feb;70(2):939–947. doi: 10.1016/S0006-3495(96)79637-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun D., Dencher N. A., Fahr A., Lindau M., Heyn M. P. Nonlinear voltage dependence of the light-driven proton pump current of bacteriorhodopsin. Biophys J. 1988 Apr;53(4):617–621. doi: 10.1016/S0006-3495(88)83140-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown L. S., Sasaki J., Kandori H., Maeda A., Needleman R., Lanyi J. K. Glutamic acid 204 is the terminal proton release group at the extracellular surface of bacteriorhodopsin. J Biol Chem. 1995 Nov 10;270(45):27122–27126. doi: 10.1074/jbc.270.45.27122. [DOI] [PubMed] [Google Scholar]
- Dancsházy Z., Drachev L. A., Ormos P., Nagy K., Skulachev V. P. Kinetics of the blue light-induced inhibition of photoelectric activity of bacteriorhodopsin. FEBS Lett. 1978 Dec 1;96(1):59–63. doi: 10.1016/0014-5793(78)81062-x. [DOI] [PubMed] [Google Scholar]
- Dancsházy Z., Tokaji Z. Actinic light density dependence of the bacteriorhodopsin protocycle. Biophys J. 1993 Aug;65(2):823–831. doi: 10.1016/S0006-3495(93)81115-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickopf S., Alexiev U., Krebs M. P., Otto H., Mollaaghababa R., Khorana H. G., Heyn M. P. Proton transport by a bacteriorhodopsin mutant, aspartic acid-85-->asparagine, initiated in the unprotonated Schiff base state. Proc Natl Acad Sci U S A. 1995 Dec 5;92(25):11519–11523. doi: 10.1073/pnas.92.25.11519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Druckmann S., Friedman N., Lanyi J. K., Needleman R., Ottolenghi M., Sheves M. The back photoreaction of the M intermediate in the photocycle of bacteriorhodopsin: mechanism and evidence for two M species. Photochem Photobiol. 1992;56(6):1041–1047. doi: 10.1111/j.1751-1097.1992.tb09727.x. [DOI] [PubMed] [Google Scholar]
- Friedman N., Gat Y., Sheves M., Ottolenghi M. On the heterogeneity of the M population in the photocycle of bacteriorhodopsin. Biochemistry. 1994 Dec 13;33(49):14758–14767. doi: 10.1021/bi00253a014. [DOI] [PubMed] [Google Scholar]
- Govindjee R., Misra S., Balashov S. P., Ebrey T. G., Crouch R. K., Menick D. R. Arginine-82 regulates the pKa of the group responsible for the light-driven proton release in bacteriorhodopsin. Biophys J. 1996 Aug;71(2):1011–1023. doi: 10.1016/S0006-3495(96)79302-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grieger I., Atkinson G. H. Photolytic interruptions of the bacteriorhodopsin photocycle examined by time-resolved resonance raman spectroscopy. Biochemistry. 1985 Sep 24;24(20):5660–5665. doi: 10.1021/bi00341a056. [DOI] [PubMed] [Google Scholar]
- Holz M., Lindau M., Heyn M. P. Distributed kinetics of the charge movements in bacteriorhodopsin: evidence for conformational substates. Biophys J. 1988 Apr;53(4):623–633. doi: 10.1016/S0006-3495(88)83141-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang S. B., Korenbrot J. I., Stoeckenius W. Transient photovoltages in purple membrane multilayers. Charge displacement in bacteriorhodopsin and its photointermediates. Biochim Biophys Acta. 1978 May 18;509(2):300–317. doi: 10.1016/0005-2736(78)90049-4. [DOI] [PubMed] [Google Scholar]
- Kalisky O., Ottolenghi M., Honig B., Korenstein R. Environmental effects on formation and photoreaction of the M412 photoproduct of bacteriorhodopsin: implications for the mechanism of proton pumping. Biochemistry. 1981 Feb 3;20(3):649–655. doi: 10.1021/bi00506a031. [DOI] [PubMed] [Google Scholar]
- Karvaly B., Dancsházy Z. Bacteriorhodopsin: a molecular photoelectric regulator. Quenching of photovoltaic effect of bimolecular lipid membranes containing bacteriorhodopsin by blue light. FEBS Lett. 1977 Apr 1;76(1):36–40. doi: 10.1016/0014-5793(77)80115-4. [DOI] [PubMed] [Google Scholar]
- Kleinschmidt C., Hess B. Influence of an electrical potential on the charge transfer kinetics of bacteriorhodopsin. Biophys J. 1990 Sep;58(3):653–663. doi: 10.1016/S0006-3495(90)82408-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kono M., Misra S., Ebrey T. G. pH dependence of light-induced proton release by bacteriorhodopsin. FEBS Lett. 1993 Sep 27;331(1-2):31–34. doi: 10.1016/0014-5793(93)80291-2. [DOI] [PubMed] [Google Scholar]
- Lanyi J. K. Proton translocation mechanism and energetics in the light-driven pump bacteriorhodopsin. Biochim Biophys Acta. 1993 Dec 7;1183(2):241–261. doi: 10.1016/0005-2728(93)90226-6. [DOI] [PubMed] [Google Scholar]
- Liu S. Y. Light-induced currents from oriented purple membrane: I. Correlation of the microsecond component (B2) with the L-M photocycle transition. Biophys J. 1990 May;57(5):943–950. doi: 10.1016/S0006-3495(90)82614-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieto-Frausto J., Lüger P., Apell H. J. Electrostatic coupling of ion pumps. Biophys J. 1992 Jan;61(1):83–95. doi: 10.1016/S0006-3495(92)81818-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno K., Govindjee R., Ebrey T. G. Blue light effect on proton pumping by bacteriorhodopsin. Biophys J. 1983 Aug;43(2):251–254. doi: 10.1016/S0006-3495(83)84347-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ormos P., Dancsházy Z., Karvaly B. Mechanism of generation and regulation of photopotential by bacteriorhodopsin in bimolecular lipid membrane. Biochim Biophys Acta. 1978 Aug 8;503(2):304–315. doi: 10.1016/0005-2728(78)90190-1. [DOI] [PubMed] [Google Scholar]
- Ormos P., Dancsházy Z., Keszthelyi L. Electric response of a back photoreaction in the bacteriorhodopsin photocycle. Biophys J. 1980 Aug;31(2):207–213. doi: 10.1016/S0006-3495(80)85051-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto H., Marti T., Holz M., Mogi T., Lindau M., Khorana H. G., Heyn M. P. Aspartic acid-96 is the internal proton donor in the reprotonation of the Schiff base of bacteriorhodopsin. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9228–9232. doi: 10.1073/pnas.86.23.9228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto H., Marti T., Holz M., Mogi T., Stern L. J., Engel F., Khorana H. G., Heyn M. P. Substitution of amino acids Asp-85, Asp-212, and Arg-82 in bacteriorhodopsin affects the proton release phase of the pump and the pK of the Schiff base. Proc Natl Acad Sci U S A. 1990 Feb;87(3):1018–1022. doi: 10.1073/pnas.87.3.1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintanilha A. T. Control of the photocycle in bacteriorhodopsin by electrochemical gradients. FEBS Lett. 1980 Aug 11;117(1):8–12. doi: 10.1016/0014-5793(80)80901-x. [DOI] [PubMed] [Google Scholar]
- Richter H. T., Brown L. S., Needleman R., Lanyi J. K. A linkage of the pKa's of asp-85 and glu-204 forms part of the reprotonation switch of bacteriorhodopsin. Biochemistry. 1996 Apr 2;35(13):4054–4062. doi: 10.1021/bi952883q. [DOI] [PubMed] [Google Scholar]
- Sampogna R. V., Honig B. Electrostatic coupling between retinal isomerization and the ionization state of Glu-204: a general mechanism for proton release in bacteriorhodopsin. Biophys J. 1996 Sep;71(3):1165–1171. doi: 10.1016/S0006-3495(96)79320-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockburger M., Klusmann W., Gattermann H., Massig G., Peters R. Photochemical cycle of bacteriorhodopsin studied by resonance Raman spectroscopy. Biochemistry. 1979 Oct 30;18(22):4886–4900. doi: 10.1021/bi00589a017. [DOI] [PubMed] [Google Scholar]
- Takei H., Gat Y., Sheves M., Lewis A. Low temperature FTIR study of the Schiff base reprotonation during the M-to-bR backphotoreaction: Asp 85 reprotonates two distinct types of Schiff base species at different temperatures. Biophys J. 1992 Dec;63(6):1643–1653. doi: 10.1016/S0006-3495(92)81757-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trissl H. W. Photoelectric measurements of purple membranes. Photochem Photobiol. 1990 Jun;51(6):793–818. [PubMed] [Google Scholar]
- Zimányi L., Váró G., Chang M., Ni B., Needleman R., Lanyi J. K. Pathways of proton release in the bacteriorhodopsin photocycle. Biochemistry. 1992 Sep 15;31(36):8535–8543. doi: 10.1021/bi00151a022. [DOI] [PubMed] [Google Scholar]
- le Coutre J., Gerwert K. Kinetic isotope effects reveal an ice-like and a liquid-phase-type intramolecular proton transfer in bacteriorhodopsin. FEBS Lett. 1996 Dec 2;398(2-3):333–336. doi: 10.1016/s0014-5793(96)01254-9. [DOI] [PubMed] [Google Scholar]


