Abstract
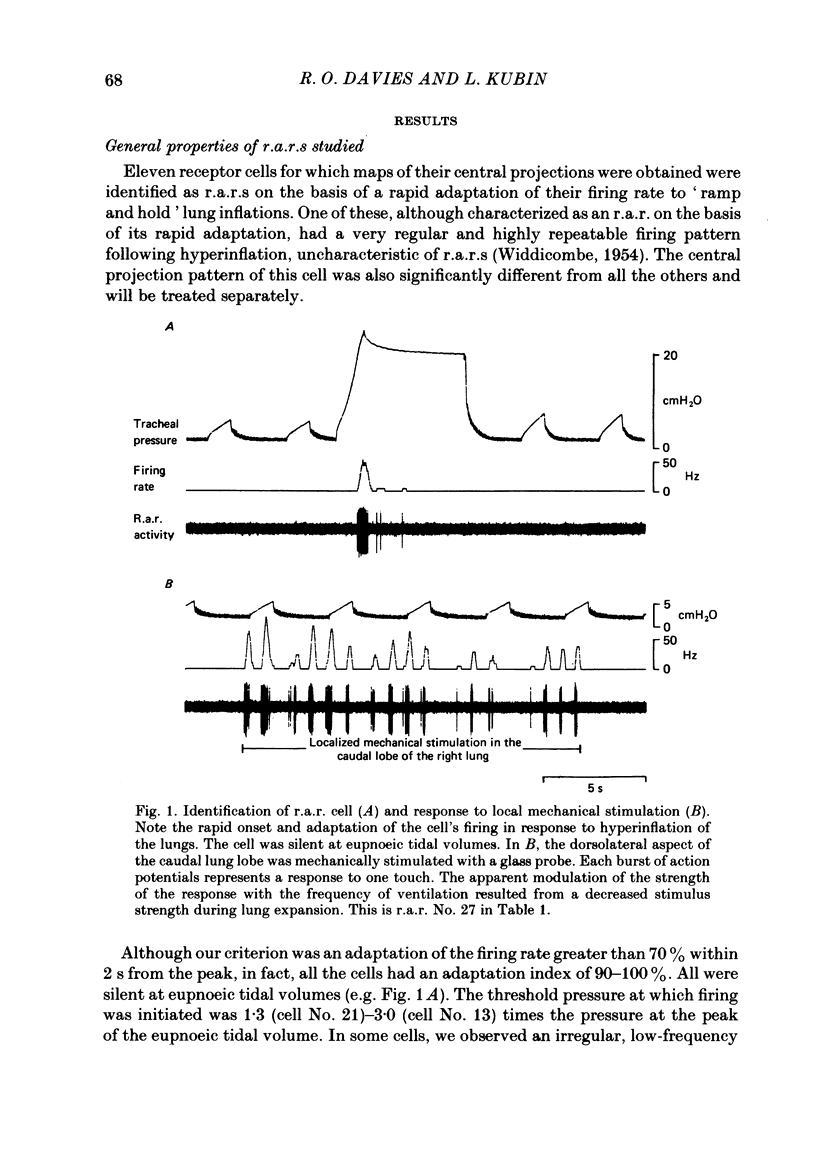
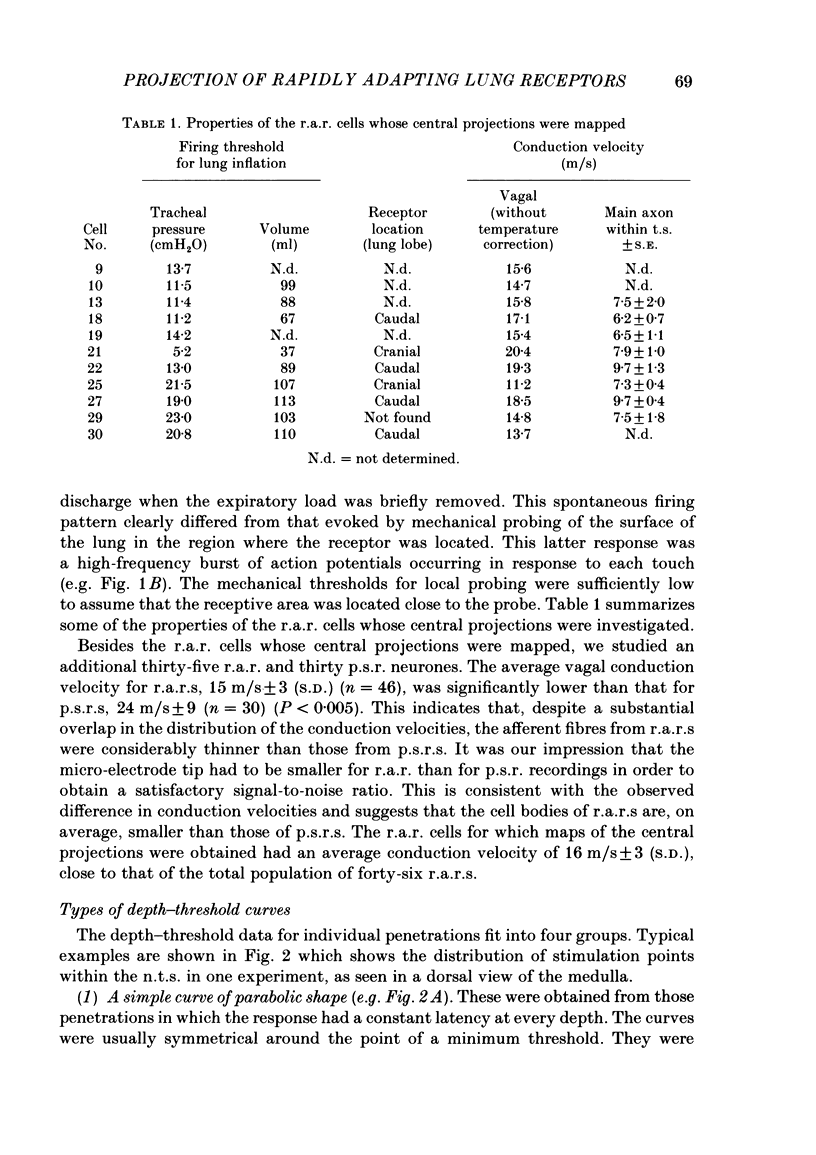
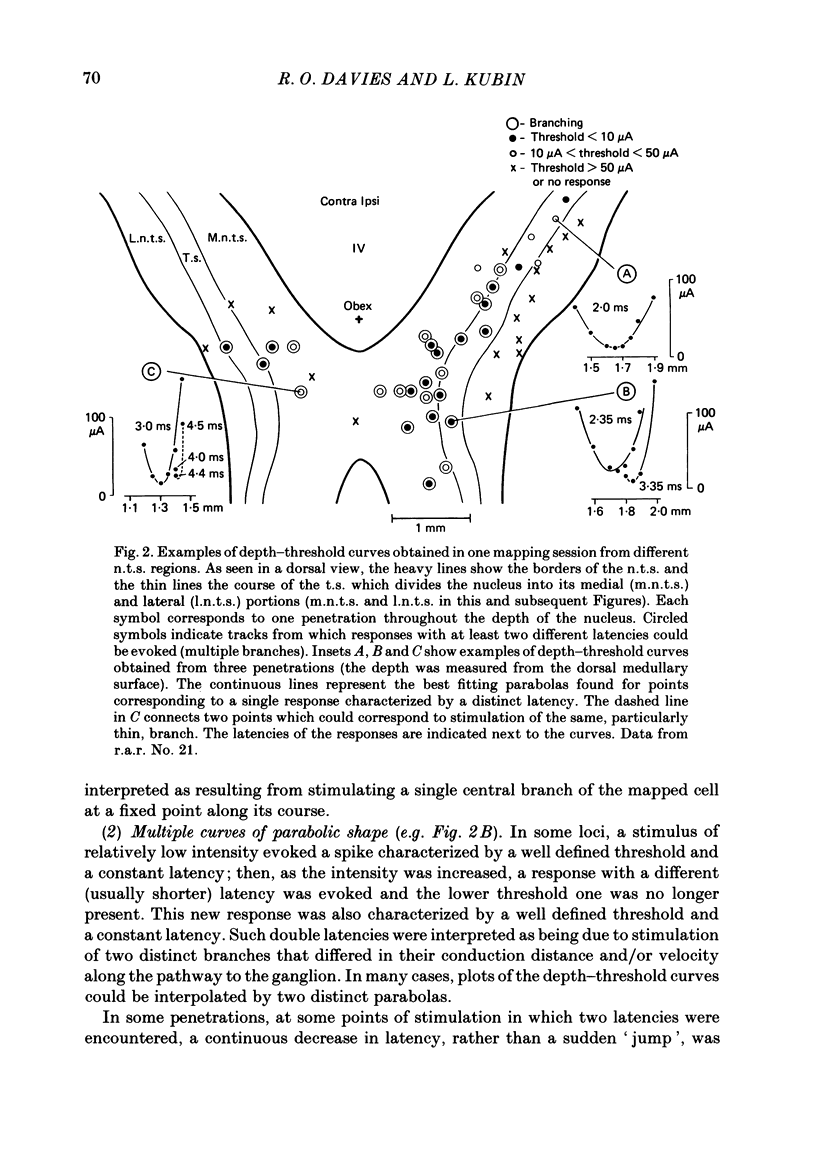
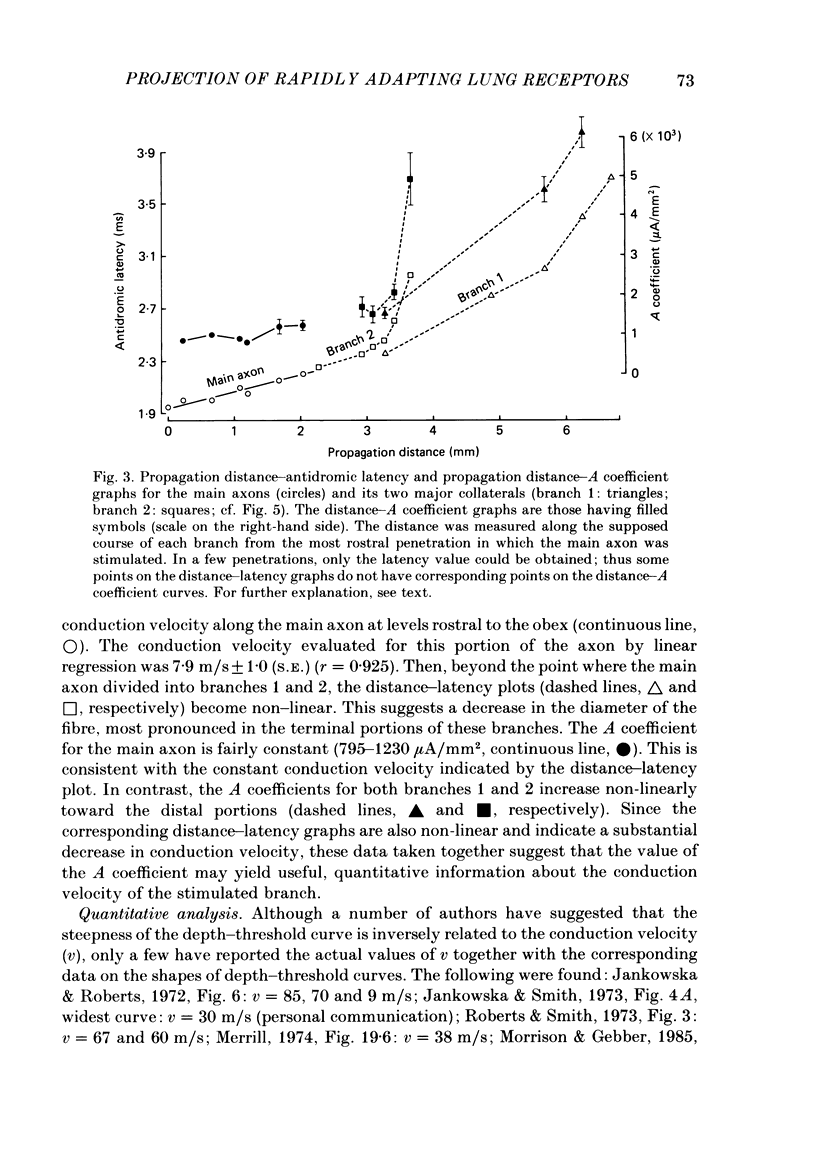
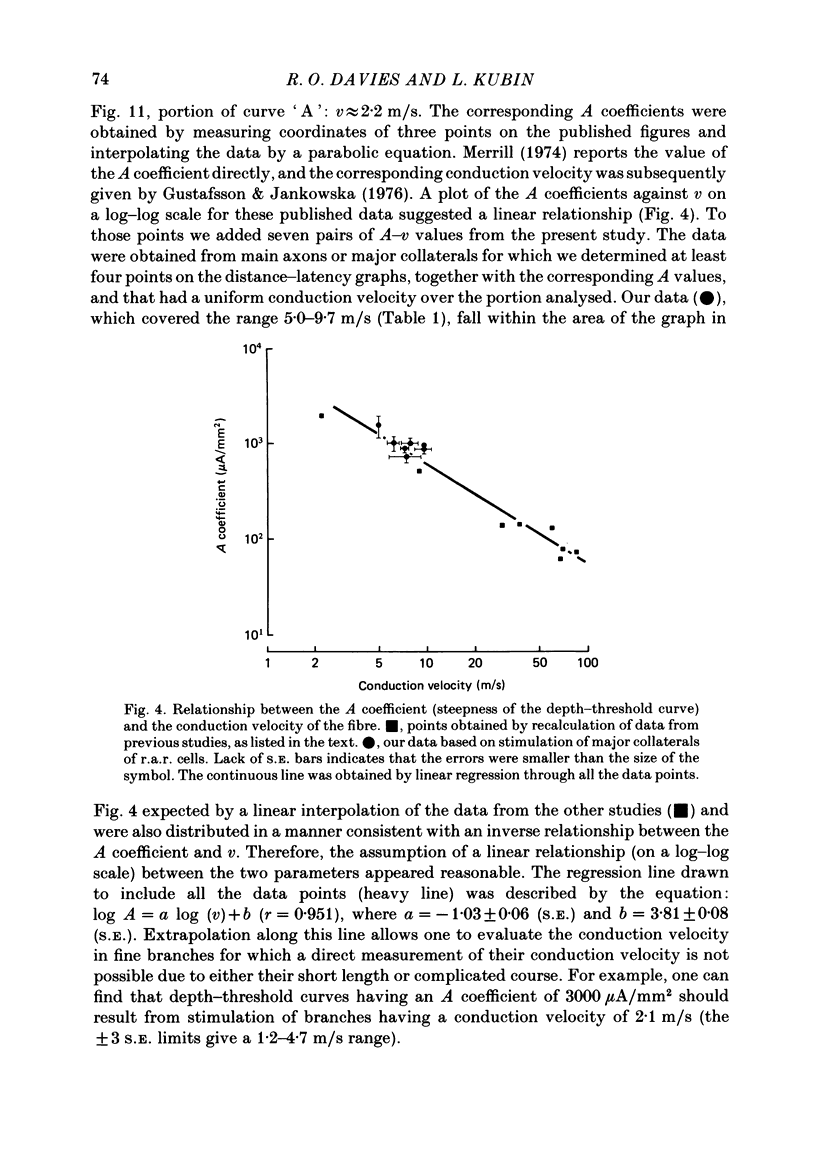
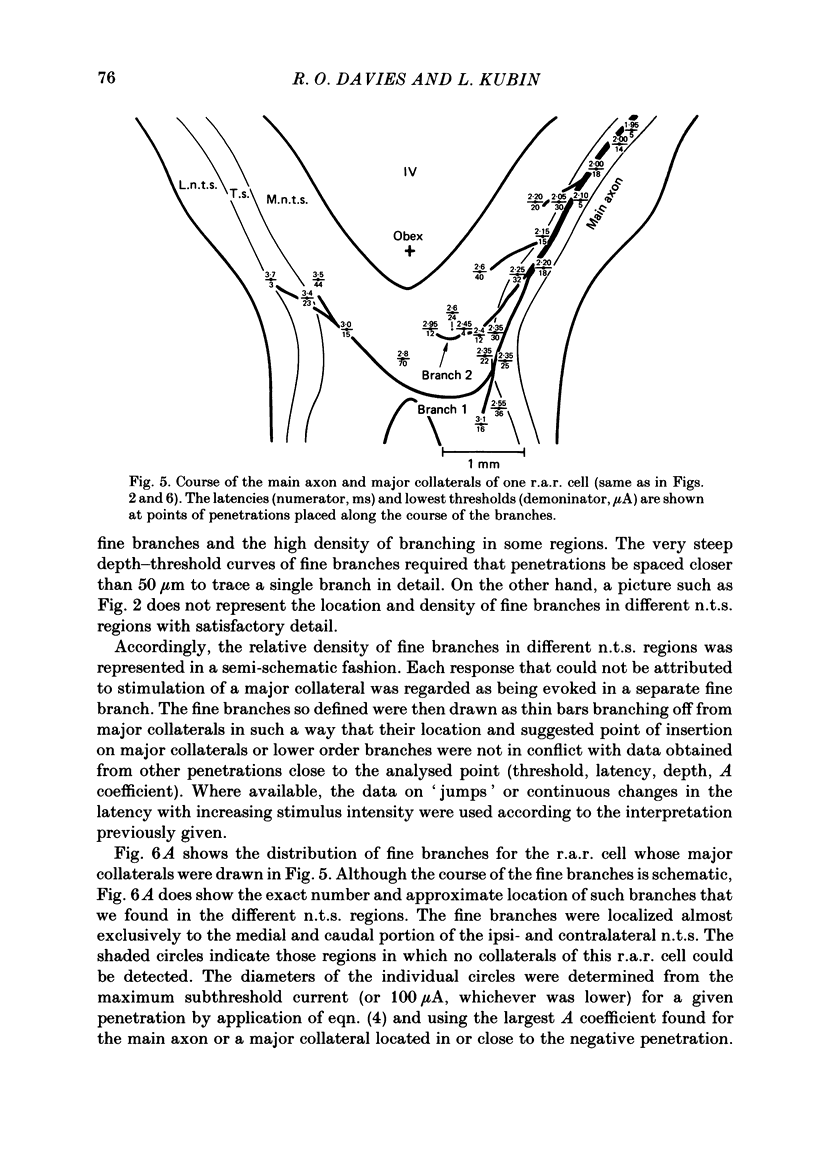
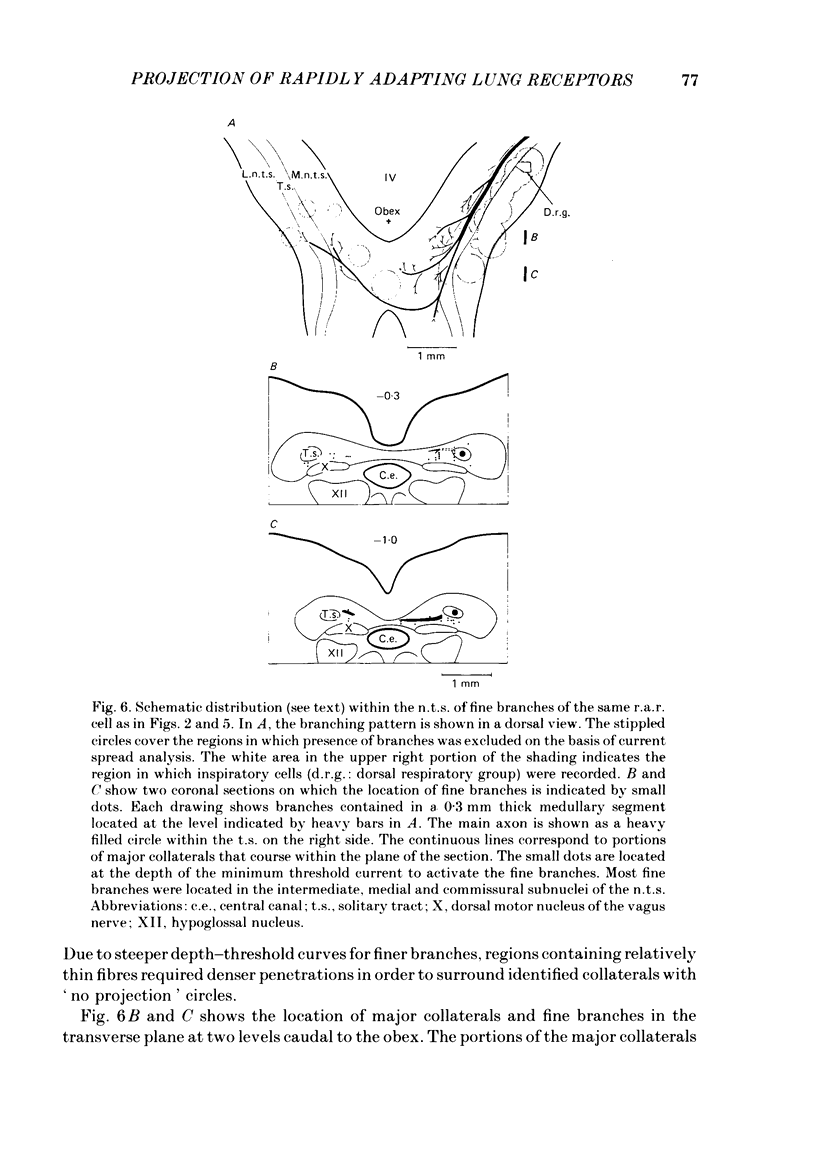
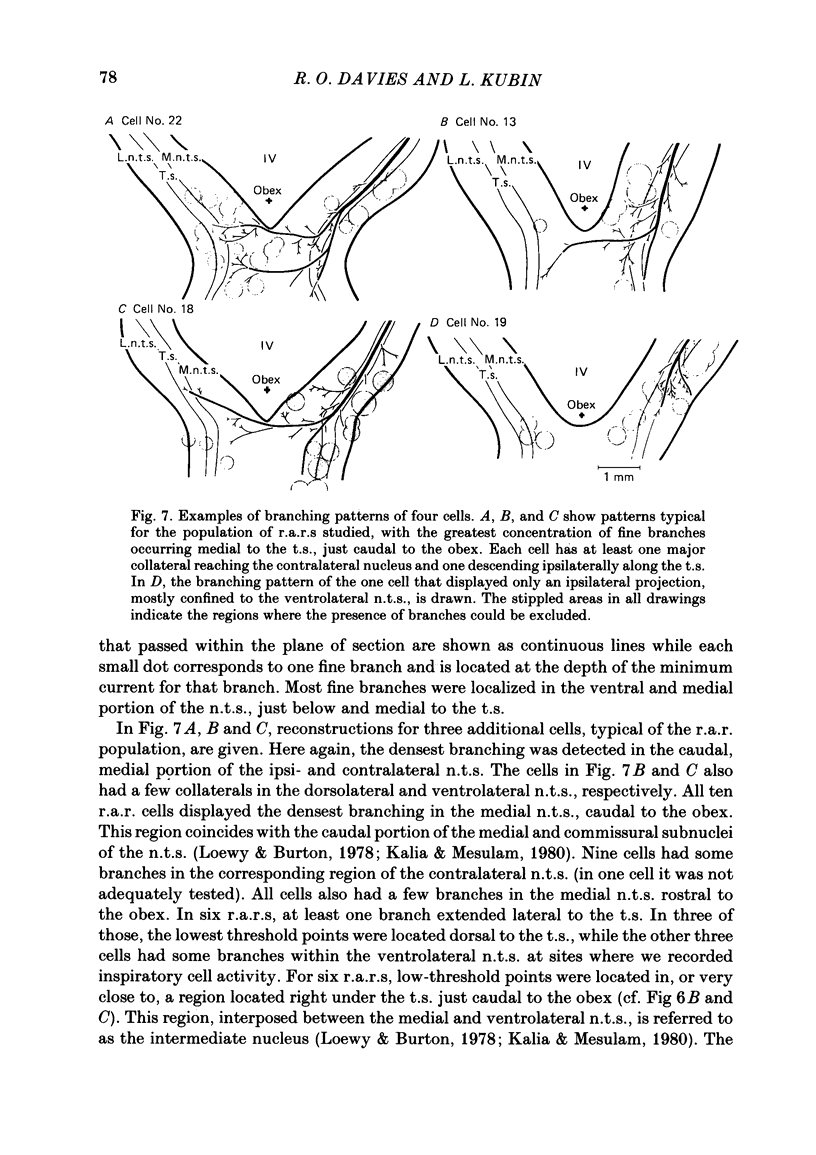
The activity of pulmonary rapidly adapting receptor (r.a.r.) neurones was recorded extracellularly in the nodose ganglion of the decerebrate cat. The receptors were identified by their rapid adaptation to 'ramp and hold' hyperinflations of the lung. The antidromic mapping technique was used to determine the sites of projection and branching patterns within the nucleus of the tractus solitarius (n.t.s.) of eleven r.a.r.s. The medulla was explored with a stimulating electrode to activate the r.a.r.s. antidromically. In each penetration, depth-threshold measurements were made for each antidromic response characterized by a distinct latency. Using the anatomical sites of the minimum threshold points, the locations of central branches of individual r.a.r.s. were determined. The main axons of all of them coursed within the tractus solitarius (t.s.) at levels from 2 mm rostral to 0.5 mm caudal to the obex. The axonal conduction velocities within the t.s. were 6.2-9.7 m/s, where the peripheral conduction velocities were 11.2-20.4 m/s (28 degrees C). Different latencies of response evoked in a single penetration were considered to indicate branching. The densest branching was found in the ipsilateral commissural subnucleus of the n.t.s. at levels 0.3-1.3 mm caudal to the obex and, to a lesser degree, in the contralateral commissural subnucleus. All r.a.r.s. sent a few branches to the medial n.t.s. rostral to the obex. Four r.a.r.s. ramified in the ventrolateral n.t.s. where inspiratory cells are located. Depth-threshold graphs were interpolated by best fitting parabolic equations: Ith = Ad2 + Bd + C; where Ith is the threshold current, d the corresponding depth of stimulation, and A, B and C are coefficients. Coefficient A is a measure of steepness of the parabola. The A coefficients were inversely related to the conduction velocity (v) of the stimulated branch. An analysis of the data from the present study (v = 5.0-9.7 m/s) combined with data from the literature (v = 2.2-85 m/s) led to a simple relationship between the A coefficient and the conduction velocity of the stimulated fibre: A = 6500/v, where A is expressed in microA/mm2 and v is expressed in m/s. Within the range 3-35 m/s, the formula is useful in predicting the effective current spread when the conduction velocity is known, or to estimate the conduction velocity from the shape of a depth-threshold curve. Two slowly adapting pulmonary stretch receptors (p.s.r.s) were studied.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahlsén G. Brain stem neurones with differential projection to functional subregions of the dorsal lateral geniculate complex in the cat. Neuroscience. 1984 Jul;12(3):817–838. doi: 10.1016/0306-4522(84)90173-8. [DOI] [PubMed] [Google Scholar]
- Armstrong D. M., Harvey R. J., Schild R. F. The spatial organisation of climbing fibre branching in the cat cerebellum. Exp Brain Res. 1973 Aug 31;18(1):40–58. doi: 10.1007/BF00236555. [DOI] [PubMed] [Google Scholar]
- Averill D. B., Cameron W. E., Berger A. J. Monosynaptic excitation of dorsal medullary respiratory neurons by slowly adapting pulmonary stretch receptors. J Neurophysiol. 1984 Oct;52(4):771–785. doi: 10.1152/jn.1984.52.4.771. [DOI] [PubMed] [Google Scholar]
- Backman S. B., Anders C., Ballantyne D., Röhrig N., Camerer H., Mifflin S., Jordan D., Dickhaus H., Spyer K. M., Richter D. W. Evidence for a monosynaptic connection between slowly adapting pulmonary stretch receptor afferents and inspiratory beta neurones. Pflugers Arch. 1984 Oct;402(2):129–136. doi: 10.1007/BF00583324. [DOI] [PubMed] [Google Scholar]
- BeMent S. L., Ranck J. B., Jr A model for electrical stimulation of central myelinated fibers with monopolar electrodes. Exp Neurol. 1969 Jun;24(2):171–186. doi: 10.1016/0014-4886(69)90013-2. [DOI] [PubMed] [Google Scholar]
- Belmonte C., Gallego R. Membrane properties of cat sensory neurones with chemoreceptor and baroreceptor endings. J Physiol. 1983 Sep;342:603–614. doi: 10.1113/jphysiol.1983.sp014871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger A. J., Averill D. B. Projection of single pulmonary stretch receptors to solitary tract region. J Neurophysiol. 1983 Mar;49(3):819–830. doi: 10.1152/jn.1983.49.3.819. [DOI] [PubMed] [Google Scholar]
- COTTLE M. K. DEGENERATION STUDIES OF PRIMARY AFFERENTS OF IXTH AND XTH CRANIAL NERVES IN THE CAT. J Comp Neurol. 1964 Jun;122:329–345. doi: 10.1002/cne.901220304. [DOI] [PubMed] [Google Scholar]
- Donoghue S., Garcia M., Jordan D., Spyer K. M. The brain-stem projections of pulmonary stretch afferent neurones in cats and rabbits. J Physiol. 1982 Jan;322:353–363. doi: 10.1113/jphysiol.1982.sp014041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREEN J. D. A simple microelectrode for recording from the central nervous system. Nature. 1958 Oct 4;182(4640):962–962. doi: 10.1038/182962a0. [DOI] [PubMed] [Google Scholar]
- Gustafsson B., Jankowska E. Direct and indirect activation of nerve cells by electrical pulses applied extracellularly. J Physiol. 1976 Jun;258(1):33–61. doi: 10.1113/jphysiol.1976.sp011405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hentall I. D., Zorman G., Kansky S., Fields H. L. Relations among threshold, spike height, electrode distance, and conduction velocity in electrical stimulation of certain medullospinal neurons. J Neurophysiol. 1984 May;51(5):968–977. doi: 10.1152/jn.1984.51.5.968. [DOI] [PubMed] [Google Scholar]
- Jankowska E., Roberts W. J. An electrophysiological demonstration of the axonal projections of single spinal interneurones in the cat. J Physiol. 1972 May;222(3):597–622. doi: 10.1113/jphysiol.1972.sp009817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E., Smith D. O. Antidromic activation of Renshaw cells and their axonal projections. Acta Physiol Scand. 1973 Jun;88(2):198–214. doi: 10.1111/j.1748-1716.1973.tb05447.x. [DOI] [PubMed] [Google Scholar]
- KERR F. W. Facial, vagal and glossopharyngeal nerves in the cat. Afferent connections. Arch Neurol. 1962 Apr;6:264–281. doi: 10.1001/archneur.1962.00450220006003. [DOI] [PubMed] [Google Scholar]
- Kalia M., Mesulam M. M. Brain stem projections of sensory and motor components of the vagus complex in the cat: I. The cervical vagus and nodose ganglion. J Comp Neurol. 1980 Sep 15;193(2):435–465. doi: 10.1002/cne.901930210. [DOI] [PubMed] [Google Scholar]
- Lipski J. Antidromic activation of neurones as an analytic tool in the study of the central nervous system. J Neurosci Methods. 1981 Jun;4(1):1–32. doi: 10.1016/0165-0270(81)90015-7. [DOI] [PubMed] [Google Scholar]
- Lipski J., Kubin L., Jodkowski J. Synaptic action of R beta neurons on phrenic motoneurons studied with spike-triggered averaging. Brain Res. 1983 Dec 12;288(1-2):105–118. doi: 10.1016/0006-8993(83)90085-9. [DOI] [PubMed] [Google Scholar]
- Loewy A. D., Burton H. Nuclei of the solitary tract: efferent projections to the lower brain stem and spinal cord of the cat. J Comp Neurol. 1978 Sep 15;181(2):421–449. doi: 10.1002/cne.901810211. [DOI] [PubMed] [Google Scholar]
- Marino P. L., Davies R. O., Pack A. I. The responses of I beta cells to increases in the rate of lung inflation. Brain Res. 1981 Aug 31;219(2):289–305. doi: 10.1016/0006-8993(81)90292-4. [DOI] [PubMed] [Google Scholar]
- Morrison S. F., Gebber G. L. Axonal branching patterns and funicular trajectories of raphespinal sympathoinhibitory neurons. J Neurophysiol. 1985 Mar;53(3):759–772. doi: 10.1152/jn.1985.53.3.759. [DOI] [PubMed] [Google Scholar]
- Pack A. I. Sensory inputs to the medulla. Annu Rev Physiol. 1981;43:73–90. doi: 10.1146/annurev.ph.43.030181.000445. [DOI] [PubMed] [Google Scholar]
- Paintal A. S. Effects of temperature on conduction in single vagal and saphenous myelinated nerve fibres of the cat. J Physiol. 1965 Sep;180(1):20–49. [PMC free article] [PubMed] [Google Scholar]
- Paintal A. S. Vagal sensory receptors and their reflex effects. Physiol Rev. 1973 Jan;53(1):159–227. doi: 10.1152/physrev.1973.53.1.159. [DOI] [PubMed] [Google Scholar]
- Ranck J. B., Jr Which elements are excited in electrical stimulation of mammalian central nervous system: a review. Brain Res. 1975 Nov 21;98(3):417–440. doi: 10.1016/0006-8993(75)90364-9. [DOI] [PubMed] [Google Scholar]
- Roberts W. J., Smith D. O. Analysis of threshold currents during microstimulation of fibres in the spinal cord. Acta Physiol Scand. 1973 Nov;89(3):384–394. doi: 10.1111/j.1748-1716.1973.tb05533.x. [DOI] [PubMed] [Google Scholar]
- Sant'Ambrogio G. Information arising from the tracheobronchial tree of mammals. Physiol Rev. 1982 Apr;62(2):531–569. doi: 10.1152/physrev.1982.62.2.531. [DOI] [PubMed] [Google Scholar]
- Shinoda Y., Arnold A. P., Asanuma H. Spinal branching of corticospinal axons in the cat. Exp Brain Res. 1976 Oct 28;26(3):215–234. doi: 10.1007/BF00234928. [DOI] [PubMed] [Google Scholar]
- WIDDICOMBE J. G. Receptors in the trachea and bronchi of the cat. J Physiol. 1954 Jan;123(1):71–104. doi: 10.1113/jphysiol.1954.sp005034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widdicombe J. G. Pulmonary and respiratory tract receptors. J Exp Biol. 1982 Oct;100:41–57. doi: 10.1242/jeb.100.1.41. [DOI] [PubMed] [Google Scholar]


