Abstract
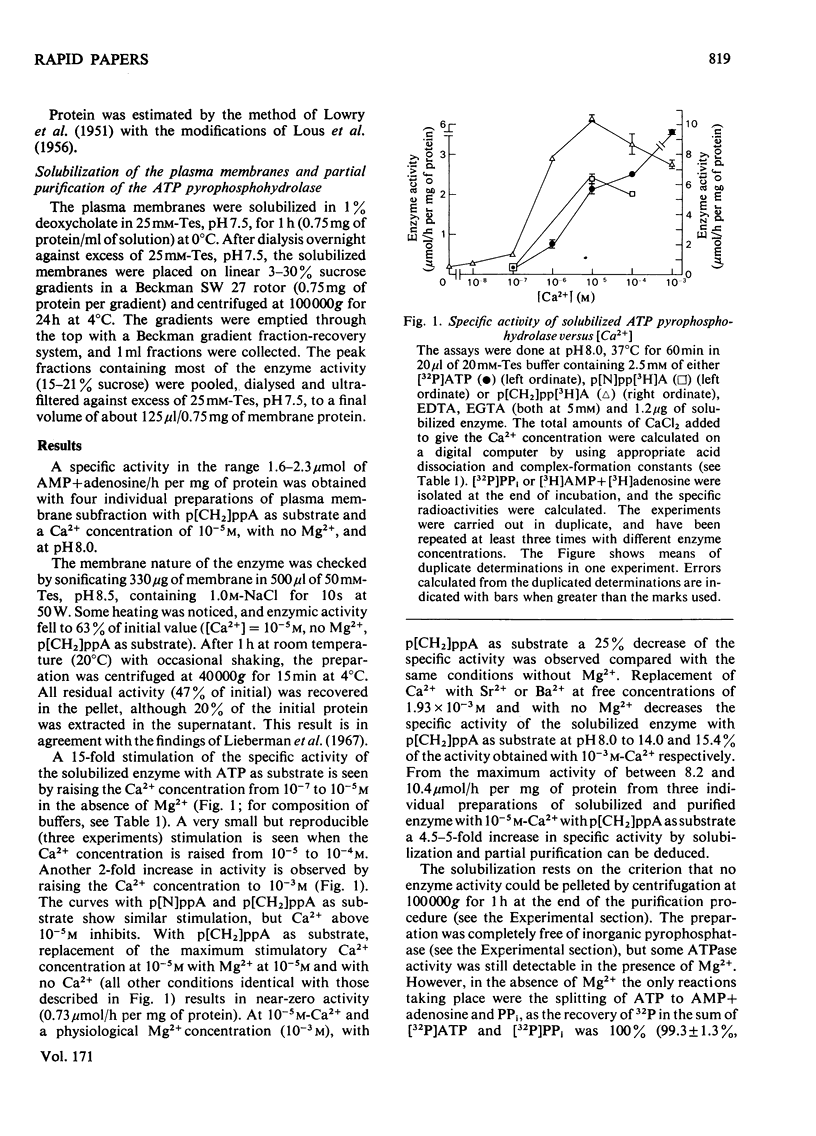
An ATP pyrophosphohydrolase in a rat liver plasma-membrane subfraction was studied with respect to specific Ca2+ activation of the beta-phosphate bond hydrolysis. ATP and, in addition, adenosine 5'-[betagamma-imido]triphosphate and adenosine 5'-[betagamma-methlylene]triphosphate were substrates for Ca2+-stimulated enzymic hydrolysis of the beta-phosphate bond. A 15-fold activation was observed by raising the free Ca2+ concentration from 10(-7) to 10(-5) M. Mg2+ had little effect. Solubilization in 1% deoxycholate and partial purification on a sucrose density gradient resulted in a 5-fold increase in specific activity with unaltered Ca2+-stimulation pattern. The possible importance of the enzyme in Ca2+ transport is discussed.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Flodgaard H., Fleron P. Thermodynamic parameters for the hydrolysis of inorganic pyrophosphate at pH 7.4 as a function of (Mg2+), (K+), and ionic strength determined from equilibrium studies of the reaction. J Biol Chem. 1974 Jun 10;249(11):3465–3474. [PubMed] [Google Scholar]
- Formby B., Capito K., Egeberg J., Hedeskov C. J. Ca-activated ATPase activity in subcellular fractions of mouse pancreatic islets. Am J Physiol. 1976 Feb;230(2):441–448. doi: 10.1152/ajplegacy.1976.230.2.441. [DOI] [PubMed] [Google Scholar]
- Franklin J. E., Trams E. G. Metabolism of coenzyme A and related nucleotides by liver plasma membranes. Biochim Biophys Acta. 1971 Jan 26;230(1):105–116. doi: 10.1016/0304-4165(71)90058-4. [DOI] [PubMed] [Google Scholar]
- House P. D., Poulis P., Weidemann M. J. Isolation of a plasma-membrane subfraction from rat liver containing an insulin-sensitive cyclic-AMP phosphodiesterase. Eur J Biochem. 1972 Jan 21;24(3):429–437. doi: 10.1111/j.1432-1033.1972.tb19703.x. [DOI] [PubMed] [Google Scholar]
- LOUIS P., PLUM C. M., SCHOU M. Kolorimetrisk bestemmelse af totalprotein og globulin i spinalvaeske; afprøvning af Lowry-metoden. Nord Med. 1956 May 17;55(20):693–695. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lieberman I., Lansing A. I., Lynch W. E. Nucleoside triphosphate pyrophosphohydrolase of the plasma membrane of the liver cell. J Biol Chem. 1967 Feb 25;242(4):736–739. [PubMed] [Google Scholar]
- RANDERATH E., RANDERATH K. RESOLUTION OF COMPLEX NUCLEOTIDE MIXTURES BY TWO-DIMENSIONAL ANION-EXCHANGE THIN-LAYER CHROMATOGRAPHY. J Chromatogr. 1964 Oct;16:126–129. doi: 10.1016/s0021-9673(01)82446-8. [DOI] [PubMed] [Google Scholar]
- Ray T. K., Tomasi V., Marinetti G. V. Hormone action at the membrane level. I. Properties of adenyl cyclase in isolated plasma membranes of rat liver. Biochim Biophys Acta. 1970 Jul 7;211(1):20–30. doi: 10.1016/0005-2736(70)90119-7. [DOI] [PubMed] [Google Scholar]
- WURTMAN R. J., AXELROD J. A SENSITIVE AND SPECIFIC ASSAY FOR THE ESTIMATION OF MONOAMINE OXIDASE. Biochem Pharmacol. 1963 Dec;12:1439–1441. doi: 10.1016/0006-2952(63)90215-6. [DOI] [PubMed] [Google Scholar]
- Yount R. G., Babcock D., Ballantyne W., Ojala D. Adenylyl imidodiphosphate, an adenosine triphosphate analog containing a P--N--P linkage. Biochemistry. 1971 Jun 22;10(13):2484–2489. doi: 10.1021/bi00789a009. [DOI] [PubMed] [Google Scholar]


