Abstract
Supercoiled rat liver mitochondrial DNA is relaxed by treatment with ribonucleases A, T1 or H. All the supercoiled mitochondrial DNA is sensitive to ribonuclease H and ribonuclease A, but only 35% of the supercoiled population is sensitive to ribonuclease T1. Removal of the ribonucleotides with calf thymus ribonuclease H, followed by denaturation of the mitochondrial DNA and analysis of the single-strand fragment lengths in the electron microscope, showed that the ribonucleotides were randomly located on both strands of the DNA. Endonuclease-S1 digestion of mitochondrial DNA after removal of the ribonucleotides reveals that no unique fragments are produced and ribonucleotides are randomly distributed with respect to one another. The average number of ribonucleotide sites per molecule was estimated to be between 8 and 13. Two possible mechanisms for the origin of ribonucleotide sites are discussed.
Full text
PDF




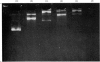
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaij C., Borst P. The gel electrophoresis of DNA. Biochim Biophys Acta. 1972 May 10;269(2):192–200. doi: 10.1016/0005-2787(72)90426-1. [DOI] [PubMed] [Google Scholar]
- Berkower I., Leis J., Hurwitz J. Isolation and characterization of an endonuclease from Escherichia coli specific for ribonucleic acid in ribonucleic acid-deoxyribonucleic acid hybrid structures. J Biol Chem. 1973 Sep 10;248(17):5914–5921. [PubMed] [Google Scholar]
- Bode V. C. Single-strand scissions induced in circular and linear lambda DNA by the presence of dithiothreitol and other reducing agents. J Mol Biol. 1967 May 28;26(1):125–129. doi: 10.1016/0022-2836(67)90266-5. [DOI] [PubMed] [Google Scholar]
- Borst P. Mitochondrial nucleic acids. Annu Rev Biochem. 1972;41:333–376. doi: 10.1146/annurev.bi.41.070172.002001. [DOI] [PubMed] [Google Scholar]
- Bruner R., Vinograd J. The evaluation of standard sedimentation coefficients of sodium RNA and sodium DNA from sedimentation velocity data in concentrated NaCl and CsCl solutions. Biochim Biophys Acta. 1965 Sep 6;108(1):18–29. doi: 10.1016/0005-2787(65)90104-8. [DOI] [PubMed] [Google Scholar]
- Espejo R. T., Canelo E. S. Properties of bacteriophage PM2: a lipid-containing bacterial virus. Virology. 1968 Apr;34(4):738–747. doi: 10.1016/0042-6822(68)90094-9. [DOI] [PubMed] [Google Scholar]
- Espejo R. T., Canelo E. S., Sinsheimer R. L. DNA of bacteriophage PM2: a closed circular double-stranded molecule. Proc Natl Acad Sci U S A. 1969 Aug;63(4):1164–1168. doi: 10.1073/pnas.63.4.1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gefter M. L. DNA replication. Annu Rev Biochem. 1975;44:45–78. doi: 10.1146/annurev.bi.44.070175.000401. [DOI] [PubMed] [Google Scholar]
- Godson G. N. Action of the single-stranded DNA specific nuclease S1 on double-stranded DNA. Biochim Biophys Acta. 1973 Apr 21;308(7):59–67. doi: 10.1016/0005-2787(73)90122-6. [DOI] [PubMed] [Google Scholar]
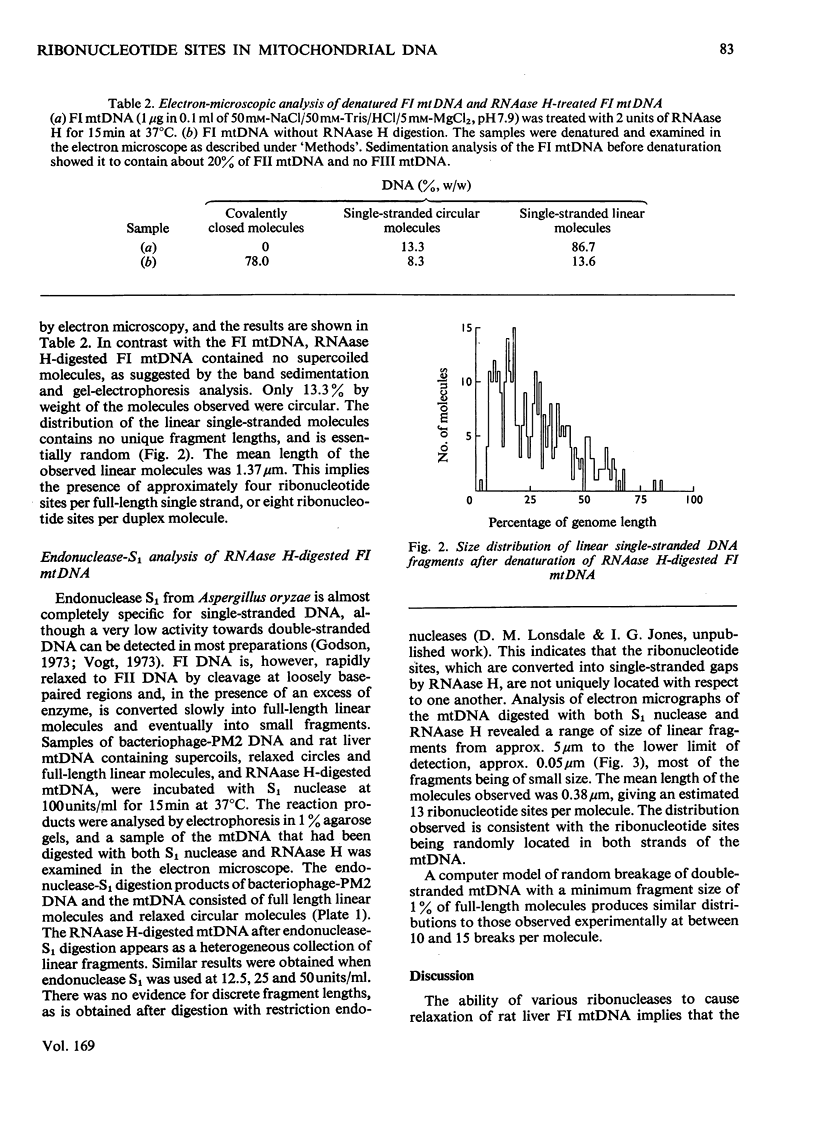
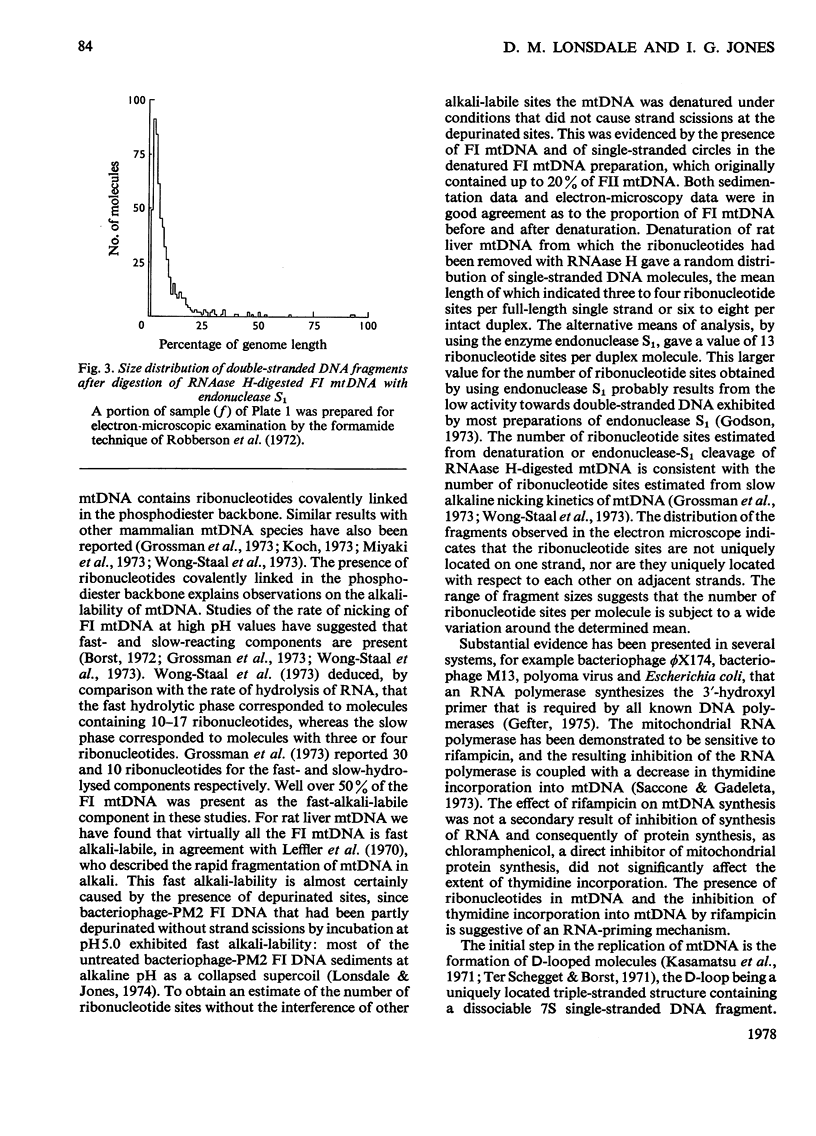
- Grossman L. I., Watson R., Vinograd J. The presence of ribonucleotides in mature closed-circular mitochondrial DNA. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3339–3343. doi: 10.1073/pnas.70.12.3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasamatsu H., Grossman L. I., Robberson D. L., Watson R., Vinograd J. The replication and structure of mitochondrial DNA in animal cells. Cold Spring Harb Symp Quant Biol. 1974;38:281–288. doi: 10.1101/sqb.1974.038.01.031. [DOI] [PubMed] [Google Scholar]
- Kasamatsu H., Robberson D. L., Vinograd J. A novel closed-circular mitochondrial DNA with properties of a replicating intermediate. Proc Natl Acad Sci U S A. 1971 Sep;68(9):2252–2257. doi: 10.1073/pnas.68.9.2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch J. Introduction of "nicks" and "chops" into human mitochondrial DNA in vivo and in vitro. Eur J Biochem. 1973 Feb 15;33(1):98–103. doi: 10.1111/j.1432-1033.1973.tb02659.x. [DOI] [PubMed] [Google Scholar]
- Leffler A. T., 2nd, Creskoff E., Luborsky S. W., McFarland V., Mora P. T. Isolation and characterization of rat liver mitochondrial DNA. J Mol Biol. 1970 Mar;48(3):455–468. doi: 10.1016/0022-2836(70)90058-6. [DOI] [PubMed] [Google Scholar]
- Lindahl T., Nyberg B. Rate of depurination of native deoxyribonucleic acid. Biochemistry. 1972 Sep 12;11(19):3610–3618. doi: 10.1021/bi00769a018. [DOI] [PubMed] [Google Scholar]
- Lonsdale D. M., Jones I. G. Ribonuclease-sensitivity of covalently closed rat liver mitochondrial deoxyribonucleic acid. Biochem J. 1974 Jul;141(1):155–158. doi: 10.1042/bj1410155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer R. R., Simpson M. V. Deoxyribonucleic acid biosynthesis in mitochondria. Purification and general properties of rat liver mitochondrial deoxyribonucleic acid polymerase. J Biol Chem. 1970 Jul 10;245(13):3426–3435. [PubMed] [Google Scholar]
- Miyaki M., Koide K., Ono T. RNase and alkali sensitivity of closed circular mitochondrial DNA of rat ascites hepatoma cells. Biochem Biophys Res Commun. 1973 Jan 23;50(2):252–258. doi: 10.1016/0006-291x(73)90833-4. [DOI] [PubMed] [Google Scholar]
- Nass M. M. Mitochondrial DNA. II. Structure and physicochemical properties of isolated DNA. J Mol Biol. 1969 Jun 28;42(3):529–545. doi: 10.1016/0022-2836(69)90241-1. [DOI] [PubMed] [Google Scholar]
- Pikó L., Blair D. G., Tyler A., Vinograd J. Cytoplasmic DNA in the unfertilized sea urchin egg: physical properties of circular mitochondrial DNA and the occurrence of catenated forms. Proc Natl Acad Sci U S A. 1968 Mar;59(3):838–845. doi: 10.1073/pnas.59.3.838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhaese H. J., Freese E. Chemical analysis of DNA alterations. I. Base liberation and backbone breakage of DNA and oligodeoxyadenylic acid induced by hydrogen peroxide and hydroxylamine. Biochim Biophys Acta. 1968 Feb 26;155(2):476–490. [PubMed] [Google Scholar]
- Robberson D. L., Kasamatsu H., Vinograd J. Replication of mitochondrial DNA. Circular replicative intermediates in mouse L cells. Proc Natl Acad Sci U S A. 1972 Mar;69(3):737–741. doi: 10.1073/pnas.69.3.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saccone C., Gadaleta M. N. The effect of rifampicin on mitochondrial DNA synthesis. FEBS Lett. 1973 Aug 1;34(1):106–108. doi: 10.1016/0014-5793(73)80714-8. [DOI] [PubMed] [Google Scholar]
- Stavrianopoulos J. G., Chargaff E. Purification and properties of ribonuclease H of calf thymus. Proc Natl Acad Sci U S A. 1973 Jul;70(7):1959–1963. doi: 10.1073/pnas.70.7.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinograd J., Lebowitz J., Radloff R., Watson R., Laipis P. The twisted circular form of polyoma viral DNA. Proc Natl Acad Sci U S A. 1965 May;53(5):1104–1111. doi: 10.1073/pnas.53.5.1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt V. M. Purification and further properties of single-strand-specific nuclease from Aspergillus oryzae. Eur J Biochem. 1973 Feb 15;33(1):192–200. doi: 10.1111/j.1432-1033.1973.tb02669.x. [DOI] [PubMed] [Google Scholar]
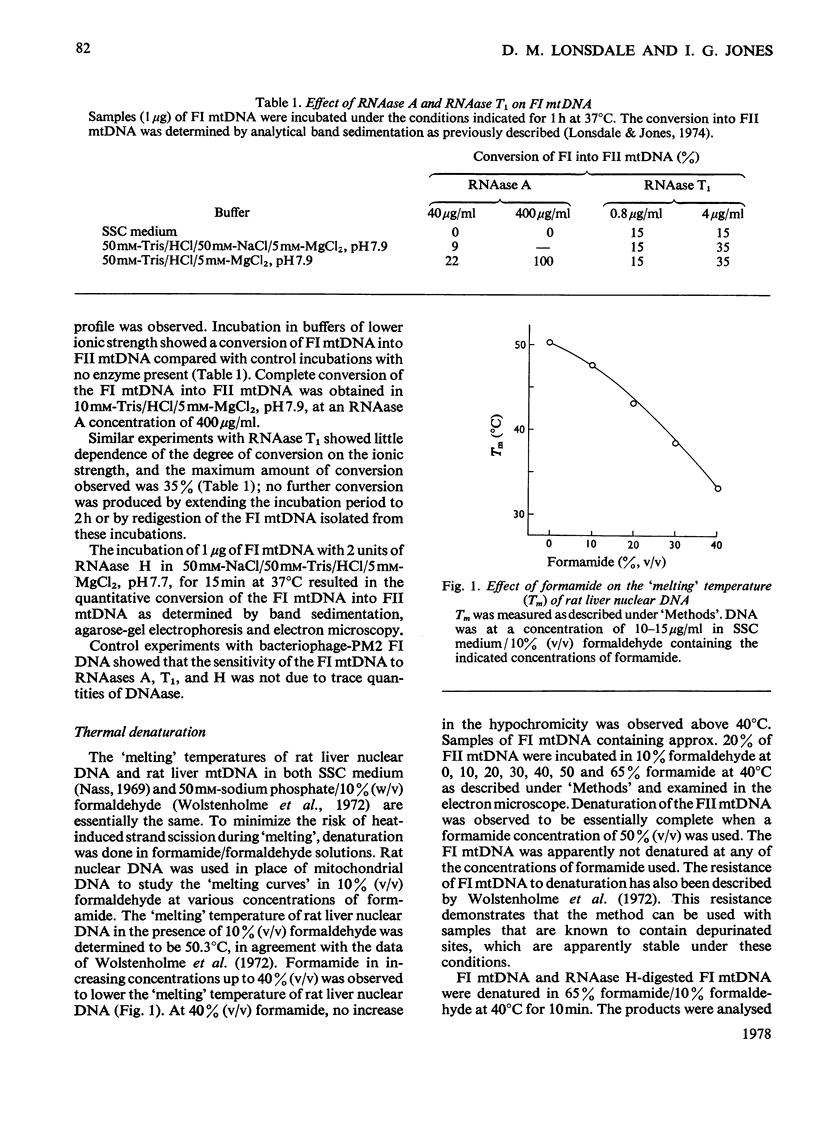
- Wolstonholme D. R., Kirschner R. G., Gross N. J. Heart denaturation studies of rat liver mitrochondrial DNA. A denaturation map and changes in molecular configurations. J Cell Biol. 1972 May;53(2):393–406. doi: 10.1083/jcb.53.2.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong-Staal F., Mendelsohn J., Goulian M. Ribonucleotides in closed circular mitochondrial DNA from HeLa cells. Biochem Biophys Res Commun. 1973 Jul 2;53(1):140–148. doi: 10.1016/0006-291x(73)91412-5. [DOI] [PubMed] [Google Scholar]
- ter Schegget J., Borst P. DNA synthesis by isolated mitochondria. I. Effect of inhibitors and characterization of the product. Biochim Biophys Acta. 1971 Aug 26;246(2):239–248. [PubMed] [Google Scholar]