Abstract
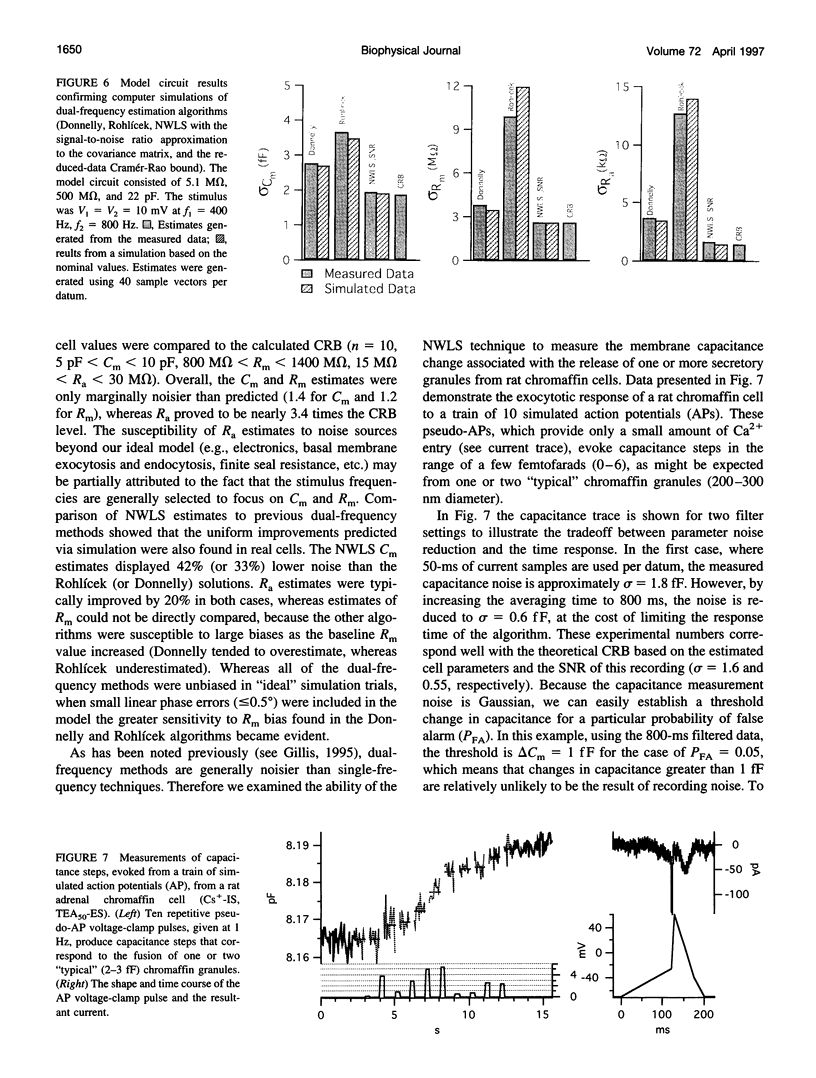
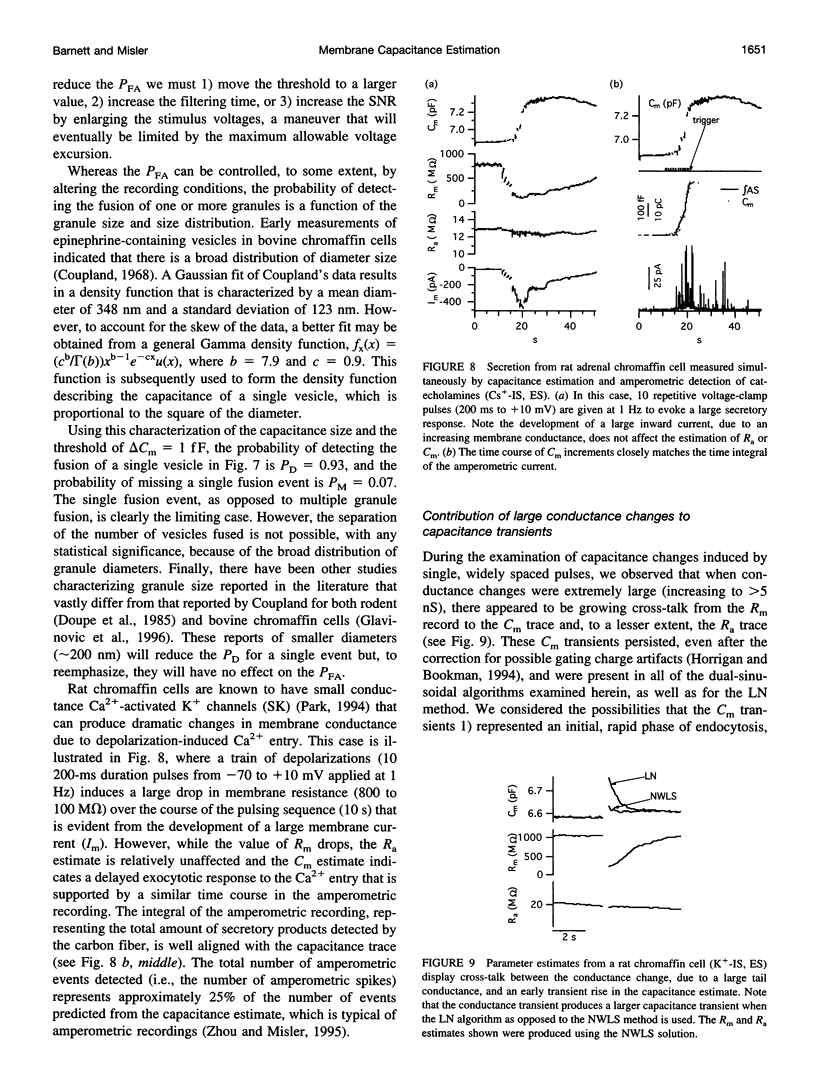
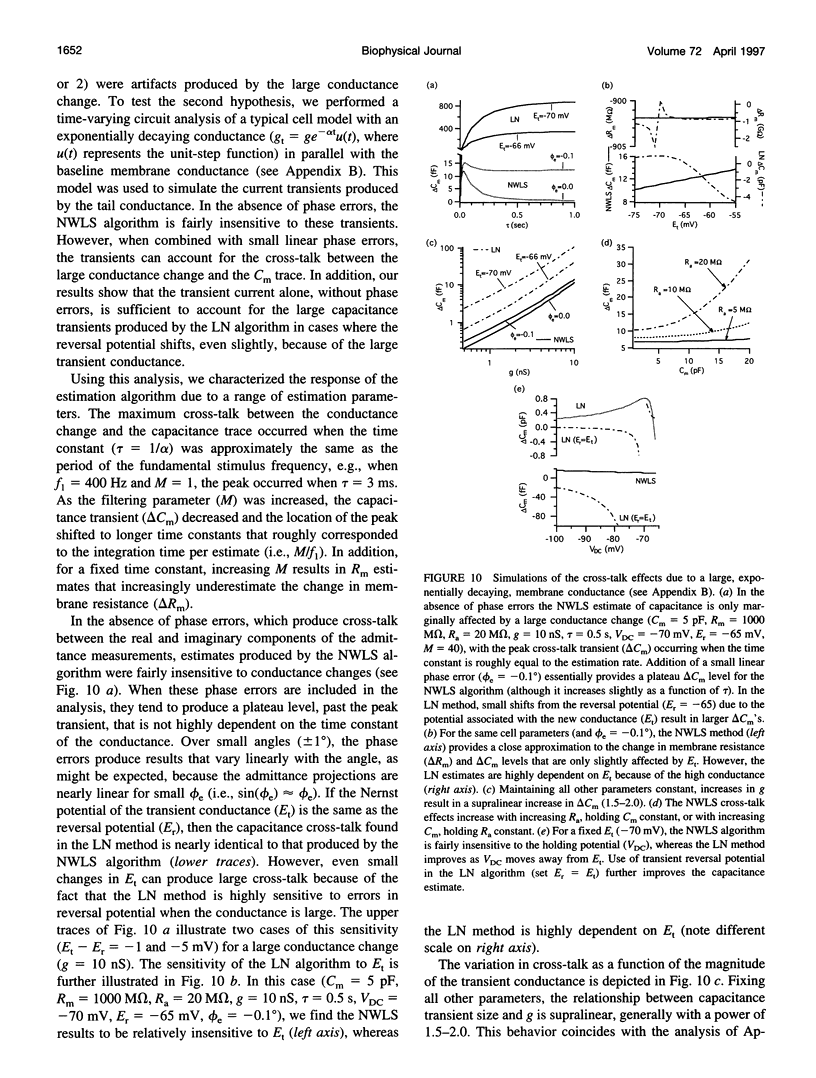
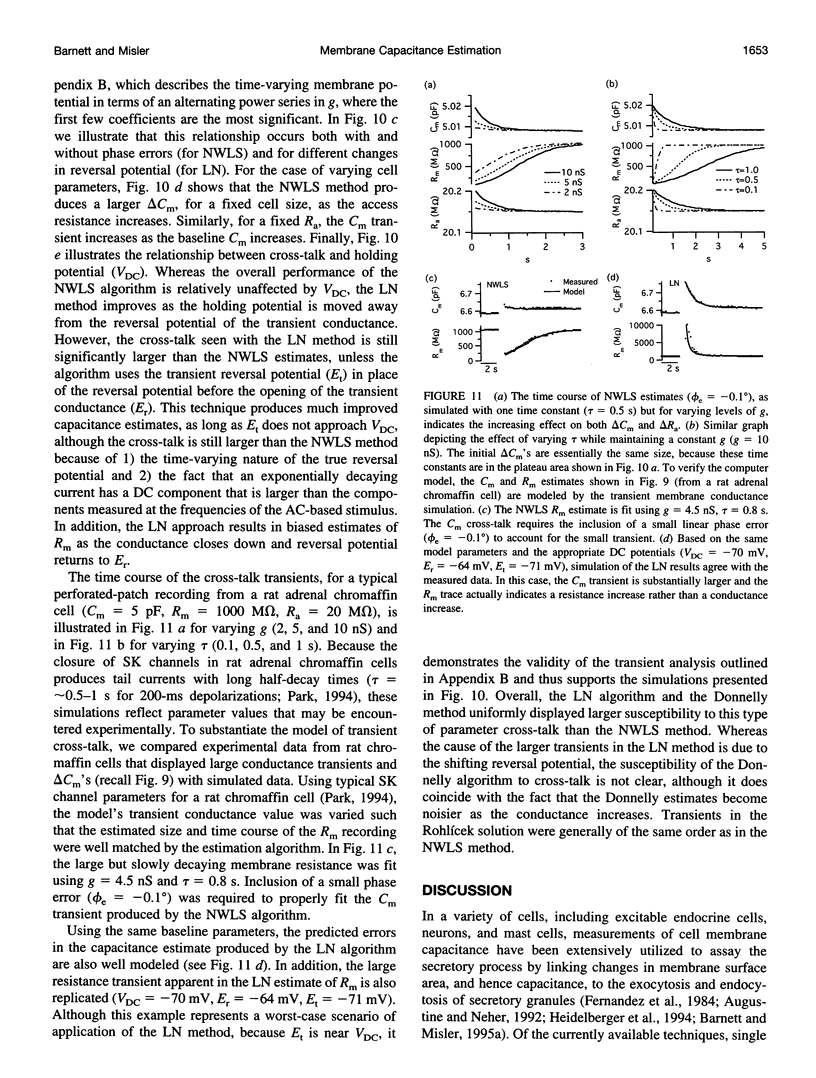
We present an optimized solution to the problem of membrane impedance estimation when a patch-clamped cell is stimulated by a dual-frequency, sinusoidal excitation. The complete data set of raw whole-cell current samples is typically reduced, via digital lock-in detection, to measurements of the complex cell model admittance at the two stimulus frequencies. We describe a statistical model of both data sets and demonstrate that the admittance data adequately represent the essential features obtained from the raw data. The parameter estimates obtained by a nonlinear weighted least-squares solution (NWLS), which under normal recording conditions is equivalent to the maximum likelihood solution, essentially obtain the theoretical lower bound on variance established by the Cramér-Rao bound. Our software implementation of the NWLS solution produces estimates of the cell model parameters that are less noisy than other dual-frequency systems. Our system can be used 1) to measure slow changes in membrane capacitance-in the face of large, slow changes in membrane resistance, 2) to detect with confidence capacitance changes expected from the exocytosis of moderate-sized dense core granules, and 3) to reduce the cross-talk between transient changes in membrane conductance and membrane capacitance.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Augustine G. J., Neher E. Calcium requirements for secretion in bovine chromaffin cells. J Physiol. 1992 May;450:247–271. doi: 10.1113/jphysiol.1992.sp019126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett D. W., Liu J., Misler S. Single-cell measurements of quantal secretion induced by alpha-latrotoxin from rat adrenal chromaffin cells: dependence on extracellular Ca2+. Pflugers Arch. 1996 Oct;432(6):1039–1046. doi: 10.1007/s004240050232. [DOI] [PubMed] [Google Scholar]
- Barnett D. W., Misler S. Coupling of exocytosis to depolarization in rat pancreatic islet beta-cells: effects of Ca2+, Sr2+ and Ba(2+)-containing extracellular solutions. Pflugers Arch. 1995 Aug;430(4):593–595. doi: 10.1007/BF00373898. [DOI] [PubMed] [Google Scholar]
- Barnett D. W., Pressel D. M., Misler S. Voltage-dependent Na+ and Ca2+ currents in human pancreatic islet beta-cells: evidence for roles in the generation of action potentials and insulin secretion. Pflugers Arch. 1995 Dec;431(2):272–282. doi: 10.1007/BF00410201. [DOI] [PubMed] [Google Scholar]
- Clausen C., Fernandez J. M. A low-cost method for rapid transfer function measurements with direct application to biological impedance analysis. Pflugers Arch. 1981 Jun;390(3):290–295. doi: 10.1007/BF00658279. [DOI] [PubMed] [Google Scholar]
- Coupland R. E. Determining sizes and distribution of sizes of spherical bodies such as chromaffin granules in tissue sections. Nature. 1968 Jan 27;217(5126):384–388. doi: 10.1038/217384a0. [DOI] [PubMed] [Google Scholar]
- Debus K., Hartmann J., Kilic G., Lindau M. Influence of conductance changes on patch clamp capacitance measurements using a lock-in amplifier and limitations of the phase tracking technique. Biophys J. 1995 Dec;69(6):2808–2822. doi: 10.1016/S0006-3495(95)80154-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly D. F. A novel method for rapid measurement of membrane resistance, capacitance, and access resistance. Biophys J. 1994 Mar;66(3 Pt 1):873–877. doi: 10.1016/s0006-3495(94)80863-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly D. F. Response to cyanide of two types of glomoid cells in mature rat carotid body. Brain Res. 1993 Dec 10;630(1-2):157–168. doi: 10.1016/0006-8993(93)90653-5. [DOI] [PubMed] [Google Scholar]
- Doupe A. J., Landis S. C., Patterson P. H. Environmental influences in the development of neural crest derivatives: glucocorticoids, growth factors, and chromaffin cell plasticity. J Neurosci. 1985 Aug;5(8):2119–2142. doi: 10.1523/JNEUROSCI.05-08-02119.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez J. M., Lindau M., Eckstein F. Intracellular stimulation of mast cells with guanine nucleotides mimic antigenic stimulation. FEBS Lett. 1987 May 25;216(1):89–93. doi: 10.1016/0014-5793(87)80762-7. [DOI] [PubMed] [Google Scholar]
- Fernandez J. M., Neher E., Gomperts B. D. Capacitance measurements reveal stepwise fusion events in degranulating mast cells. 1984 Nov 29-Dec 5Nature. 312(5993):453–455. doi: 10.1038/312453a0. [DOI] [PubMed] [Google Scholar]
- Fidler N., Fernandez J. M. Phase tracking: an improved phase detection technique for cell membrane capacitance measurements. Biophys J. 1989 Dec;56(6):1153–1162. doi: 10.1016/S0006-3495(89)82762-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillis K. D., Misler S. Single cell assay of exocytosis from pancreatic islet B cells. Pflugers Arch. 1992 Jan;420(1):121–123. doi: 10.1007/BF00378654. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Heidelberger R., Heinemann C., Neher E., Matthews G. Calcium dependence of the rate of exocytosis in a synaptic terminal. Nature. 1994 Oct 6;371(6497):513–515. doi: 10.1038/371513a0. [DOI] [PubMed] [Google Scholar]
- Heinemann C., Chow R. H., Neher E., Zucker R. S. Kinetics of the secretory response in bovine chromaffin cells following flash photolysis of caged Ca2+. Biophys J. 1994 Dec;67(6):2546–2557. doi: 10.1016/S0006-3495(94)80744-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horrigan F. T., Bookman R. J. Releasable pools and the kinetics of exocytosis in adrenal chromaffin cells. Neuron. 1994 Nov;13(5):1119–1129. doi: 10.1016/0896-6273(94)90050-7. [DOI] [PubMed] [Google Scholar]
- Joshi C., Fernandez J. M. Capacitance measurements. An analysis of the phase detector technique used to study exocytosis and endocytosis. Biophys J. 1988 Jun;53(6):885–892. doi: 10.1016/S0006-3495(88)83169-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korn S. J., Horn R. Influence of sodium-calcium exchange on calcium current rundown and the duration of calcium-dependent chloride currents in pituitary cells, studied with whole cell and perforated patch recording. J Gen Physiol. 1989 Nov;94(5):789–812. doi: 10.1085/jgp.94.5.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindau M., Fernandez J. M. IgE-mediated degranulation of mast cells does not require opening of ion channels. Nature. 1986 Jan 9;319(6049):150–153. doi: 10.1038/319150a0. [DOI] [PubMed] [Google Scholar]
- Lindau M., Neher E. Patch-clamp techniques for time-resolved capacitance measurements in single cells. Pflugers Arch. 1988 Feb;411(2):137–146. doi: 10.1007/BF00582306. [DOI] [PubMed] [Google Scholar]
- Neely A., Lingle C. J. Two components of calcium-activated potassium current in rat adrenal chromaffin cells. J Physiol. 1992;453:97–131. doi: 10.1113/jphysiol.1992.sp019220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E., Marty A. Discrete changes of cell membrane capacitance observed under conditions of enhanced secretion in bovine adrenal chromaffin cells. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6712–6716. doi: 10.1073/pnas.79.21.6712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park Y. B. Ion selectivity and gating of small conductance Ca(2+)-activated K+ channels in cultured rat adrenal chromaffin cells. J Physiol. 1994 Dec 15;481(Pt 3):555–570. doi: 10.1113/jphysiol.1994.sp020463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penner R., Neher E. Stimulus-secretion coupling in mast cells. Soc Gen Physiol Ser. 1989;44:295–310. [PubMed] [Google Scholar]
- Penner R., Neher E. The role of calcium in stimulus-secretion coupling in excitable and non-excitable cells. J Exp Biol. 1988 Sep;139:329–345. doi: 10.1242/jeb.139.1.329. [DOI] [PubMed] [Google Scholar]
- Rohlicek V., Schmid A. Dual-frequency method for synchronous measurement of cell capacitance, membrane conductance and access resistance on single cells. Pflugers Arch. 1994 Aug;428(1):30–38. doi: 10.1007/BF00374749. [DOI] [PubMed] [Google Scholar]
- Zhou Z., Misler S. Action potential-induced quantal secretion of catecholamines from rat adrenal chromaffin cells. J Biol Chem. 1995 Feb 24;270(8):3498–3505. [PubMed] [Google Scholar]
- Zhou Z., Misler S. Amperometric detection of quantal secretion from patch-clamped rat pancreatic beta-cells. J Biol Chem. 1996 Jan 5;271(1):270–277. doi: 10.1074/jbc.271.1.270. [DOI] [PubMed] [Google Scholar]
- Zierler K. Simplified method for setting the phase angle used in capacitance measurements in studies of exocytosis. Biophys J. 1992 Sep;63(3):854–856. doi: 10.1016/S0006-3495(92)81639-9. [DOI] [PMC free article] [PubMed] [Google Scholar]