Abstract
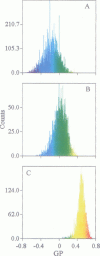
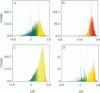
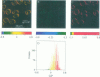
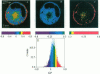
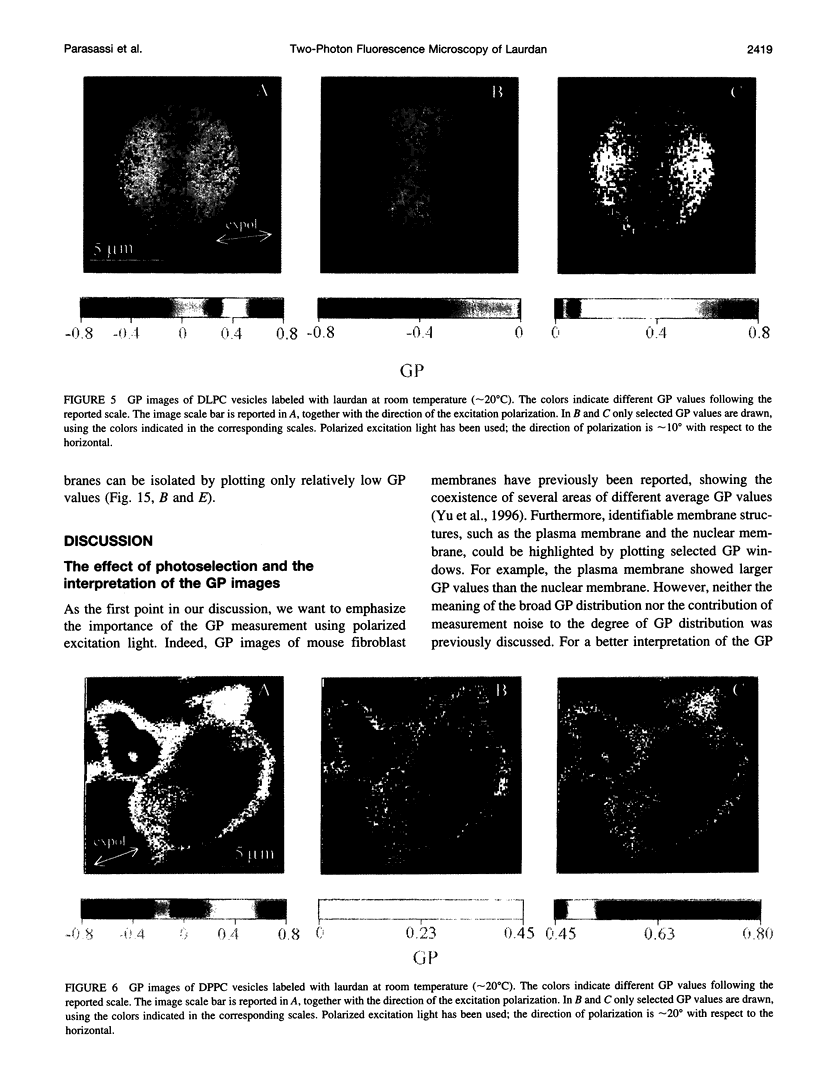
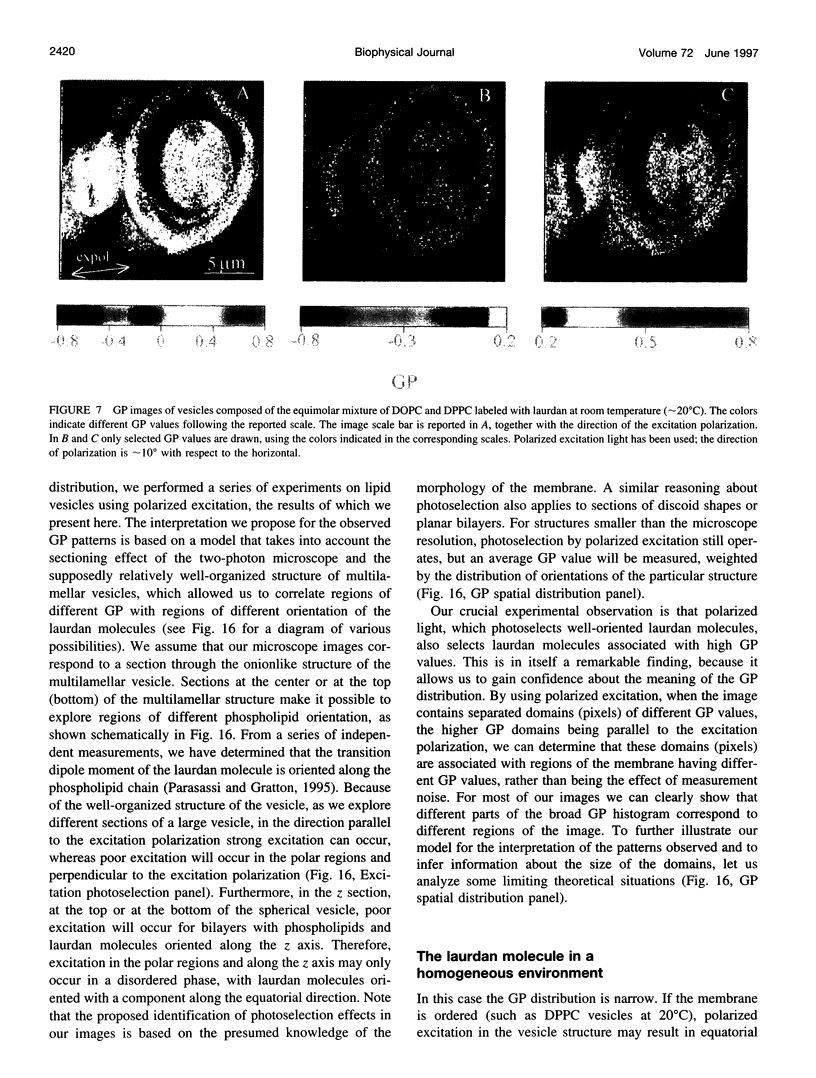
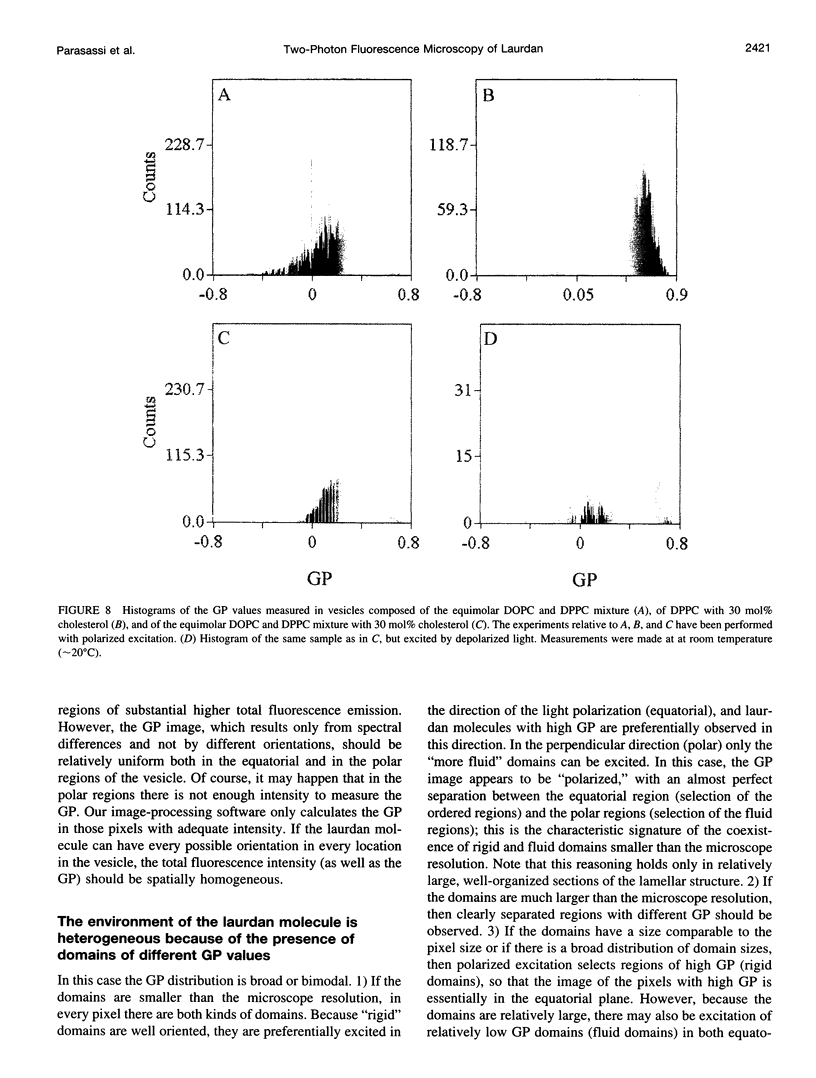
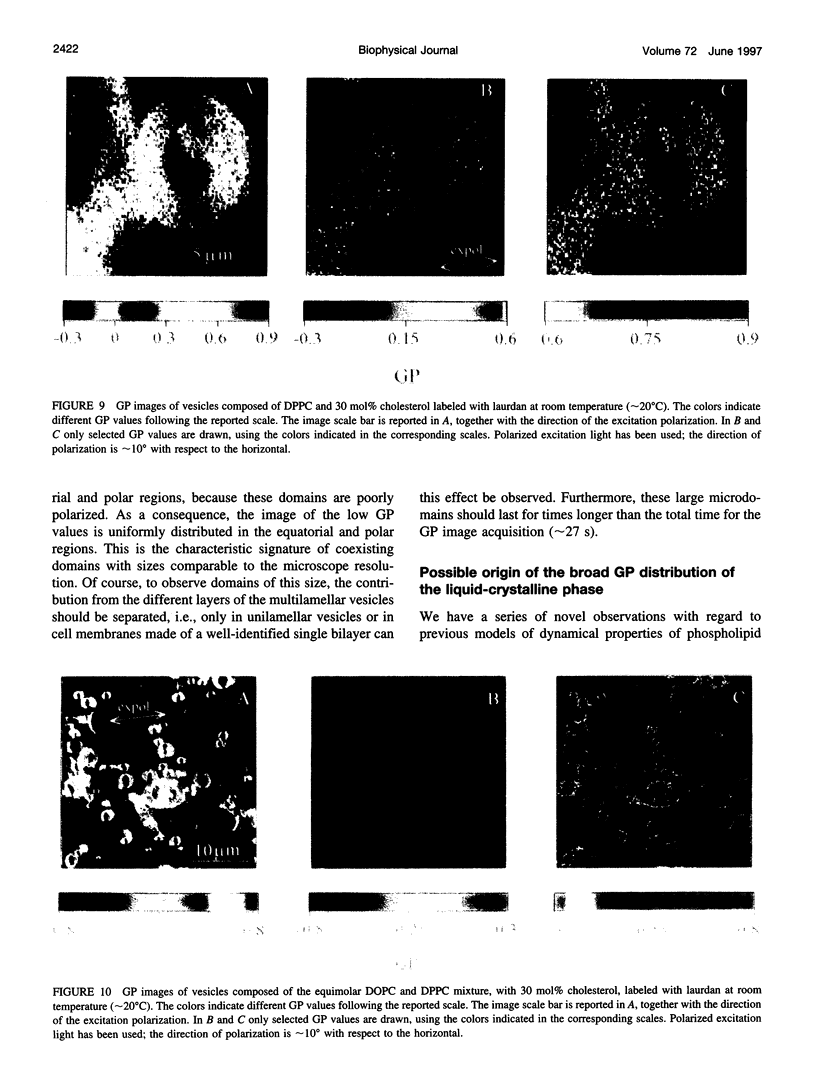
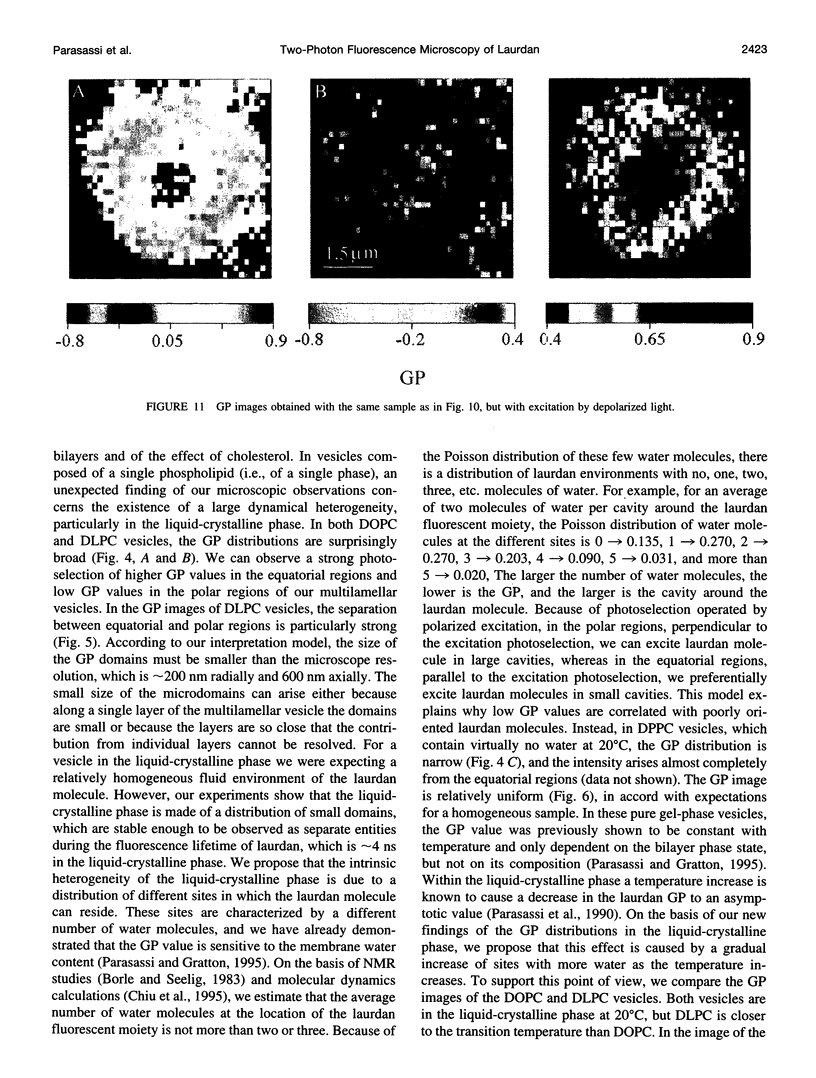
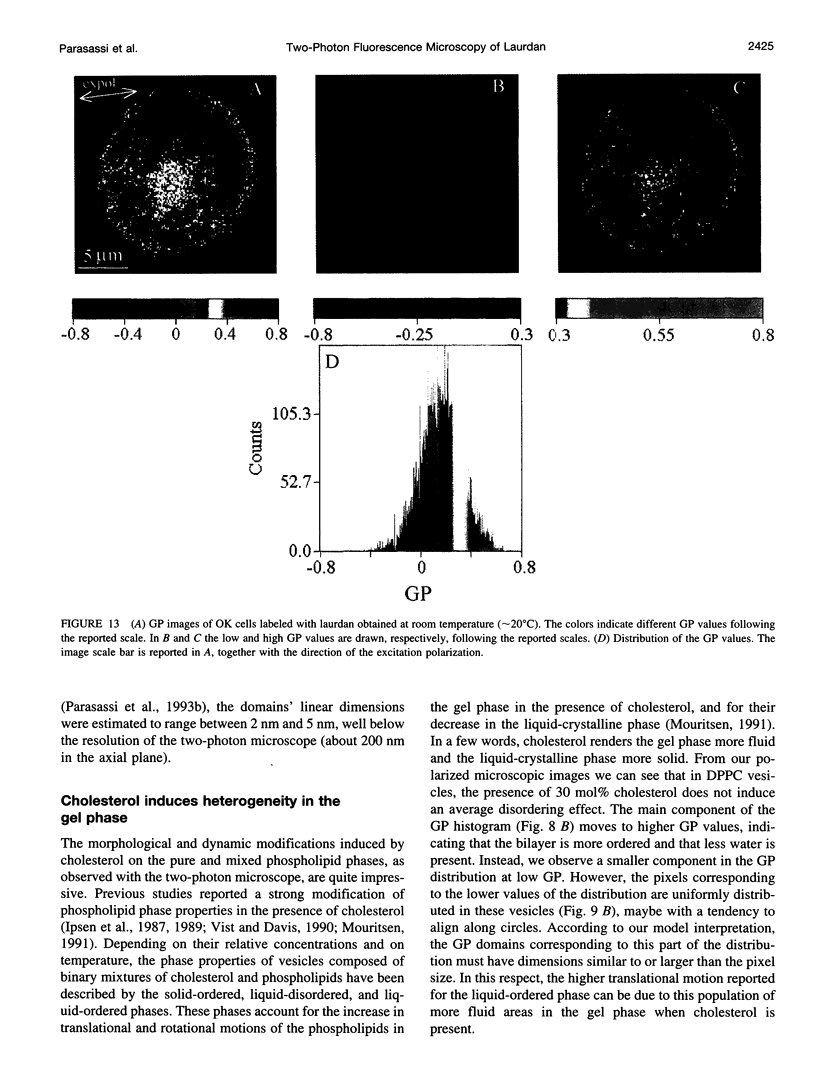
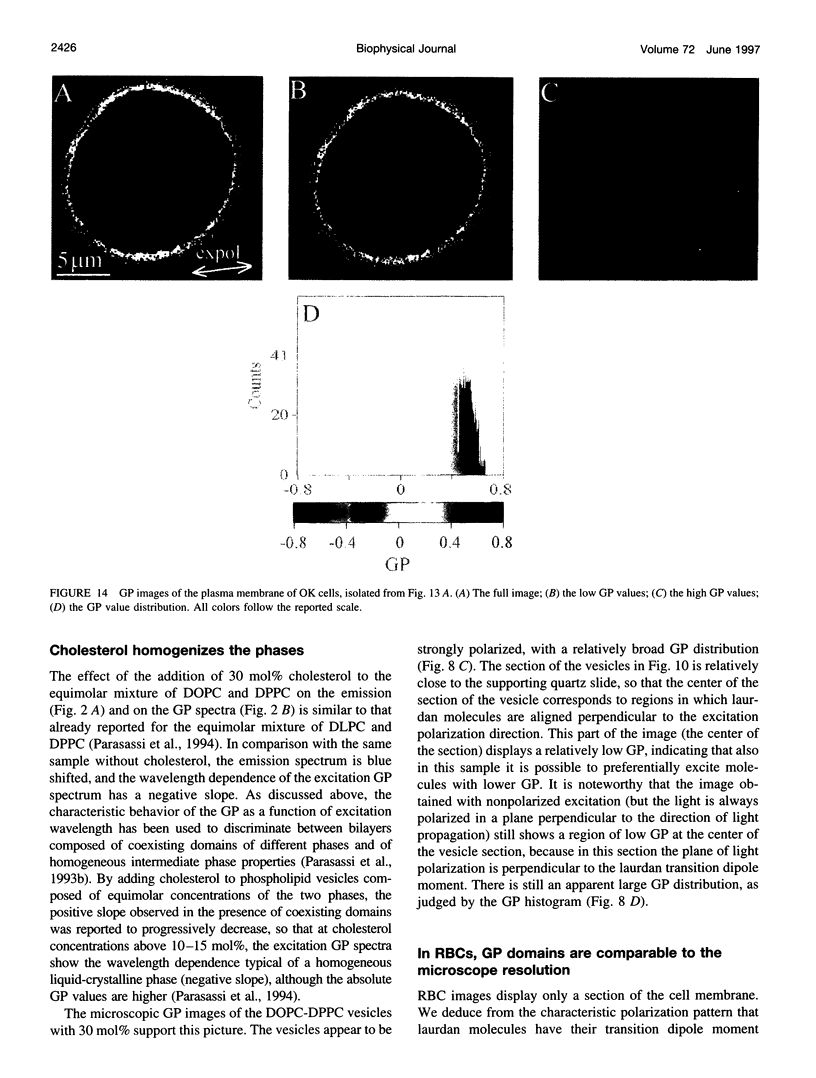
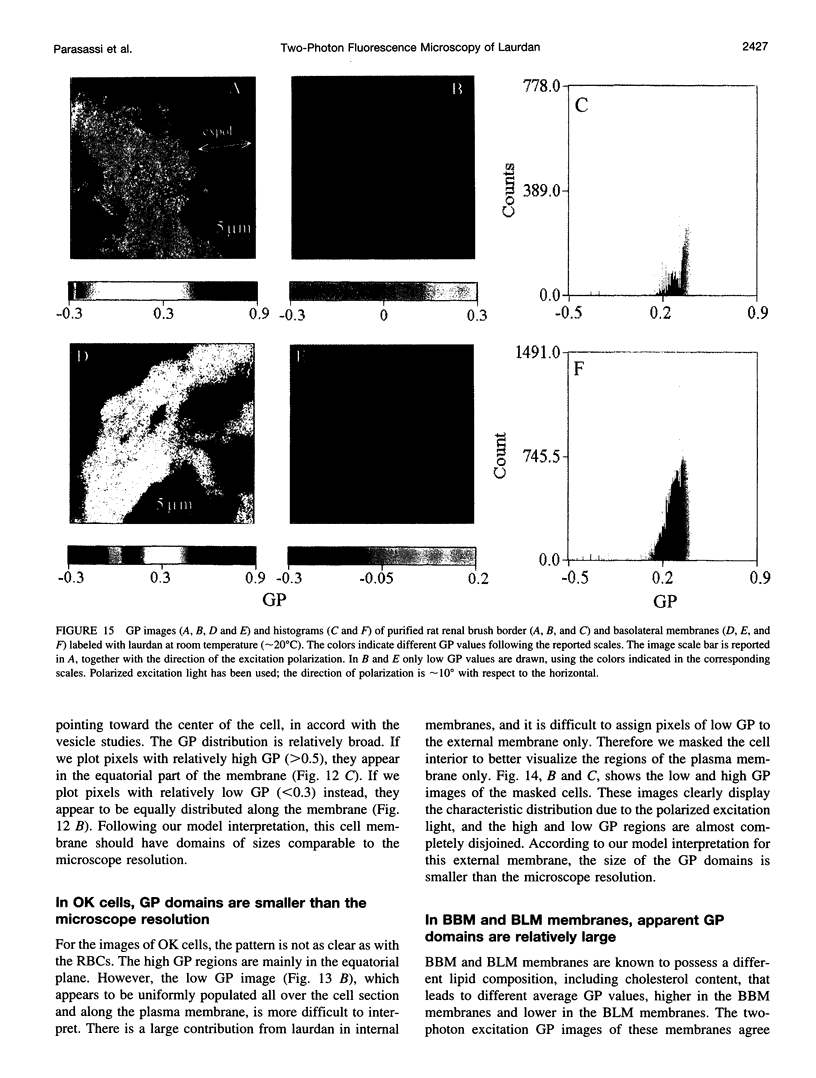
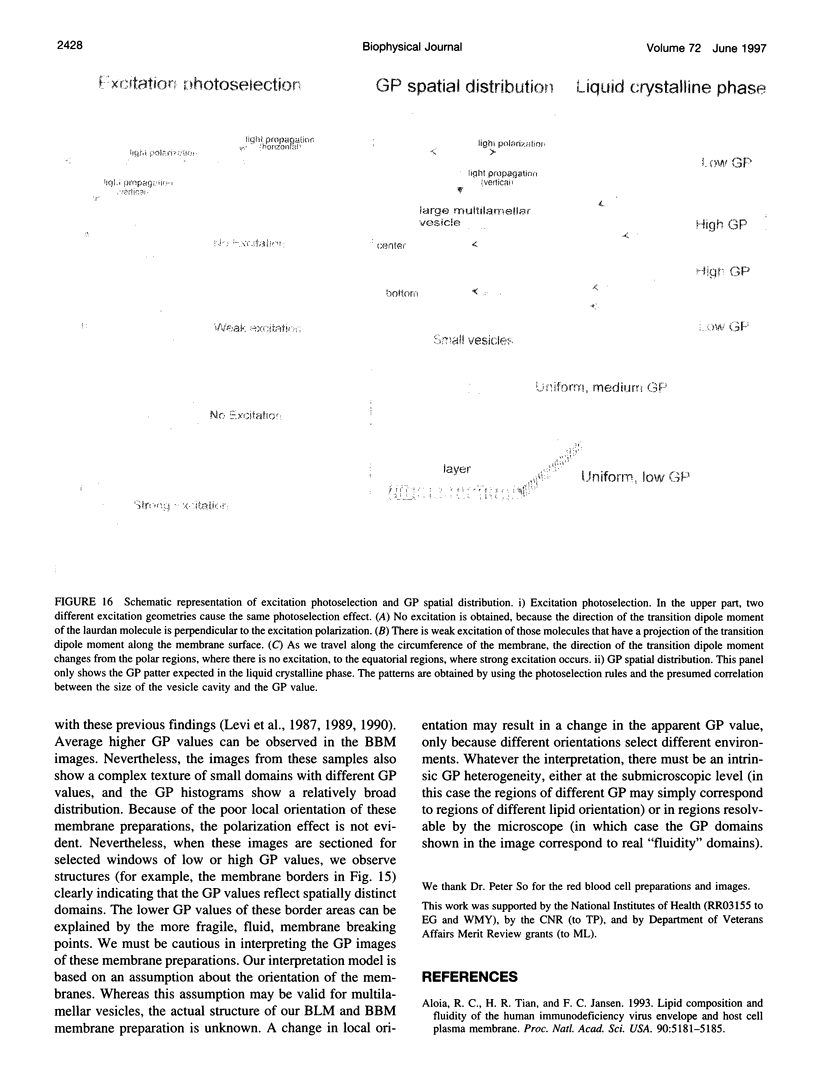
Two-photon excitation microscopy shows coexisting regions of different generalized polarization (GP) in phospholipid vesicles, in red blood cells, in a renal tubular cell line, and in purified renal brushborder and basolateral membranes labeled with the fluorescent probe laurdan. The GP function measures the relative water content of the membrane. In the present study we discuss images obtained with polarized laser excitation, which selects different molecular orientations of the lipid bilayer corresponding to different spatial regions. The GP distribution in the gel-phase vesicles is relatively narrow, whereas the GP distribution in the liquid-crystalline phase vesicles (DOPC and DLPC) is broad. Analysis of images obtained with polarized excitation of the liquid-crystalline phase vesicles leads to the conclusion that coexisting regions of different GP must have dimensions smaller than the microscope resolution (approximately 200 nm radially and 600 nm axially). Vesicles of an equimolar mixture of DOPC and DPPC show coexisting rigid and fluid domains (high GP and low GP), but the rigid domains, which are preferentially excited by polarized light, have GP values lower than the pure gel-phase domains. Cholesterol strongly modifies the domain morphology. In the presence of 30 mol% cholesterol, the broad GP distribution of the DOPC/DPPC equimolar sample becomes narrower. The sample is still very heterogeneous, as demonstrated by the separations of GP disjoined regions, which are the result of photoselection of regions of different lipid orientation. In intact red blood cells, microscopic regions of different GP can be resolved, whereas in the renal cells GP domains have dimensions smaller than the microscope resolution. Preparations of renal apical brush border membranes and basolateral membranes show well-resolved GP domains, which may result from a different local orientation, or the domains may reflect a real heterogeneity of these membranes.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aloia R. C., Tian H., Jensen F. C. Lipid composition and fluidity of the human immunodeficiency virus envelope and host cell plasma membranes. Proc Natl Acad Sci U S A. 1993 Jun 1;90(11):5181–5185. doi: 10.1073/pnas.90.11.5181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arar M., Baum M., Biber J., Murer H., Levi M. Epidermal growth factor inhibits Na-Pi cotransport and mRNA in OK cells. Am J Physiol. 1995 Feb;268(2 Pt 2):F309–F314. doi: 10.1152/ajprenal.1995.268.2.F309. [DOI] [PubMed] [Google Scholar]
- Borle F., Seelig J. Hydration of Escherichia coli lipids. Deuterium T1 relaxation time studies of phosphatidylglycerol, phosphatidylethanolamine and phosphatidylcholine. Biochim Biophys Acta. 1983 Oct 26;735(1):131–136. doi: 10.1016/0005-2736(83)90268-7. [DOI] [PubMed] [Google Scholar]
- Chiu S. W., Clark M., Balaji V., Subramaniam S., Scott H. L., Jakobsson E. Incorporation of surface tension into molecular dynamics simulation of an interface: a fluid phase lipid bilayer membrane. Biophys J. 1995 Oct;69(4):1230–1245. doi: 10.1016/S0006-3495(95)80005-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong P. L. Evidence for regular distribution of sterols in liquid crystalline phosphatidylcholine bilayers. Proc Natl Acad Sci U S A. 1994 Oct 11;91(21):10069–10073. doi: 10.1073/pnas.91.21.10069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong P. L., Tang D., Sugar I. P. Exploration of physical principles underlying lipid regular distribution: effects of pressure, temperature, and radius of curvature on E/M dips in pyrene-labeled PC/DMPC binary mixtures. Biophys J. 1994 Jun;66(6):2029–2038. doi: 10.1016/S0006-3495(94)80996-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ipsen J. H., Karlström G., Mouritsen O. G., Wennerström H., Zuckermann M. J. Phase equilibria in the phosphatidylcholine-cholesterol system. Biochim Biophys Acta. 1987 Nov 27;905(1):162–172. doi: 10.1016/0005-2736(87)90020-4. [DOI] [PubMed] [Google Scholar]
- Ipsen J. H., Mouritsen O. G., Zuckermann M. J. Theory of thermal anomalies in the specific heat of lipid bilayers containing cholesterol. Biophys J. 1989 Oct;56(4):661–667. doi: 10.1016/S0006-3495(89)82713-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi M., Baird B. M., Wilson P. V. Cholesterol modulates rat renal brush border membrane phosphate transport. J Clin Invest. 1990 Jan;85(1):231–237. doi: 10.1172/JCI114417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi M., Jameson D. M., van der Meer B. W. Role of BBM lipid composition and fluidity in impaired renal Pi transport in aged rat. Am J Physiol. 1989 Jan;256(1 Pt 2):F85–F94. doi: 10.1152/ajprenal.1989.256.1.F85. [DOI] [PubMed] [Google Scholar]
- Levi M., Molitoris B. A., Burke T. J., Schrier R. W., Simon F. R. Effects of vitamin D-induced chronic hypercalcemia on rat renal cortical plasma membranes and mitochondria. Am J Physiol. 1987 Feb;252(2 Pt 2):F267–F275. doi: 10.1152/ajprenal.1987.252.2.F267. [DOI] [PubMed] [Google Scholar]
- Maresca B., Cossins A. R. Cell physiology. Fatty feedback and fluidity. Nature. 1993 Oct 14;365(6447):606–607. doi: 10.1038/365606a0. [DOI] [PubMed] [Google Scholar]
- Molitoris B. A., Simon F. R. Renal cortical brush-border and basolateral membranes: cholesterol and phospholipid composition and relative turnover. J Membr Biol. 1985;83(3):207–215. doi: 10.1007/BF01868695. [DOI] [PubMed] [Google Scholar]
- Mouritsen O. G. Theoretical models of phospholipid phase transitions. Chem Phys Lipids. 1991 Mar;57(2-3):179–194. doi: 10.1016/0009-3084(91)90075-m. [DOI] [PubMed] [Google Scholar]
- Parasassi T., De Stasio G., d'Ubaldo A., Gratton E. Phase fluctuation in phospholipid membranes revealed by Laurdan fluorescence. Biophys J. 1990 Jun;57(6):1179–1186. doi: 10.1016/S0006-3495(90)82637-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parasassi T., Di Stefano M., Loiero M., Ravagnan G., Gratton E. Influence of cholesterol on phospholipid bilayers phase domains as detected by Laurdan fluorescence. Biophys J. 1994 Jan;66(1):120–132. doi: 10.1016/S0006-3495(94)80763-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parasassi T., Giusti A. M., Raimondi M., Gratton E. Abrupt modifications of phospholipid bilayer properties at critical cholesterol concentrations. Biophys J. 1995 May;68(5):1895–1902. doi: 10.1016/S0006-3495(95)80367-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parasassi T., Loiero M., Raimondi M., Ravagnan G., Gratton E. Absence of lipid gel-phase domains in seven mammalian cell lines and in four primary cell types. Biochim Biophys Acta. 1993 Dec 12;1153(2):143–154. doi: 10.1016/0005-2736(93)90399-k. [DOI] [PubMed] [Google Scholar]
- Parasassi T., Ravagnan G., Rusch R. M., Gratton E. Modulation and dynamics of phase properties in phospholipid mixtures detected by Laurdan fluorescence. Photochem Photobiol. 1993 Mar;57(3):403–410. doi: 10.1111/j.1751-1097.1993.tb02309.x. [DOI] [PubMed] [Google Scholar]
- Rodgers W., Glaser M. Characterization of lipid domains in erythrocyte membranes. Proc Natl Acad Sci U S A. 1991 Feb 15;88(4):1364–1368. doi: 10.1073/pnas.88.4.1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sankaram M. B., Thompson T. E. Modulation of phospholipid acyl chain order by cholesterol. A solid-state 2H nuclear magnetic resonance study. Biochemistry. 1990 Nov 27;29(47):10676–10684. doi: 10.1021/bi00499a015. [DOI] [PubMed] [Google Scholar]
- Virtanen J. A., Ruonala M., Vauhkonen M., Somerharju P. Lateral organization of liquid-crystalline cholesterol-dimyristoylphosphatidylcholine bilayers. Evidence for domains with hexagonal and centered rectangular cholesterol superlattices. Biochemistry. 1995 Sep 12;34(36):11568–11581. doi: 10.1021/bi00036a033. [DOI] [PubMed] [Google Scholar]
- Vist M. R., Davis J. H. Phase equilibria of cholesterol/dipalmitoylphosphatidylcholine mixtures: 2H nuclear magnetic resonance and differential scanning calorimetry. Biochemistry. 1990 Jan 16;29(2):451–464. doi: 10.1021/bi00454a021. [DOI] [PubMed] [Google Scholar]
- Yu W., So P. T., French T., Gratton E. Fluorescence generalized polarization of cell membranes: a two-photon scanning microscopy approach. Biophys J. 1996 Feb;70(2):626–636. doi: 10.1016/S0006-3495(96)79646-7. [DOI] [PMC free article] [PubMed] [Google Scholar]