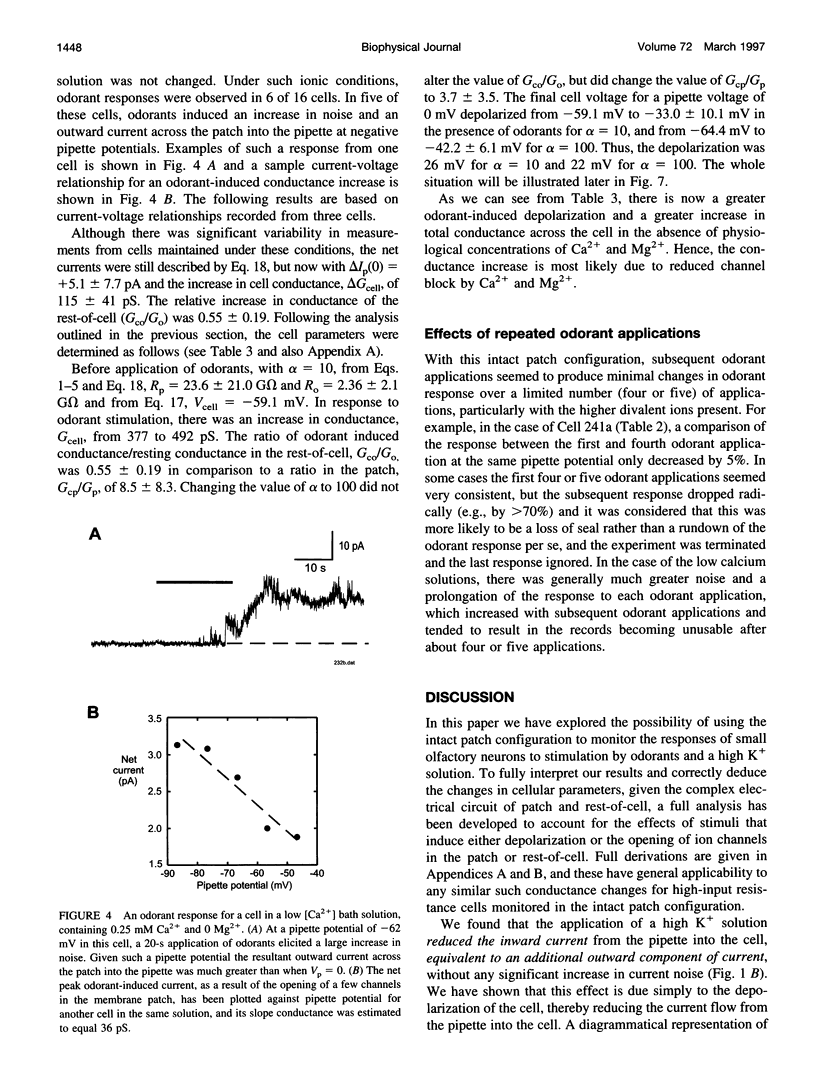
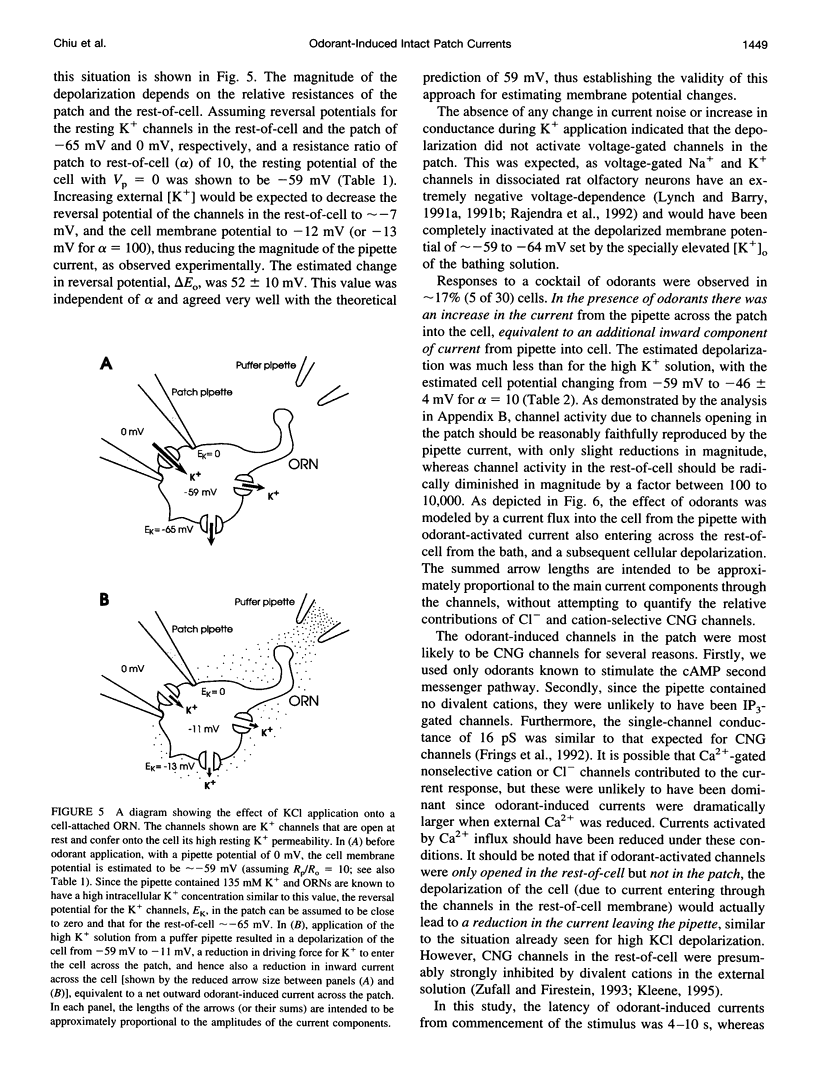
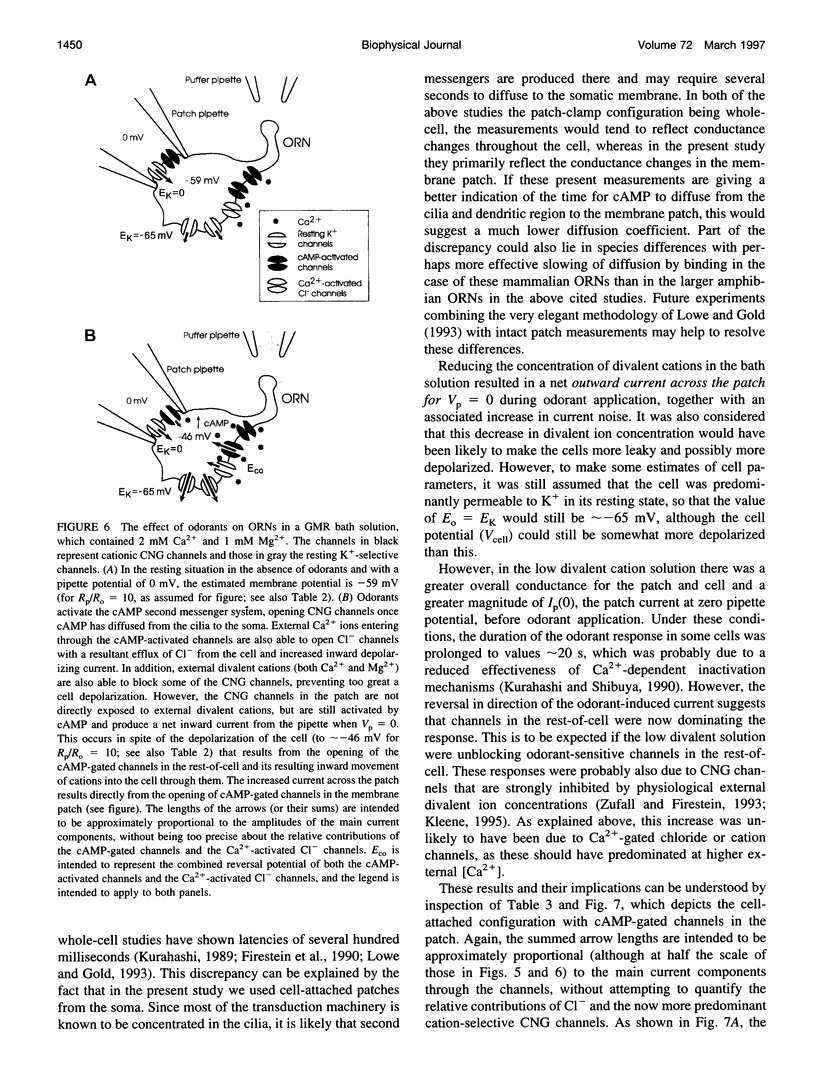
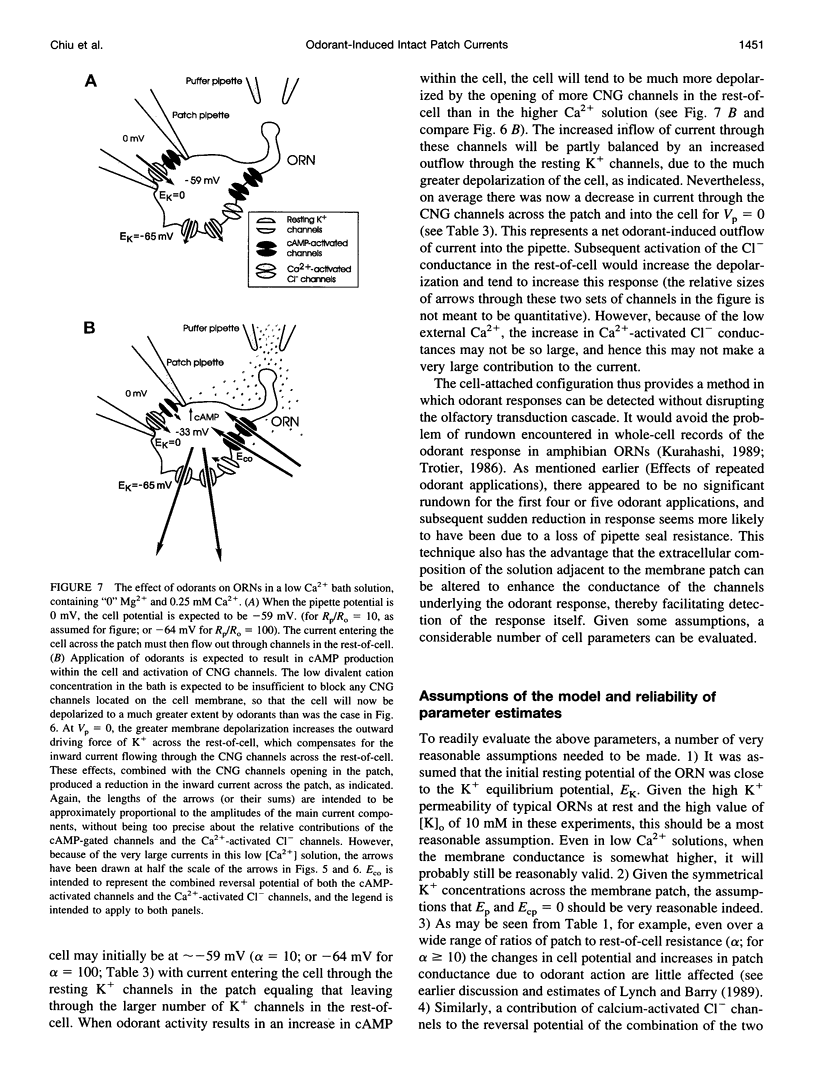
Abstract
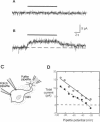
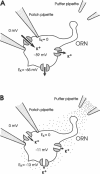
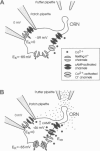
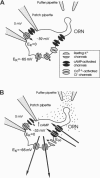
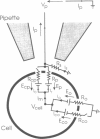
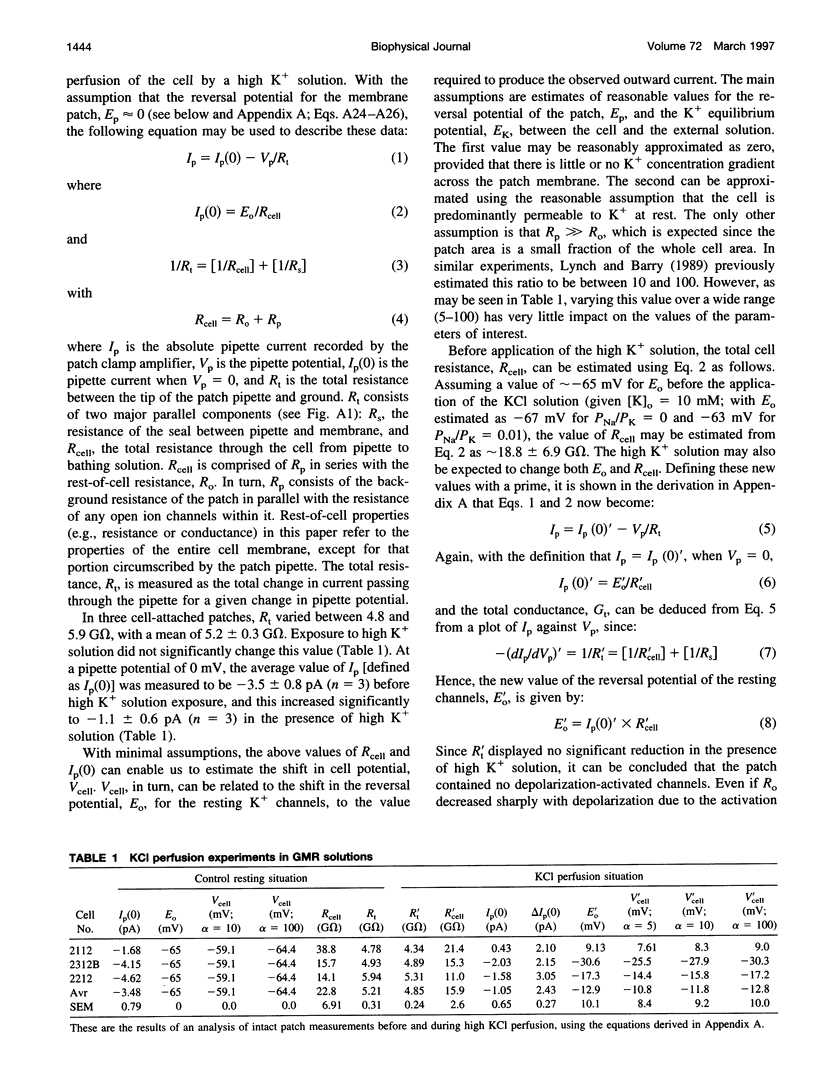
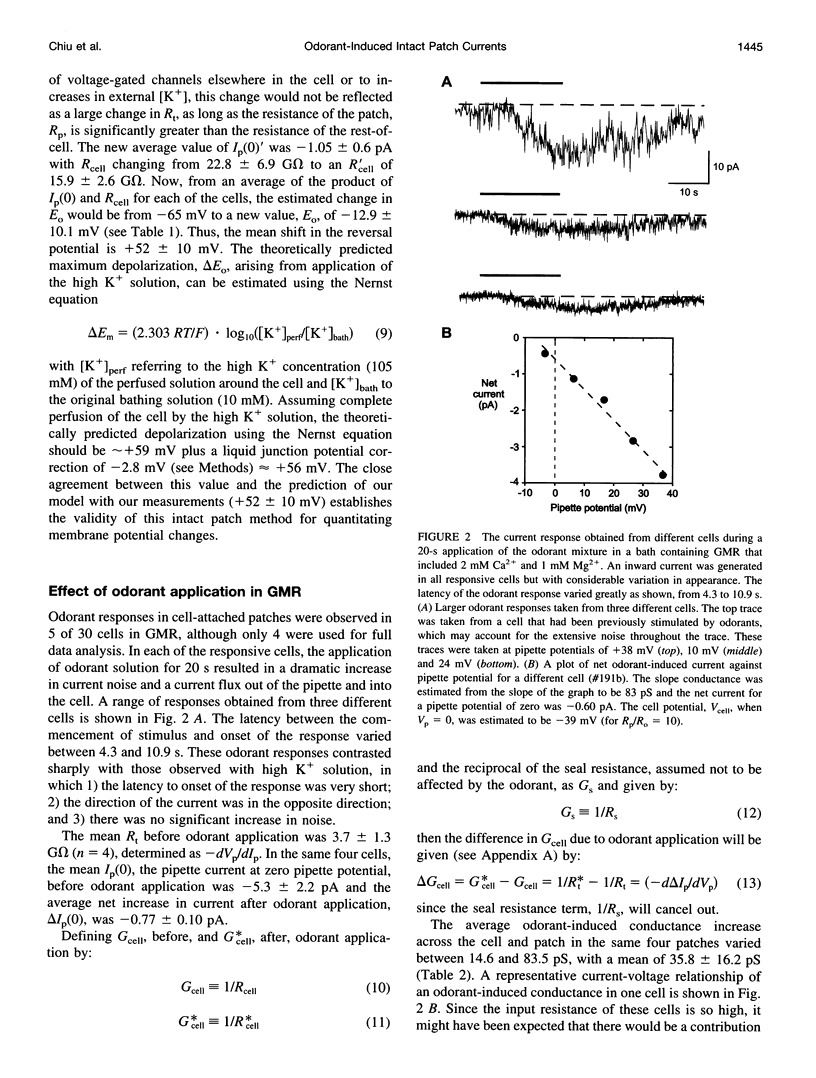
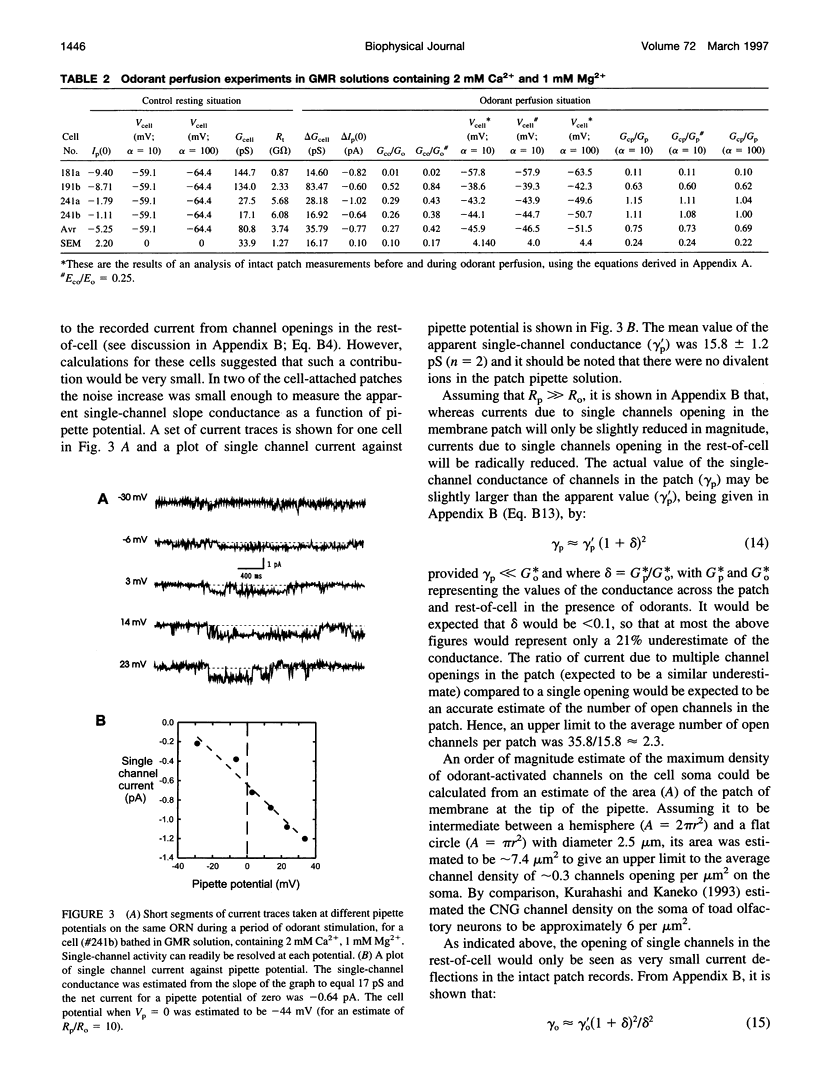
Odorant-induced currents in mammalian olfactory receptor neurons have proved difficult to obtain reliably using conventional whole-cell recording. By using a mathematical model of the electrical circuit of the patch and rest-of-cell, we demonstrate how cell-attached patch measurements can be used to quantitatively analyze responses to odorants or a high (100 mM) K+ solution. High K+ induced an immediate current flux from cell to pipette, which was modeled as a depolarization of approximately 52 mV, close to that expected from the Nernst equation (56 mV), and no change in the patch conductance. By contrast, a cocktail of cAMP-stimulating odorants induced a current flux from pipette into cell following a significant (4-10 s) delay. This was modeled as an average patch conductance increase of 36 pS and a depolarization of 13 mV. Odorant-induced single channels had a conductance of 16 pS. In cells bathed with no Mg2+ and 0.25 mM Ca2+, odorants induced a current flow from cell to pipette, which was modeled as a patch conductance increase of approximately 115 pS and depolarization of approximately 32 mV. All these results are consistent with cAMP-gated cation channels dominating the odorant response. This approach, which provides useful estimates of odorant-induced voltage and conductance changes, is applicable to similar measurements in any small cells.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ache B. W., Zhainazarov A. Dual second-messenger pathways in olfactory transduction. Curr Opin Neurobiol. 1995 Aug;5(4):461–466. doi: 10.1016/0959-4388(95)80006-9. [DOI] [PubMed] [Google Scholar]
- Barry P. H. JPCalc, a software package for calculating liquid junction potential corrections in patch-clamp, intracellular, epithelial and bilayer measurements and for correcting junction potential measurements. J Neurosci Methods. 1994 Jan;51(1):107–116. doi: 10.1016/0165-0270(94)90031-0. [DOI] [PubMed] [Google Scholar]
- Barry P. H., Quartararo N. PNSCROLL, a software package for graphical interactive analysis of single channel patch clamp currents and other binary file records: under mouse control. Comput Biol Med. 1990;20(3):193–204. doi: 10.1016/0010-4825(90)90005-a. [DOI] [PubMed] [Google Scholar]
- Bezanilla F. A high capacity data recording device based on a digital audio processor and a video cassette recorder. Biophys J. 1985 Mar;47(3):437–441. doi: 10.1016/S0006-3495(85)83935-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dionne V. E., Dubin A. E. Transduction diversity in olfaction. J Exp Biol. 1994 Sep;194:1–21. doi: 10.1242/jeb.194.1.1. [DOI] [PubMed] [Google Scholar]
- Dubin A. E., Dionne V. E. Action potentials and chemosensitive conductances in the dendrites of olfactory neurons suggest new features for odor transduction. J Gen Physiol. 1994 Feb;103(2):181–201. doi: 10.1085/jgp.103.2.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubin A. E., Dionne V. E. Modulation of Cl-, K+, and nonselective cation conductances by taurine in olfactory receptor neurons of the mudpuppy Necturus maculosus. J Gen Physiol. 1993 Apr;101(4):469–485. doi: 10.1085/jgp.101.4.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadool D. A., Ache B. W. Plasma membrane inositol 1,4,5-trisphosphate-activated channels mediate signal transduction in lobster olfactory receptor neurons. Neuron. 1992 Nov;9(5):907–918. doi: 10.1016/0896-6273(92)90243-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firestein S., Shepherd G. M., Werblin F. S. Time course of the membrane current underlying sensory transduction in salamander olfactory receptor neurones. J Physiol. 1990 Nov;430:135–158. doi: 10.1113/jphysiol.1990.sp018286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frings S., Lynch J. W., Lindemann B. Properties of cyclic nucleotide-gated channels mediating olfactory transduction. Activation, selectivity, and blockage. J Gen Physiol. 1992 Jul;100(1):45–67. doi: 10.1085/jgp.100.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frings S., Seifert R., Godde M., Kaupp U. B. Profoundly different calcium permeation and blockage determine the specific function of distinct cyclic nucleotide-gated channels. Neuron. 1995 Jul;15(1):169–179. doi: 10.1016/0896-6273(95)90074-8. [DOI] [PubMed] [Google Scholar]
- Hatt H., Ache B. W. Cyclic nucleotide- and inositol phosphate-gated ion channels in lobster olfactory receptor neurons. Proc Natl Acad Sci U S A. 1994 Jul 5;91(14):6264–6268. doi: 10.1073/pnas.91.14.6264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleene S. J. Block by external calcium and magnesium of the cyclic-nucleotide-activated current in olfactory cilia. Neuroscience. 1995 Jun;66(4):1001–1008. doi: 10.1016/0306-4522(94)00634-h. [DOI] [PubMed] [Google Scholar]
- Kleene S. J. Origin of the chloride current in olfactory transduction. Neuron. 1993 Jul;11(1):123–132. doi: 10.1016/0896-6273(93)90276-w. [DOI] [PubMed] [Google Scholar]
- Kurahashi T. Activation by odorants of cation-selective conductance in the olfactory receptor cell isolated from the newt. J Physiol. 1989 Dec;419:177–192. doi: 10.1113/jphysiol.1989.sp017868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurahashi T., Kaneko A. Gating properties of the cAMP-gated channel in toad olfactory receptor cells. J Physiol. 1993 Jul;466:287–302. [PMC free article] [PubMed] [Google Scholar]
- Kurahashi T., Shibuya T. Ca2(+)-dependent adaptive properties in the solitary olfactory receptor cell of the newt. Brain Res. 1990 May 7;515(1-2):261–268. doi: 10.1016/0006-8993(90)90605-b. [DOI] [PubMed] [Google Scholar]
- Kurahashi T., Yau K. W. Co-existence of cationic and chloride components in odorant-induced current of vertebrate olfactory receptor cells. Nature. 1993 May 6;363(6424):71–74. doi: 10.1038/363071a0. [DOI] [PubMed] [Google Scholar]
- Lowe G., Gold G. H. Nonlinear amplification by calcium-dependent chloride channels in olfactory receptor cells. Nature. 1993 Nov 18;366(6452):283–286. doi: 10.1038/366283a0. [DOI] [PubMed] [Google Scholar]
- Lowe G., Gold G. H. Olfactory transduction is intrinsically noisy. Proc Natl Acad Sci U S A. 1995 Aug 15;92(17):7864–7868. doi: 10.1073/pnas.92.17.7864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch J. W., Barry P. H. Action potentials initiated by single channels opening in a small neuron (rat olfactory receptor). Biophys J. 1989 Apr;55(4):755–768. doi: 10.1016/S0006-3495(89)82874-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch J. W., Barry P. H. Inward rectification in rat olfactory receptor neurons. Proc Biol Sci. 1991 Feb 22;243(1307):149–153. doi: 10.1098/rspb.1991.0024. [DOI] [PubMed] [Google Scholar]
- Lynch J. W., Barry P. H. Properties of transient K+ currents and underlying single K+ channels in rat olfactory receptor neurons. J Gen Physiol. 1991 May;97(5):1043–1072. doi: 10.1085/jgp.97.5.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales B., Ugarte G., Labarca P., Bacigalupo J. Inhibitory K+ current activated by odorants in toad olfactory neurons. Proc Biol Sci. 1994 Sep 22;257(1350):235–242. doi: 10.1098/rspb.1994.0120. [DOI] [PubMed] [Google Scholar]
- Nakamura T., Gold G. H. A cyclic nucleotide-gated conductance in olfactory receptor cilia. 1987 Jan 29-Feb 4Nature. 325(6103):442–444. doi: 10.1038/325442a0. [DOI] [PubMed] [Google Scholar]
- Okada Y., Teeter J. H., Restrepo D. Inositol 1,4,5-trisphosphate-gated conductance in isolated rat olfactory neurons. J Neurophysiol. 1994 Feb;71(2):595–602. doi: 10.1152/jn.1994.71.2.595. [DOI] [PubMed] [Google Scholar]
- Pace U., Hanski E., Salomon Y., Lancet D. Odorant-sensitive adenylate cyclase may mediate olfactory reception. Nature. 1985 Jul 18;316(6025):255–258. doi: 10.1038/316255a0. [DOI] [PubMed] [Google Scholar]
- Pun R. Y., Kleene S. J., Gesteland R. C. Guanine nucleotides modulate steady-state inactivation of voltage-gated sodium channels in frog olfactory receptor neurons. J Membr Biol. 1994 Oct;142(1):103–111. doi: 10.1007/BF00233387. [DOI] [PubMed] [Google Scholar]
- Rajendra S., Lynch J. W., Barry P. H. An analysis of Na+ currents in rat olfactory receptor neurons. Pflugers Arch. 1992 Mar;420(3-4):342–346. doi: 10.1007/BF00374468. [DOI] [PubMed] [Google Scholar]
- Reed R. R. Signaling pathways in odorant detection. Neuron. 1992 Feb;8(2):205–209. doi: 10.1016/0896-6273(92)90287-n. [DOI] [PubMed] [Google Scholar]
- Schild D., Lischka F. W. Amiloride-insensitive cation conductance in Xenopus laevis olfactory neurons: a combined patch clamp and calcium imaging analysis. Biophys J. 1994 Feb;66(2 Pt 1):299–304. doi: 10.1016/s0006-3495(94)80804-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schild D., Lischka F. W., Restrepo D. InsP3 causes an increase in apical [Ca2+]i by activating two distinct current components in vertebrate olfactory receptor cells. J Neurophysiol. 1995 Feb;73(2):862–866. doi: 10.1152/jn.1995.73.2.862. [DOI] [PubMed] [Google Scholar]
- Trotier D. A patch-clamp analysis of membrane currents in salamander olfactory receptor cells. Pflugers Arch. 1986 Dec;407(6):589–595. doi: 10.1007/BF00582636. [DOI] [PubMed] [Google Scholar]
- Zhainazarov A. B., Ache B. W. Na(+)-activated nonselective cation channels in primary olfactory neurons. J Neurophysiol. 1995 May;73(5):1774–1781. doi: 10.1152/jn.1995.73.5.1774. [DOI] [PubMed] [Google Scholar]
- Zufall F., Firestein S. Divalent cations block the cyclic nucleotide-gated channel of olfactory receptor neurons. J Neurophysiol. 1993 May;69(5):1758–1768. doi: 10.1152/jn.1993.69.5.1758. [DOI] [PubMed] [Google Scholar]