Abstract
Singlet oxygen causes the cytotoxic process of tumour cells in photodynamic therapy. The mechanism by which singlet oxygen damages cells is, however, not fully understood. To address this issue, we synthesized and used two types of endoperoxides, MNPE (1-methylnaphthalene-4-propionate endoperoxide) and NDPE (naphthalene-1,4-dipropionate endoperoxide), that generate defined amounts of singlet oxygen at 37 °C with similar half lives. MNPE, which is more hydrophobic than NDPE, induced the release of cytochrome c from mitochondria into the cytosol and exhibited cytotoxicity, but NDPE did not. RBL cells, a rat basophil leukaemia-derived line, that overexpress phospholipid hydroperoxide glutathione peroxidase in mitochondria were found to be highly resistant to the cytotoxic effect of MNPE. MNPE treatment induced much less DNA ladder formation and nuclear fragmentation in cells than etoposide treatment, even though these treatments induced a similar extent of cellular damage. Singlet oxygen inhibited caspase 9 and 3 activities directly and also suppressed the activation of the caspase cascade. Collectively, these data suggest that singlet oxygen triggers an apoptotic pathway by releasing cytochrome c from mitochondria via the peroxidation of mitochondrial components and results in cell death that is different from typical apoptosis, because of the abortive apoptotic pathway caused by impaired caspase activation.
Keywords: apoptosis, caspase, cytochrome c, endoperoxide, singlet oxygen
Abbreviations: BHT, butylated hydroxytoluene; BSO, L-buthionine sulphoximine; DABCO, 1,4-diazadicyclo[2.2.2]octane; DAPI, 4,6-diamidino-2-phenylindole; DMEM, Dulbecco's modified minimum essential medium; DTT, dithiothreitol; ERK, extracellular-signal-regulated kinase; FBS, foetal bovine serum; GPx, glutathione peroxidase; JNK, c-Jun N-terminal kinase; LDH, lactate dehydrogenase; MCA, methyl-coumaryl-7-amide; MNP, 1-methylnaphthalene-4-propionate; MNPE, 1-methylnaphthalene-4-propionate endoperoxide; NDP, naphthalene-1,4-dipropionate; NDPE, naphthalene-1,4-dipropionate endoperoxide; PARP, poly(ADP-ribose) polymerase; PHGPx, phospholipid hydroperoxide GPx; ROS, reactive oxygen species; SNAP, S-nitroso-N-acetyl-D,L-penicillamine; SOD, superoxide dismutase; TBS, Tris-buffered saline; THF, tetrahydrofuran; WST-1, 2-(4-iodophenyl)-3-(4-nitrophenyl)-5-(2,4-disulphophenyl)-2H-tetrazolium
INTRODUCTION
ROS (reactive oxygen species) are produced during various biological process, such as inflammation and aging, and result in the dysfunction of susceptible cells [1]. Considerable efforts have been made to comprehend the mechanism of damage, including apoptosis, by ROS. In the intrinsic apoptotic pathway, ROS commonly triggers the release of cytochrome c from mitochondria into the cytosol [2,3]. The released cytochrome c, together with dATP and magnesium ions, stimulates protein factors, including Apaf-1 and procaspase 9, to form an apoptosome, resulting in proteolytic activation of the caspase cascade [4]. At a later stage, procaspase 3 is converted into the active form by the activated caspase 9, cleaves protein components, such as ICAD [inhibitor of caspase-dependent proteinase CAD (caspase-activated DNase)] and PARP [poly(ADP-ribose) polymerase], and leads to DNA fragmentation. From morphological points of view, chromatin condensation and nuclear fragmentation occur during apoptosis.
The hydroxyl radical, which is produced from H2O2 via a one-electron reduction, is regarded as the most reactive and, hence, is an extremely detrimental ROS. Singlet oxygen is produced during photochemical reactions in the presence of a photosensitizer. Superoxide-mediated radical reactions [5] and the decomposition of peroxynitrite [6] may also be sources of singlet oxygen.
Singlet oxygen is assumed to cause skin photoaging [7] and the cytotoxic process for tumour cells during photodynamic therapy [8]. Singlet oxygen kills bacteria [9–11] and is utilized to exterminate viruses without adverse effects [12]. When viruses are exposed to singlet oxygen, infection is prohibited without causing viral DNA damage. NAD(P)H appears to be one of primary targets of singlet oxygen in mitochondria [13]. Since proteins are present at high concentrations in the body and contain reactive residues, such as tryptophan, tyrosine, histidine and cysteine, approx. 68% of singlet oxygen is consumed by proteins [14].
Singlet oxygen produced by UVA irradiation is known to induce apoptosis in T-cells [15,16] and HL-60 cells [17]. Singlet oxygen and UV activate mitogen-activated protein kinases, p38, JNK (c-Jun N-terminal kinase) and ERK (extracellular-signal-regulated kinase) [18–20]. Singlet oxygen, as well as H2O2, result in the cleavage of Bid, which causes the release of cytochrome c from mitochondria. Zhuang et al. [21] compared the effects of singlet oxygen and H2O2, and concluded that singlet oxygen activates both the p38- and caspase-8-mediated pathways, but H2O2 activates only the caspase-8-mediated pathway. Despite its significant role in photoaging and for therapeutic purposes, the biological effects of singlet oxygen, especially on proteins, are poorly characterized. One of reasons is that an adequate system for producing pure singlet oxygen quantitatively is barely available.
We synthesized water-soluble endoperoxides that enabled us to investigate the function of singlet oxygen in biological systems. In the present paper, we report that singlet oxygen produced within cells is highly toxic and causes cell death. Although the release of cytochrome c from mitochondria was observed, cell death did not show the typical profile of apoptosis mainly due to the suppression of caspases by singlet oxygen.
EXPERIMENTAL
Materials
MNPE (1-methylnaphthalene-4-propionate endoperoxide) and NDPE (naphthalene-1,4-dipropionate endoperoxide) were synthesized as described previously [22]. The structure and purity of the endoperoxides were determined by NMR. The half-lives of MNPE and NDPE were approx. 25 min and 27 min at 37 °C respectively. The generation of singlet oxygen from endoperoxides was detected by ESR using DRD156 [4,4′-bis(1-p-carboxyphenyl-3-methyl-5-hydroxyl)pyrazole] as a sensitive singlet-oxygen-detecting probe, which specifically reacts with singlet oxygen among the ROS [23]. The efficiency of singlet oxygen formation was similar between MNPE and NDPE, although reported yield varies from 45 to 98%, depending on the assay methods used [24,25]. Vitamin E, vitamin E succinate, DABCO (1,4-diazadicyclo[2.2.2]octane) and β-carotene were purchased from Sigma.
Cell culture
HepG2 cells were maintained in DMEM (Dulbecco's modified minimal essential medium) (Sigma) containing 100 units/ml penicillin and 100 μg/ml streptomycin supplemented with 10% FBS (foetal bovine serum) (Invitrogen). RBL cells, a rat basophil leukaemia-derived line, and their transfectants were maintained in DMEM containing the above antibiotics supplemented with 5% FBS and 0.5 mg/ml geneticin (Gibco BRL). Cells were grown at 37 °C in a humidified atmosphere containing 5% CO2.
HPLC analysis of MNP (1-methylnaphthalene-4-propionate) and NDP (naphthalene-1,4-dipropionate) in cells
The delivery of endoperoxides to cells was assessed by measuring decomposition products, MNP and NDP, as described by Klotz et al. [19]. After incubation with 2 mM MNPE or NDPE for 2 h, HepG2 cells (1×107) were washed with PBS and scraped. Cells were disrupted for 1 min by a Bioruptor (Cosmo Bio, Tokyo, Japan) at 200 W with cooling in ice-cold water. The sample was centrifuged at 15000 g for 10 min followed by centrifugation at 100000 g for 60 min. The supernatant was partially deproteinized by Microcon (cut-off molecular mass 30 kDa) (Millipore) and subjected to HPLC (LC-2000; Jasco) on a reversed-phase C18 column (Toso, Tokyo, Japan). The decomposition product, MNP or NDP, was eluted at 25 °C at a flow rate of 1 ml/min. The mobile phase was methanol/50 mM ammonium acetate, pH 7.0, with 20% methanol for 1 min, increased to 100% methanol in 9 min, and was kept at 100% methanol for 5 min. Absorbance of the eluent was monitored at 290 nm. Amounts of the decomposed compounds that had been incorporated into cells were standardized based on the recovery of a known amount of MNP or NDP added to the supernatant prepared from untreated control cells.
HPLC analysis of vitamin E in cells
The delivery of vitamin E or vitamin E succinate into cells was determined according to the method described in [26]. After incubation with 10 μM vitamin E or vitamin E succinate for 16 h, the cells (1×107) were washed with PBS, scraped and suspended into 100 μl of PBS containing 0.01% BHT (butylated hydroxytoluene). The cell suspension was mixed with 500 μl of n-hexane. After 2 min, this mixture was deproteinized with cool ethanol, denatured with 5% methanol, and vortex-mixed for 5 min. The upper phase was separated after centrifugation at 8000 g for 10 min, and dried under nitrogen. The residue was dissolved in 100 μl of methanol and analysed by HPLC on a reversed-phase C18 column. The sample was eluted with 100% methanol at 25 °C at a flow rate of 1 ml/min. Absorbance of the eluent was monitored at 284 nm. Amounts of vitamin E or vitamin E succinate incorporated into cells were standardized based on the recovery of known amount of each compound added to the control cell suspension.
HPLC analysis of β-carotene in cells
The delivery of β-carotene was determined according to the method described by Offord et al. [27]. β-Carotene (10 mM) was dissolved in THF (tetrahydrofuran) and was added to a final concentration of 10 μM to DMEM containing 10% FBS and 0.01% Tween 20. After sonication for 1 min, the medium was sterilized by passing through an Acrodisc syringe filter with a 0.2 μm pore-size HT Tuffryn membrane (Pall, Ann Arbor, MI, U.S.A.). The cells (1×107) treated for 16 h with DMEM containing β-carotene were washed with PBS and lysed with 600 μl of lysis buffer (150 mM NaCl, 2 mM EDTA, 1% Tween 20 and 100 mM Tris/HCl, pH 8.0). The cells were scraped and sonicated for 30 s in 15 ml plastic tubes, then mixed thoroughly with 0.8 ml of water, 1.6 ml of ethanol and 8 μl of deferoxamine mesylate (10 mg/ml) in the dark. The samples were extracted twice with 3.6 ml of n-hexane containing 0.03% (w/v) BHT. After centrifugation for 5 min at 2000 g at room temperature (25 °C), the two supernatants were mixed and dried under nitrogen. The residue was dissolved in 100 μl of THF containing 0.03% BHT and analysed by HPLC on a reversed-phase C18 column. The sample was eluted with 100% methanol at 40 °C at a flow rate of 2 ml/min. The eluent was monitored by absorbance at 450 nm. The amount of β-carotene incorporated into cells was standardized based on the recovery of a known amount of each compound added to the control cell lysate.
Establishment of selenium-deficient cells
In order to establish selenium-deficient HepG2 cells, the serum concentration of the medium was decreased stepwise from 10% to 1% over 7 days as described previously [28]. Cells were used for experiments at least 7 days after being maintained in medium containing 1% FBS. For the replenishment of selenium, sodium selenite (Wako, Osaka, Japan) was added to a concentration of 1 μM.
Measurement of total glutathione content in cells
Depletion of glutathione in HepG2 cells was achieved by treatment with 5 mM BSO (L-buthionine sulphoximine) (Sigma) for 24 h. Total glutathione was measured by the method described by Anderson [29]. Briefly, cells (1×106) were washed twice with PBS and suspended in 100 μl of 5% sulphosalicylic acid by vigorous vortex-mixing. After centrifugation at 8000 g for 10 min, the supernatant was recovered for assay. Samples (10 μl) were mixed with the 980 μl of reaction mixture containing 100 mM sodium phosphate, pH 7.4, 5 mM EDTA, 0.6 mM 5,5′-dithiobis(2-nitrobenzoic acid) (Wako) and 0.3 mM NADPH. The increase in absorbance at 412 nm was monitored after the addition of 3 units of glutathione reductase (Roche, Mannheim, Germany). Authentic glutathione (Roche) solutions were used as a standard.
Enzyme assays
After washing once with PBS, cells were scraped from a culture dish and were collected by centrifugation at 500 g for 4 min. Cells were suspended in an appropriate volume of 10 mM Tris/HCl, pH 7.4, and were disrupted by a Bioruptor at 200 W for 30 s with cooling in ice-cold water. The supernatant was collected after centrifugation at 15000 g for 15 min, and was used in the assay of enzyme activities. SOD (superoxide dismutase) activity was determined as described previously [30] using WST-1 [2-(4-iodophenyl)-3-(4-nitrophenyl)-5-(2,4-disulphophenyl)-2H-tetrazolium] (Wako) for the detection of generated superoxide anion. Briefly, the reaction mixture contained an appropriate amount of diluted xanthine oxidase (Roche), 0.1 mM xanthine (Wako), 0.025 mM WST-1, 0.1 mM EDTA, 50 mM NaHCO3, pH 10.2, in a total volume of 3 ml. The increase in the absorbance at 438 nm was monitored at 25 °C for 1 min. One unit was defined as the amount of enzyme required to inhibit 50% of the absorbance change of 0.060/min. This unit of enzyme activity is equivalent to that determined by the standard procedure using Nitro Blue Tetrazolium. CuZnSOD and MnSOD activities were defined as 2 mM NaCN-inhibitable activity and -resistant activity respectively.
GPx (glutathione peroxidase) activity was determined as described previously [30]. One unit was defined as the amount of enzyme required to oxidize 1 μmol of NADPH, corresponding to 2 μmol of GSH.
Caspase activity was determined essentially as described by Stennicke and Salvesen [31]. Acetyl-Leu-Glu-His-Asp-4-MCA (methyl-coumaryl-7-amide) and acetyl-Asp-Glu-Val-Asp-4-MCA were used for the measurement of caspase 9 and caspase 3 activity respectively. The reaction mixture contained 25 mM Pipes/KOH, pH 7.2, 5 mM MgCl2, 10 mM DTT (dithiothreitol), 0.1% CHAPS, 10% sucrose and 0.05 mM substrate in a 200 μl volume. The initial rates of enzymatic hydrolysis were measured by the release of MCA from the substrate as the emission at 460 nm upon excitation at 380 nm using a BioLumin 960 Fluorometer (Molecular Dynamics, Tokyo, Japan) equipped with a thermostatically controlled plate reader.
Evaluation of the viability of cells by LDH (lactate dehydrogenase) activity
LDH activity was measured to assess the sensitivity of the cells to singlet oxygen [30]. At 24 h after treatment of cells in 24-well plates, portions of the medium were collected for measurement of LDH activity. The cells were collected and disrupted by brief sonication in PBS containing 0.1% Tween 20. Cellular extracts free of debris were prepared by centrifugation at 15000 g for 10 min. The assay for LDH activity was performed using an LDH CII kit (Wako). The viability of the cells was calculated as the percentage of the LDH activity recovered in the cellular extract against the total (cellar extract plus medium) recovered activity.
SDS/PAGE and immunoblot analysis
Protein samples were subjected to SDS/PAGE with appropriate concentrations of polyacrylamide and then transferred on to a Hybond-P membrane (Amersham Biosciences, Little Chalfont, Bucks., U.K.) under semi-dry conditions by means of a Transfer-blot SD Semi-dry transfer cell (Bio-Rad). The membrane was then blocked by incubation with 5% (w/v) skimmed milk in TBS (Tris-buffered saline; 137 mM NaCl, 2.7 mM KCl and 25 mM Tris/HCl, pH 7.6) containing 0.1% Tween 20 for 2 h at room temperature. The membranes were then incubated with a mouse anti-(cytochrome c) Ig (1:1000 dilution) (Pharmingen, San Diego, CA, U.S.A.), rabbit anti-(caspase 3) IgG (H-277, Santa Cruz Biotechnology, Santa Cruz, CA, U.S.A.), rabbit anti-(human caspase 9) Ig (Cell Signaling Technology, Beverly, MA, U.S.A.), or rabbit anti-PARP IgG (H-250, Santa Cruz Biotechnology) for 16 h at 4 °C. After washing with TBS containing 0.1% Tween 20, the membrane was incubated with 1:1000 diluted peroxidase-conjugated goat anti-mouse IgG or goat anti-rabbit IgG (Santa Cruz Biotechnology) for 1 h at room temperature. After washing, the peroxidase activity on the membranes was detected by a chemiluminescence method using an ECL® (enhanced chemiluminescence) Plus kit (Amersham Biosciences) and exposed to X-ray films (Kodak, Rochester, NY, U.S.A.). The amounts of protein were quantified by densitometric scanning of X-ray films using an Atto Densitograph (Atto, Tokyo, Japan).
Measurement of cytochrome c release from mitochondria in cells
Control cells or glutathione-depleted cells by incubation in 5 mM BSO-containing medium for 24 h were treated with endoperoxides or a precursor for 2 h, then incubated for an additional 4 h in fresh medium. Cytosolic fractions were prepared as described previously [32]. Briefly, 1×107 cells were scraped and washed in PBS, and suspended in 500 μl of a lysis buffer (250 mM sucrose, 50 mM Pipes/KOH, pH 7.4, 50 mM KCl, 5 mM EGTA, 2 mM MgCl2, 1 mM DTT and 1 mM PMSF). After 30 min on ice, the cells were lysed with 40 strokes using pestle B in a Dounce homogenizer. After centrifugation at 14000 g for 15 min, the supernatant was regarded as the cytosolic fraction. Cytosolic proteins (10 μg) were subjected to immunoblot analysis.
Effects on cytochrome c release from isolated mitochondria
Mitochondria were isolated from rat livers by conventional subcellular fractionation. Briefly, 6 g of rat liver was homogenized in 3 vol. of solution A (0.32 M sucrose, 3 mM MgCl2 and 20 mM Tris/HCl, pH 7.6), and the homogenate was filtered through cheesecloth. The homogenate was gently poured on to 20 ml of solution A in a 50 ml centrifuge tube, and centrifuged at 800 g for 10 min. The supernatant was centrifuged further at 8000 g for 10 min. The precipitate was suspended gently in 4 ml of a buffer (0.25 M sucrose, 1 mM MgCl2 and 10 mM Tris/HCl, pH 7.6) by means of Dounce homogenizer using pestle B. All of the above procedures were performed at 4 °C. The isolated mitochondria were treated with each endoperoxide for 60 min followed by centrifugation at 12000 g for 10 min. Equal volumes of supernatants, containing approx. 20 μg of proteins, were separated and analysed as above.
Quantification of fragmented nuclei using fluorescence microscopy
After treated with MNPE or etoposide, cells were trypsinized, washed in PBS and suspended into PBS. Approx. 5×105 cells in 10 μl were stained with 0.03 mM DAPI (4,6-diamidino-2-phenylindole), and photographs were taken with a digital camera using a fluorescence microscope (Olympus BX 50, Tokyo, Japan). At least 500 cells on photographs were counted for calculating the percentages of the cells that had an intact, condensed or fragmented nucleus.
Detection of fragmented DNA by agarose gel electrophoresis
DNA was isolated from RBL-S1 cells (1×106 cells) according to the methods described by Ishizawa et al. [33]. Isolated DNA samples were electrophoresed on a 2% agarose gel in Tris/borate/EDTA buffer. DNA was visualized on a UV illuminator after staining with 0.5 μg/ml ethidium bromide.
Evaluation of caspase activation in a cell-free system
Caspase activation in a cell-free system was achieved essentially according to a method described previously [34]. Briefly, HepG2 cell lysate was prepared in 4 vol. of buffer containing 0.32 M sucrose, 3 mM MgCl2, 20 mM Tris/HCl, pH 7.5, 2 μg/ml aprotinin, 2 μg/ml pepstatin A, 50 μM (p-amidinophenyl)methanesulphonyl fluoride hydrochloride by homogenizing the cells with 40 strokes using pestle B in a Dounce homogenizer. The homogenates were centrifuged at 8000 g for 10 min, and the resulting supernatants were centrifuged at 100000 g for 1 h. The caspases in the 100000 g supernatants were activated by incubation at 37 °C for 1 h with 75 μg/ml cytochrome c, 2 mM dATP and 2 mM MgCl2 with or without MNPE or SNAP (S-nitroso-N-acetyl-D,L-penicillamine).
Statistics
Data are presented as the means±S.D. for triplicate experiments. Student's t test was used to compare the significance of the differences between data. Values of P<0.05 were considered to be significant.
RESULTS
Cellular damage is caused by hydrophobic endoperoxides
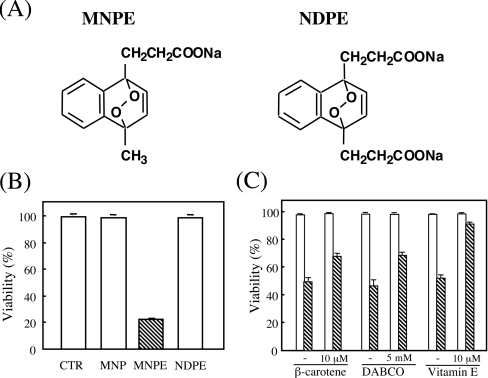
We synthesized two types of endoperoxides, MNPE and NDPE (Figure 1A) that generate defined amounts of singlet oxygen with half lives of approx. 25 and 27 min at 37 °C in aqueous solution respectively. After incubation of HepG2 cells with each endoperoxide for 2 h, the media were changed to fresh media, and the cytotoxic effects of the compounds were evaluated by measuring LDH activity after 22 h (Figure 1B). MNPE decreased cellular viability in a dose-dependent manner, while NDPE, as well as MNP, a precursor of MNPE, had essentially no effect under these conditions. To attribute the cytotoxic effect of MNPE to generated singlet oxygen, we examined the protective action of singlet oxygen inhibitors, β-carotene, DABCO and vitamin E, on cellular viability (Figure 1C). An assay of LDH activity indicated that all three compounds significantly protected the cells from the cytotoxic effects of MNPE. Vitamin E succinate, a soluble form of vitamin E, had about the same protective effect as vitamin E, but histidine and vitamin C had virtually no effect under these conditions (results not shown). Table 1 shows intracellular contents of the decomposition products of the endoperoxides, MNP and NDP, and inhibitors of singlet oxygen, β-carotene, vitamin E and vitamin E succinate. Values were consistent with the harmful effects of corresponding peroxides. A low level of β-carotene incorporation into cells may explain the lower efficiency than for vitamin E in protection against cytotoxicity of endoperoxides.
Figure 1. MNPE, but not NDPE, exerted cytotoxic effects on HepG2 cells.
(A) Structures of MNPE and NDPE. (B) HepG2 cells were incubated with 4 mM MNPE, NDPE or MNP (a precursor of MNPE) for 2 h and then incubated in fresh medium for an additional 22 h. LDH activity in the medium and cells were measured. CTR, control. (C) Effects of β-carotene, DABCO and vitamin E on the protection of cells from the cytotoxicity of singlet oxygen were examined. Cells were pre-incubated in medium containing 10 μM β-carotene, 5 mM DABCO or 10 μM vitamin E for 16 h and then treated without (open bar) or with 2 mM MNPE (hatched column) for 2 h. In the case of DABCO, 5 mM DABCO was present in the medium during MNPE treatment. Viability of the cells was assessed by measuring LDH activity after 22 h. The means±S.D. for triplicate assays are shown.
Table 1. MNP, NDP, vitamin E, vitamin E succinate and β-carotene contents in HepG2 cells.
The concentrations of the compounds were measured by HPLC under conditions described in the Experimental section. The values of these compounds in untreated cells were regarded as baseline levels. Data are presented as means±S.D. for triplicate samples.
| Compound | Concentration (nmol/107 cells) |
|---|---|
| Degradation product of endoperoxide | |
| MNP | 8.5±1.2 |
| NDP | 1.2±0.3 |
| Singlet oxygen inhibitor | |
| Vitamin E | 4.3±0.1 |
| Vitamin E succinate | 5.1±0.5 |
| β-Carotene | 0.023±0.003 |
Effects of MNPE on selenium-deficient or GSH-depleted cells
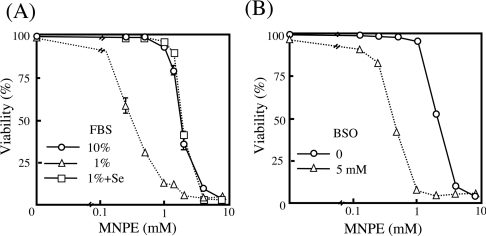
We then examined the dose-dependent cytotoxicity of MNPE on cells that were cultivated in medium supplemented with 1% FBS, instead of conventional 10% FBS, which resulted in a selenium deficiency, or in medium containing 5 mM BSO, an inhibitor for γ-glutamylcysteine synthetase. Treatment of these cells with MNPE indicated an enhanced cytotoxicity in cells cultivated under conditions of selenium-deficiency or with decreased GSH by BSO treatment (Figure 2). When selenium was replenished to the medium with 1% FBS, a resistance similar to the conventional culture was observed (Figure 2). No significant difference was observed in SOD activities among these cells (Table 2). While GPx activity was significantly decreased in cells cultivated in 1% FBS, supplementation by selenium elevated the activity, as has been reported previously [28]. The depletion of GSH had no effect on GPx activity, which was assayed using t-butyl hydroperoxide as a substrate in the presence of a large excess of GSH. However, since BSO treatment lowered intracellular GSH levels, the potential for peroxide detoxification by GPx would be limited by the low GSH content within the cells. These data suggest a pivotal role of GSH-dependent detoxification of peroxides in protection against the toxicity of singlet oxygen.
Figure 2. Enhanced susceptibility to singlet oxygen of cells cultivated under selenium-deficient or GSH-deficient conditions.
(A) Cells cultivated in medium containing 10% FBS (○), 1% FBS (△) or 1% FBS with replenishment of 1 μM sodium selenite (□) were exposed to various concentrations of MNPE. (B) Cells were incubated without (○) or with (△) 5 mM BSO for 24 h and then treated with various concentrations of MNPE. Viability of the cells was assessed by measuring LDH activity.
Table 2. MnSOD, CuZnSOD and GPx activities, and GSH content in HepG2 cells cultured under the indicated conditions.
Control, conventional 10% FBS; GSH-deficiency, +5 mM BSO; selenium-deficiency, 1% FBS; selenium replenishment, added 1 μM sodium selenite to selenium-deficient medium.
| Conditions | MnSOD (units/mg of protein) | CuZnSOD (units/mg of protein) | GPx (m-units/mg of protein) | GSH (μg/mg protein) |
|---|---|---|---|---|
| Control | 20.9±0.6 | 21.5±1.0 | 11.2±0.2 | 12.1±1.4 |
| (+)5 mM BSO | 19.2±0.4 | 20.8±1.7 | 9.8±0.3 | 0.2±0.0 |
| 1% FBS | 24.9±0.4 | 18.9±1.1 | 1.8±0.3 | 11.6±1.0 |
| 1% FBS+Se | 24.4±0.5 | 16.5±1.5 | 64.3±1.4 | 9.6±1.3 |
Cells overexpressing PHGPx (phospholipid hydroperoxide GPx) in mitochondria are resistant to singlet oxygen toxicity
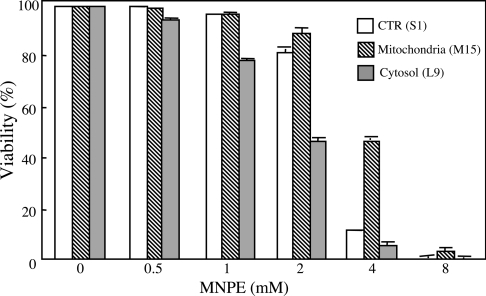
Singlet oxygen might increase the formation of peroxides, and PHGPx, among the GPx family members, is generally thought to play a significant role in protection against the ROS cytotoxicity [35]. We examined the cytotoxic effects of MNPE on control cells (S1), which were transfected with vector alone, or cells overexpressing PHGPx either at the cytoplasm (L9) or at the mitochondria (M15) [36,37] (Figure 3). The results showing that M15 cells were the most resistant to the cytotoxic effects of MNPE point to mitochondria as a potential target for singlet oxygen. It is not clear at present, however, why cells that express more PHGPx in the cytoplasm are less resistant to cytotoxicity than control cells.
Figure 3. Effects of endoperoxides on cells overexpressing PHGPx.
Cells overexpressing PHGPx at cytoplasm (L9 cells, grey bars) or mitochondria (M15 cells, hatched bars) and control cells (S1 cells, open bars) were treated with various concentrations of MNPE for 2 h. After 22 h, viability of the cells was assessed by measuring LDH activity. CTR, control.
Effect of endoperoxides on the cytochrome c release from mitochondria
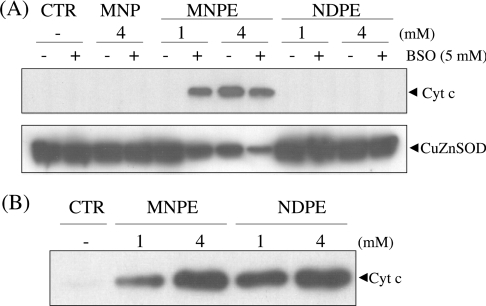
Since mitochondria appeared to be a target of singlet oxygen, we examined the levels of both cytochrome c released into cytoplasm and the portion remaining in mitochondria in cells after treatment with the endoperoxides. While the treatment of cells with MNPE caused the release of cytochrome c into the cytoplasm, no cytochrome c release was observed in the cytoplasm of cells treated with either NDPE or MNP (Figure 4A). These data indicate that the observed cytotoxic effect can be attributed to singlet oxygen generated from MNPE located near the mitochondria in cells. The depletion of glutathione by treatment with BSO enhanced the release of cytochrome c. This is consistent with the cytoprotective role of glutathione against singlet-oxygen-induced damage. Thus, despite the similar chemical nature of the two endoperoxides as singlet oxygen donors, only MNPE effectively impaired mitochondria and triggered the release of cytochrome c in cells.
Figure 4. Effect of the endoperoxides on cytochrome c release from mitochondria in cells and isolated mitochondria in the cell-free system.
(A) After treatment with 1 or 4 mM endoperoxides or 4 mM MNP for 2 h, the cytosolic fraction was prepared from HepG2 cells by gentle homogenization in a Dounce homogenizer. Cytochrome c, present in the cytosolic fraction, was assayed by immunoblotting using an anti-(cytochrome c) (Cyt c) antibody. CuZnSOD was detected by a specific antibody and was used as a marker for cytosolic proteins. (B) The mitochondrial fraction was prepared from rat liver by gentle homogenization, followed by centrifugation, and was treated with MNPE or NDPE. After centrifugation, the released cytochrome c from mitochondria was analysed by immunoblotting as described above. Typical data from triplicate experiments are shown. CTR, control.
To examine the potential detrimental effects of endoperoxides on mitochondria, we investigated their direct action on isolated mitochondria from rat liver (Figure 4B). Both MNPE and NDPE triggered the release of cytochrome c from isolated mitochondria to almost the same extent. This mitochondrial toxicity of both endoperoxides observed in the cell-free system suggest that the above results obtained in cultured cells are related to the membrane permeability of the compounds, i.e. MNPE penetrated the plasma membrane and reached the mitochondria, but NDPE did not.
Characterization of cellular damage caused by singlet oxygen
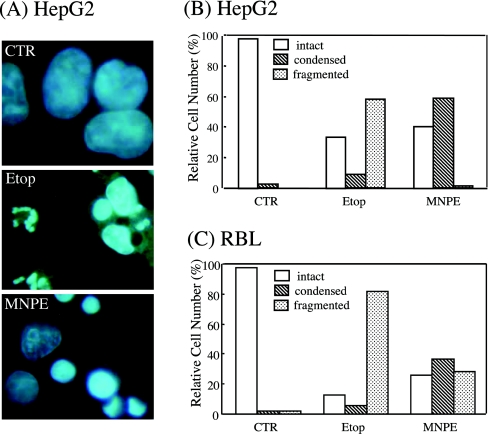
To gain insight into the mechanism of cell death, morphological changes in the cells were observed and compared with those during etoposide-induced apoptosis. When the cells were stained with DAPI and examined by fluorescence microscopy, condensed chromatin and fragmented nuclei, which are criteria for apoptosis, were observed in these cells (Figure 5A). The number of cells that contained either condensed chromatin or fragmented nuclei were counted and compared. When HepG2 cells were treated with etoposide, approx. 60% contained fragmented nuclei. However, treatment with MNPE decreased the number of cells with fragmented nuclei and increased those with condensed nuclei (Figure 5B). Although their population was different, a similar trend was observed in RBL cells by treatment with MNPE (Figure 5C). Since nuclear fragmentation follows chromatin condensation in apoptosis, the morphological process characteristic of apoptosis appeared to cease at this stage during the cell death caused by singlet oxygen.
Figure 5. Singlet oxygen induced chromatin condensation, but not nuclear fragmentation.
HepG2 cells were incubated with 0.4 mM etoposide (Etop) throughout the period or 2 mM MNPE for 2 h. After 16 h, the cells were stained with DAPI and examined under a fluorescence microscope (A). The number of intact, condensed, and fragmented nuclei of HepG2 (B) or RBL (C) cells were counted in more than 500 cells and the data are presented as a percentage. CTR, control.
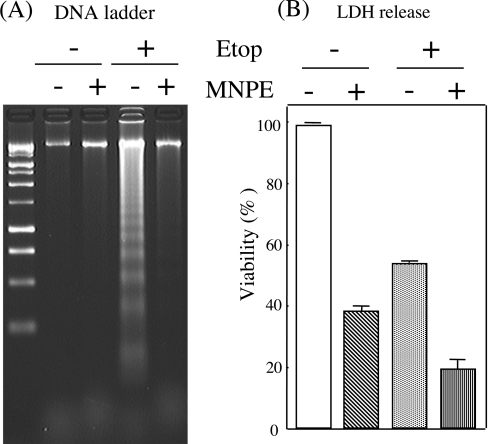
We characterized further the nature of the cellular damage by assaying DNA fragmentation, another criterion of apoptosis. DNA, prepared from RBL cells that had been treated with etoposide and/or MNPE, was run on an agarose gel (Figure 6A). Although the viability of cells was slightly different between the two groups, DNA ladder formation was much less in MNPE-treated cells than in etoposide-treated cells (Figure 6B). The presence of MNPE for the initial 2 h during etoposide treatment suppressed DNA ladder formation. When a FACS analysis was performed using propidium iodide and annexin V staining, the population of the propidium-iodide-negative and annexin-V-positive cells, corresponding to apoptotic cells, was decreased with a parallel increase in double positive cells, corresponding to necrotic cells, in MNPE-treated cells compared with etoposide-treated cells (results not shown). These data indicate that cells treated with MNPE released cytochrome c from the mitochondria, but did not undergo typical apoptotic death.
Figure 6. Effects of singlet oxygen on DNA ladder formation.
RBL cells were incubated with 2 mM MNPE for an initial 2 h, changed to fresh medium, and incubated an additional 15 h. For etoposide treatment, 0.4 mM etoposide (Etop) was present all the time. MNPE (2 mM) was included from 3 to 5 h after etoposide addition, and the cells were incubated further for an additional 13 h in fresh medium containing etoposide. (A) DNA was extracted from cells, separated on a 2% agarose gel and stained with ethidium bromide. Typical data of triplicate experiments are shown. (B) Viability of cells that had been treated under the same conditions as (A) was assessed by measuring LDH activity.
Singlet oxygen inhibits caspase activity
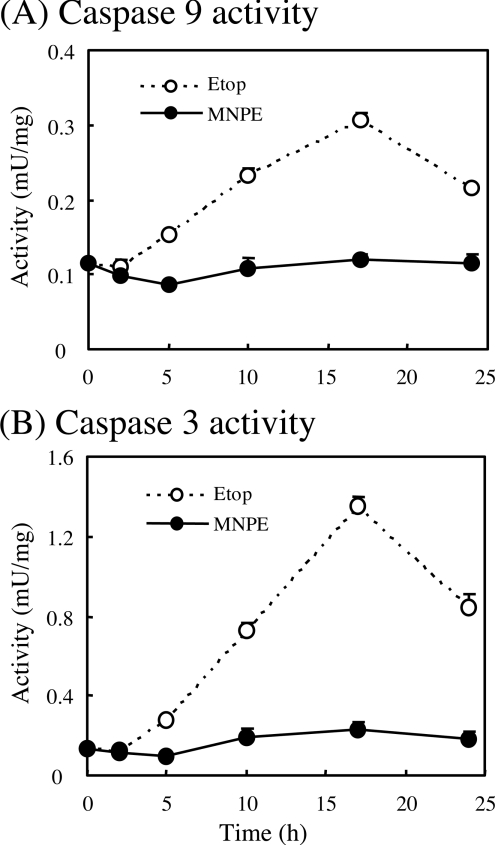
Since DNA fragmentation and the accompanying apoptotic process triggered by many stimuli is mediated by activation of the caspase cascade after the release of cytochrome c into the cytosol, we examined the caspase activity of cytosolic fractions from HepG2 cells that had been treated with MNPE and compared the findings with that from etoposide-treated cells (Figure 7). The activities of both caspase 9 and caspase 3 were gradually elevated, reaching a maximum at 17 h after etoposide treatment. In MNPE-treated cells, however, their activities were much lower than those of etoposide-treated cells through this period.
Figure 7. Comparison of activities of caspase 9 and 3 between etoposide- and singlet-oxygen-induced cell death.
HepG2 cells were incubated with 2 mM MNPE for an initial 2 h, changed to fresh medium, and incubated for an additional 15 h. For etoposide treatment, 0.5 mM etoposide (Etop) was present at all times. A cellular lysate was prepared at the indicated time points. Activities of caspase 9 (A) and 3 (B) were assayed using specific substrates.
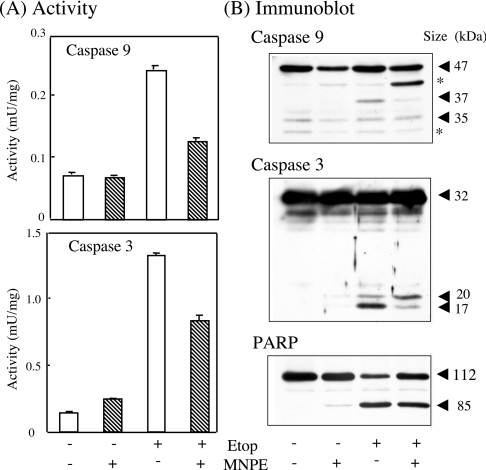
Proteins, as well as caspase 9 and 3 activities, were then assayed in HepG2 cells that had been treated with MNPE and/or etoposide (Figure 8). While both activities were markedly elevated in etoposide-treated cells, the activities in MNPE-treated cells were again very low (Figure 8A). The co-presence of MNPE from 15 to 17 h after etoposide addition significantly suppressed their activities. An immunoblot analysis of the cytosolic proteins showed that the caspase activity roughly corresponded to the processing of precursors to mature forms (Figure 8B). The low level of cleaved PARP, a nuclear protein and predominant substrate of caspase 3, was also consistent with a low caspase 3 activity in the MNPE-treated cells.
Figure 8. Correlation of activities of caspase 9 and 3 with their active forms and PARP.
(A) HepG2 cells were incubated with 2 mM MNPE for an initial 2 h, changed to fresh medium, and incubated for an additional 15 h. With etoposide treatment, 0.4 mM etoposide (Etop) was present all the time. MNPE was added to 2 mM at 15 h after etoposide and incubated further for 2 h. (B) Approx. 10 μg of protein was separated by SDS/PAGE and blotted on to a Hybond-P membrane. Immunoblot analyses were performed using anti-(caspase 9), anti-(caspase 3) or anti-PARP antibodies. Typical data from triplicate experiments are shown. Sizes of proteins are shown in kDa. *, non-specific band.
Singlet oxygen suppresses activation of the caspase cascade
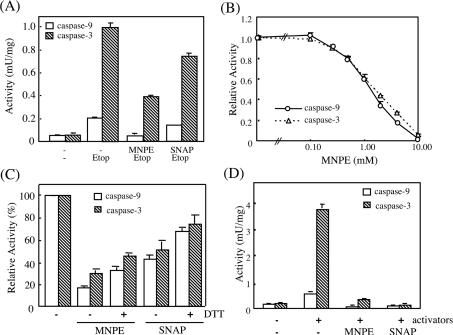
To elucidate the mechanism associated with the low caspase activity in MNPE-treated cells, we extracted soluble proteins from etoposide-treated cells and incubated them with MNPE (Figure 9). The effects of treatment with SNAP, an NO donor, and 10 mM DTT were also examined. When the protein fraction that originally contained high caspase activities was treated with MNPE, a marked suppression of the activity was observed (Figure 9A). The inhibition was dose-dependent, with a half-maximal inhibition at approx. 1 mM MNPE under these conditions (Figure 9B). A buffer pH between 6 and 9 during treatment with MNPE did not significantly affect the inhibition, although a lower pH caused a decrease in activity (results not shown). Reduction with DTT only slightly recovered the activity (Figure 9C). We then examined the effects of MNPE on caspase activities in a cell-free system that had been reconstituted from the cytosolic fraction, cytochrome c, dATP and magnesium. Full activity was obtained when all these components were included in the cytosolic fraction. The presence of MNPE as well as SNAP also suppressed the activation of caspases 9 and 3 in the cell-free reconstitution system (Figure 9D). Thus treatment with singlet oxygen appeared to suppress activation of the caspase cascade by activators of apoptosome formation.
Figure 9. Effects of singlet oxygen and NO on caspase activities from cells pre-treated with etoposide, and the effects of reduction with DTT.
(A)–(C) Apoptosis was induced by 0.4 mM etoposide in HepG2 cells. Activities of caspase 9 and 3 were measured in cytosolic fractions of the cells that had been treated as follows. (A) Cytosolic fractions were incubated further with 2 mM MNPE or 2 mM SNAP for 2 h at 37 °C. (B) Cytosolic fractions were treated with various concentrations of MNPE for 2 h at 37 °C. (C) After treatment of the cytosolic fraction with either 2 mM MNPE or 2 mM SNAP as in (A), cytosolic fractions were incubated further with 10 mM DTT for 1 h at 37 °C. (D) The cytosolic fraction was prepared from untreated HepG2 cells. The caspase cascade was activated by adding activators, cytochrome c, dATP and MgCl2. Effects of 2 mM MNPE or 2 mM SNAP on caspase activities were examined in samples by co-incubation with these activators.
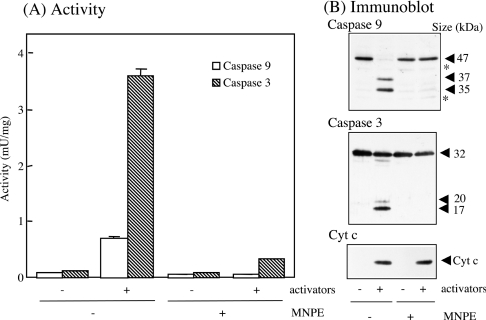
We also examined the effects of pre-treating the cells with MNPE on the activation of the caspase cascade in a cell-free system (Figure 10). After 2 h of incubation of HepG2 cells with 2 mM MNPE, the cytosolic fraction was obtained and was stimulated with activators. The activities of caspase 9 and 3 were much lower in MNPE-treated cells than in untreated cells (Figure 10A). The levels of active forms of caspase 9 and 3 present were consistent with their low activities (Figure 10B). Thus singlet oxygen suppressed the activation of the caspase cascade before the processing of caspase 9.
Figure 10. Effects of pre-treatment of cells with MNPE on caspase activation in the cell-free system.
HepG2 cells were pre-treated without (−) or with (+) 2 mM MNPE for 2 h, and cytosolic fractions were prepared immediately. Caspases in the fractions were activated by adding activators, cytochrome c, dATP and MgCl2. (A) Activities of caspase 9 and 3 were measured in triplicate. (B) Approx. 10 μg of protein was separated by SDS/PAGE and blotted on to the Hybond-P membrane. Immunoblot analyses were performed using the anti-(caspase 9), anti-(caspase 3) or anti-(cytochrome c) antibody. Sizes of proteins are shown in kDa. *, non-specific band. Typical data of triplicate experiments are shown.
DISCUSSION
We synthesized and used two naphthalene endoperoxides with different polarities, but a similar half-life as singlet oxygen donors, and found that only the less polar donor MNPE damaged the cells (Figure 1). This suggests that the production of singlet oxygen in close proximity to the target molecule in cells is required to exert a detrimental effect. Klotz et al. [19] reported a similar observation for HL-60 cells using different endoperoxides.
The suppression of GPx activity in cells either by decreasing FBS supplementation to 1%, which decreased GPx proteins, or by the inhibition of GSH biosynthesis by BSO treatment (Table 1) enhanced the sensitivity of the cells to singlet oxygen toxicity (Figure 2). Although GPx activity, which was measured in the presence of a large excess of GSH, was similar to the control, the capacity of the GSH-dependent detoxification of peroxides would be limited in GSH-deficient cells. The reactivity of singlet oxygen to the conjugated double bond is very high (∼106 M−1·s−1) and, hence, causes lipid peroxidation [1]. Although singlet oxygen is not a radical and cannot be directly detoxified by antioxidative enzymes, ROS that are generated by reactions with singlet oxygen in cells may exert harmful effects. Thus the resulting ROS, but not singlet oxygen itself, could be a substrate in the GSH-dependent detoxification reaction.
When intact cells were treated with these endoperoxides, only the less polar MNPE was effective. The half-life of singlet oxygen in water is several microseconds, and thus the distance it can penetrate is less than 50 nm. In addition, the plasma membrane contains many singlet-oxygen-scavenging compounds, e.g. vitamin E, which would decrease the amount of singlet oxygen within the lipid bilayer. Thus singlet oxygen produced near a target molecule would be effective. Mitochondria were shown to be one of the primary targets of singlet oxygen within cells (Figures 3 and 4). However, both compounds were equally effective on isolated mitochondria from rat liver. This indicates that the generation of ROS near the mitochondria would be required for singlet oxygen to exert cytotoxicity. Damage of the target molecule by singlet oxygen in mitochondria is considered to be directly or indirectly involved in the release of cytochrome c. Taking the high reactivity of singlet oxygen to the conjugated double bond into consideration, the most likely target in mitochondria are molecules containing polyunsaturated fatty acids. When we evaluated the effects of singlet oxygen, cells that express high levels of PHGPx in mitochondria exhibited the highest resistance to MNPE (Figure 3). The protection of cells by PHGPx from damage during photodynamic therapy has been documented [38]. The peroxidation of cardiolipin, a lipid that is predominantly present in the mitochondrial inner membrane and which is rich in unsaturated fatty acid, is a possible mechanism of cytochrome c release during oxidative stress in mitochondria [39,40]. Hence, PHGPx is likely to protect mitochondria by preventing this particular lipid from peroxidation triggered by singlet oxygen. The protective role of vitamin E against MNPE may be explained by high efficiency in incorporation into cells compared with β-carotene (Table 1). It is also possible that vitamin E interacts with MNPE and affects the generation of singlet oxygen.
Regarding cellular damage by singlet oxygen, several cytotoxic mechanisms have been proposed. Mitogen-activated protein kinases, p38, JNK and ERK, are activated during apoptosis induced by singlet oxygen and UV [18,19,21,41,42]. The involvement of transcription factors, such as AP-2 (activator protein 2), is also known [43]. In singlet-oxygen-induced cell damage, apoptotic machinery would be initiated by the cytochrome c released into the cytosol. The damaged cells showed chromatin condensation, a morphological profile of apoptotic cell death. However, we observed only very low levels of nuclear fragmentation (Figure 5). It appears that cellular damage ceased at the stage of chromatin condensation. On the other hand, DNA ladder formation, another criterion of apoptosis, was also suppressed in MNPE-treated cells (Figure 6). Thus these characteristics of cell death triggered by singlet oxygen were different from typical apoptosis.
To investigate the molecular mechanism of this atypical cell death, we examined caspase activities. When cells were treated with MNPE or etoposide and showed a similar viability as judged by LDH activity, the activities of caspase 9 and 3 were much lower in MNPE-treated cells than in etoposide-treated cells (Figures 7 and 8). The suppression of caspase activities by singlet oxygen appeared to occur by at least two mechanisms in the cell death pathway.
One is the direct action of singlet oxygen on the active forms of caspases. MNPE inhibited the activities of both caspase 9 and 3 directly in cells treated with etoposide or in the cytosolic fraction that contained active forms of caspases, as judged by the presence of processed forms (Figures 8 and 9). At high doses, NO is a well-known cytotoxic mediator and causes cell death. NO is known to cause caspase inhibition by modifying the reactive thiol residue in the catalytic centre [44]. Singlet oxygen, as well as NO, preferentially reacts with deprotonated thiol and results in modification of the reactive residue. Free cysteine reacts with singlet oxygen to give the disulphide and also cysteic acid [14]. Caspases would be a preferable target, because they possess a reactive cysteine residue that is involved in proteolytic reactions. While the inactivation of caspase 3 with H2O2 is reversible [45], singlet-oxygen-induced inactivation was only partially reversed by reduction with DTT (Figure 9C). It would be intriguing to know what types of modification actually occur in the case of caspases. However, the reaction of sterically constrained thiol groups in proteins with singlet oxygen has not been investigated extensively [14]. A chemical analysis of the products will be needed to understand the reaction of thiol groups with singlet oxygen.
The other mechanism of caspase suppression would be related to apoptosome formation. The cytochrome c released from mitochondria binds Apaf 1 (apoptotic protease-activating factor 1) and forms an apoptosome together with procaspase 9, dATP and other factors [46]. This process appears to be redox-sensitive, because NO modulates the process [47]. We examined the effects of singlet oxygen on caspase activation in a cell-free reconstitution system and observed the suppression of caspase activation by these activators (Figure 9D). Although it would be intriguing to determine the effect of MNPE pre-treatment on the activation of caspases, the caspase cascade was inactivated irreversibly during incubation at 37 °C, even in the absence of MNPE (results not shown). This is because the machinery involved in apoptosome formation is unstable in a cell-free system at 37 °C. Since the release of singlet oxygen from MNPE is highly temperature sensitive and only a very small amount of singlet oxygen was released from MNPE at 4 °C, we were unable to examine the effect of singlet oxygen on apoptosome formation in the MNPE-treated cytosolic fraction. Therefore we examined the effects of singlet oxygen on the activation of the caspase cascade using the cytosolic fraction from the cells pre-treated with MNPE (Figure 10). The activities of caspase 9 and 3, as well as their active forms, implied that singlet oxygen suppressed the activation of the caspase cascade. It has been shown that some of the products formed on the reaction of singlet oxygen with amino acids and peptides can have potent inhibitory effects on caspase activity [48]. It is therefore possible that the observed inhibition of caspase activity may not be directly mediated by singlet oxygen, but arise from some of its downstream products [49].
Another question is what is the fate of cells undergoing abortive apoptosis? In vivo, cells can be efficiently phagocytosed without the translocation of phosphatidylserine to the outer leaflet of the plasma membrane [50]. In addition, the involvement of other proteases in the process has been documented [51]. It has actually been reported that caspase activation is not required for apoptosis of a certain type of cells [52]. Our data also show that singlet oxygen caused cell death that was not typical apoptosis owing to the suppression of caspase activity.
In conclusion, single oxygen intracellularly generated from endoperoxides caused cell death. One of the primary target organelles within cells is the mitochondrion. Although singlet oxygen reacts with several cellular components, such as polyunsaturated fatty acids, that are involved in the retention of cytochrome c in the inner membrane are the likely targets. Caspase activities did not correspond to the levels of released cytochrome c in the cytoplasm. This is mainly due to the impairment of caspases by singlet oxygen. Consequently, the cells did not follow a typical apoptotic pathway, but showed an atypical, necrosis-like cell death.
Acknowledgments
We thank Ms Masako Seki for maintenance of laboratory equipment and secretarial services. This work was supported, in part, by Grant-in-Aid for Scientific Research (C) (No. 16590238) and 21st Century COE Program from the Japan Society for the Promotion of Science (JSPS) and by the Ichiro Kanehara Foundation.
References
- 1.Halliwell B., Gutteridge J. M. C. 3rd edn. Oxford: Clarendon Press; 1999. Free Radical Biology and Medicine. [Google Scholar]
- 2.Green D., Reed J. C. Mitochondria and apoptosis. Science. 1998;281:1309–1312. doi: 10.1126/science.281.5381.1309. [DOI] [PubMed] [Google Scholar]
- 3.Hengartner M. O. The biochemistry of apoptosis. Nature (London) 2000;407:770–776. doi: 10.1038/35037710. [DOI] [PubMed] [Google Scholar]
- 4.Cain K., Bratton S. B., Langlais C., Walker G., Brown D. G., Sun X. M., Cohen G. M. Apaf-1 oligomerizes into biologically active approximately 700-kDa and inactive approximately 1.4-MDa apoptosome complexes. J. Biol. Chem. 2000;275:6067–6070. doi: 10.1074/jbc.275.9.6067. [DOI] [PubMed] [Google Scholar]
- 5.Khan A. U., Kasha M. Singlet molecular oxygen in the Haber–Weiss reaction. Proc. Natl. Acad. Sci. U.S.A. 1994;91:12365–12367. doi: 10.1073/pnas.91.26.12365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khan A. U., Kovacic D., Kolbanovskiy A., Desai M., Frenkel K., Geacintov N. E. The decomposition of peroxynitrite to nitroxyl anion (NO−) and singlet oxygen in aqueous solution. Proc. Natl. Acad. Sci. U.S.A. 2000;97:2984–2989. doi: 10.1073/pnas.050587297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berneburg M., Grether-Beck S., Kurten V., Ruzicka T., Briviba K., Sies H., Krutmann J. Singlet oxygen mediates the UVA-induced generation of the photoaging-associated mitochondrial common deletion. J. Biol. Chem. 1999;274:15345–15349. doi: 10.1074/jbc.274.22.15345. [DOI] [PubMed] [Google Scholar]
- 8.Ahmad N., Mukhtar H. Mechanism of photodynamic therapy-induced cell death. Methods Enzymol. 2000;319:342–358. doi: 10.1016/s0076-6879(00)19034-2. [DOI] [PubMed] [Google Scholar]
- 9.Tatsuzawa H., Maruyama T., Misawa N., Fujimori K., Hori K., Sano Y., Kambayashi Y., Nakano M. Inactivation of bacterial respiratory chain enzymes by singlet oxygen. FEBS Lett. 1998;439:329–333. doi: 10.1016/s0014-5793(98)01397-0. [DOI] [PubMed] [Google Scholar]
- 10.Tatsuzawa H., Maruyama T., Hori K., Sano Y., Nakano M. Singlet oxygen (1ΔgO2) as the principal oxidant in myeloperoxidase-mediated bacterial killing in neutrophil phagosome. Biochem. Biophys. Res. Commun. 1999;262:647–650. doi: 10.1006/bbrc.1999.1265. [DOI] [PubMed] [Google Scholar]
- 11.Tatsuzawa H., Maruyama T., Misawa N., Fujimori K., Nakano M. Quenching of singlet oxygen by carotenoids produced in Escherichia coli – attenuation of singlet oxygen-mediated bacterial killing by carotenoids. FEBS Lett. 2000;484:280–284. doi: 10.1016/s0014-5793(00)02149-9. [DOI] [PubMed] [Google Scholar]
- 12.Pellieux C., Dewilde A., Pierlot C., Aubry J. M. Bactericidal and virucidal activities of singlet oxygen generated by thermolysis of naphthalene endoperoxides. Methods Enzymol. 2000;319:197–207. doi: 10.1016/s0076-6879(00)19020-2. [DOI] [PubMed] [Google Scholar]
- 13.Petrat F., Pindiur S., Kirsch M., de Groot H. NAD(P)H, a primary target of 1O2 in mitochondria of intact cells. J. Biol. Chem. 2003;278:3298–3307. doi: 10.1074/jbc.M204230200. [DOI] [PubMed] [Google Scholar]
- 14.Davies M. J. Singlet oxygen-mediated damage to proteins and its consequences. Biochem. Biophys. Res. Commun. 2003;305:761–770. doi: 10.1016/s0006-291x(03)00817-9. [DOI] [PubMed] [Google Scholar]
- 15.Morita A., Werfel T., Stege H., Ahrens C., Karmann K., Grewe M., Grether-Beck S., Ruzicka T., Kapp A., Klotz L. O., et al. Evidence that singlet oxygen-induced human T helper cell apoptosis is the basic mechanism of ultraviolet-A radiation phototherapy. J. Exp. Med. 1997;186:1763–1768. doi: 10.1084/jem.186.10.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Godar D. E. UVA1 radiation triggers two different final apoptotic pathways. J. Invest. Dermatol. 1999;112:3–12. doi: 10.1046/j.1523-1747.1999.00474.x. [DOI] [PubMed] [Google Scholar]
- 17.Kochevar I. E., Lynch M. C., Zhuang S., Lambert C. R. Singlet oxygen, but not oxidizing radicals, induces apoptosis in HL-60 cells. Photochem. Photobiol. 2000;72:548–553. doi: 10.1562/0031-8655(2000)072<0548:sobnor>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 18.Klotz L. O., Briviba K., Sies H. Singlet oxygen mediates the activation of JNK by UVA radiation in human skin fibroblasts. FEBS Lett. 1997;408:289–291. doi: 10.1016/s0014-5793(97)00440-7. [DOI] [PubMed] [Google Scholar]
- 19.Klotz L. O., Pellieux C., Briviba K., Pierlot C., Aubry J. M., Sies H. Mitogen-activated protein kinase (p38-, JNK-, ERK-) activation pattern induced by extracellular and intracellular singlet oxygen and UVA. Eur. J. Biochem. 1999;260:917–922. doi: 10.1046/j.1432-1327.1999.00255.x. [DOI] [PubMed] [Google Scholar]
- 20.Klotz L. O. Oxidant-induced signaling: effects of peroxynitrite and singlet oxygen. Biol. Chem. 2002;383:443–456. doi: 10.1515/BC.2002.047. [DOI] [PubMed] [Google Scholar]
- 21.Zhuang S., Demirs J. T., Kochevar I. E. p38 mitogen-activated protein kinase mediates bid cleavage, mitochondrial dysfunction, and caspase-3 activation during apoptosis induced by singlet oxygen but not by hydrogen peroxide. J. Biol. Chem. 2000;275:25939–25948. doi: 10.1074/jbc.M001185200. [DOI] [PubMed] [Google Scholar]
- 22.Aubry J. M., Cazin B., Duprat F. Chemical sources of singlet oxygen. 3. Peroxidation of water-soluble singlet oxygen carriers with the hydrogen peroxide–molybdate system. J. Org. Chem. 1989;54:726–728. [Google Scholar]
- 23.Liu W., Ogata T., Sato K., Ohba Y., Sakurai K., Igarashi T. Syntheses of water-soluble endoperoxides as a singlet oxygen source. ITE Lett. Batt. New Technol. Med. 2001;2:98–101. [Google Scholar]
- 24.Saito I., Matsuura T., Inoue K. Formation of superoxide ion via one-electron transfer from electron donors to singlet oxygen. J. Am. Chem. Soc. 1983;105:3200–3206. [Google Scholar]
- 25.Nakano M., Kambayashi Y., Tatsuzawa H., Komiyama T., Fujimori K. Useful 1O2 (1Δg) generator, 3-(4′-methyl-1′-naphthyl)-propionic acid, 1′,4′-endoperoxide (NEPO), for dioxygenation of squalene (a skin surface lipid) in an organic solvent and bacterial killing in aqueous medium. FEBS Lett. 1998;432:9–12. doi: 10.1016/s0014-5793(98)00822-9. [DOI] [PubMed] [Google Scholar]
- 26.Solichova D., Korecka L., Svobodova I., Musil F., Blaha V., Zdansky P., Zadak Z. Development and validation of HPLC method for the determination of α-tocopherol in human erythrocytes for clinical applications. Anal. Bioanal. Chem. 2003;376:444–447. doi: 10.1007/s00216-003-1886-1. [DOI] [PubMed] [Google Scholar]
- 27.Offord E. A., Gautier J. C., Avanti O., Scaletta C., Runge F., Kramer K., Applegate L. A. Photoprotective potential of lycopene, β-carotene, vitamin E, vitamin C and carnosic acid in UVA-irradiated human skin fibroblasts. Free Radical Biol. Med. 2002;32:1293–1303. doi: 10.1016/s0891-5849(02)00831-6. [DOI] [PubMed] [Google Scholar]
- 28.Kayanoki Y., Fujii J., Islam K. N., Suzuki K., Kawata S., Matsuzawa Y., Taniguchi N. The protective role of glutathione peroxidase in apoptosis induced by reactive oxygen species. J. Biochem. (Tokyo) 1996;119:817–822. doi: 10.1093/oxfordjournals.jbchem.a021313. [DOI] [PubMed] [Google Scholar]
- 29.Anderson M. E. Determination of glutathione and glutathione disulfide in biological samples. Methods Enzymol. 1985;113:548–555. doi: 10.1016/s0076-6879(85)13073-9. [DOI] [PubMed] [Google Scholar]
- 30.Otsu K., Ikeda Y., Fujii J. Accumulation of manganese superoxide dismutase under metal-depleted conditions: proposed role for zinc ions in cellular redox balance. Biochem. J. 2004;377:241–248. doi: 10.1042/BJ20030935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stennicke H. R., Salvesen G. S. Biochemical characteristics of caspases-3, -6, -7, and -8. J. Biol. Chem. 1997;272:25719–25723. doi: 10.1074/jbc.272.41.25719. [DOI] [PubMed] [Google Scholar]
- 32.Matsuki S., Iuchi Y., Ikeda Y., Sasagawa I., Tomita Y., Fujii J. Suppression of cytochrome c release and apoptosis in testes with heat stress by minocycline. Biochem. Biophys. Res. Commun. 2003;312:843–849. doi: 10.1016/j.bbrc.2003.10.191. [DOI] [PubMed] [Google Scholar]
- 33.Ishizawa M., Kobayashi Y., Miyamura T., Matsuura S. Simple procedure of DNA isolation from human serum. Nucleic Acids Res. 1991;19:5792. doi: 10.1093/nar/19.20.5792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cain K., Brown D. G., Langlais C., Cohen G. M. Caspase activation involves the formation of the aposome, a large (approximately 700 kDa) caspase-activating complex. J. Biol. Chem. 1999;274:22686–22692. doi: 10.1074/jbc.274.32.22686. [DOI] [PubMed] [Google Scholar]
- 35.Imai H., Nakagawa Y. Biological significance of phospholipid hydroperoxide glutathione peroxidase (PHGPx, GPx4) in mammalian cells. Free Radical Biol. Med. 2003;34:145–169. doi: 10.1016/s0891-5849(02)01197-8. [DOI] [PubMed] [Google Scholar]
- 36.Arai M., Imai H., Koumura T., Yoshida M., Emoto K., Umeda M., Chiba N., Nakagawa Y. Mitochondrial phospholipid hydroperoxide glutathione peroxidase plays a major role in preventing oxidative injury to cells. J. Biol. Chem. 1999;274:4924–4933. doi: 10.1074/jbc.274.8.4924. [DOI] [PubMed] [Google Scholar]
- 37.Nomura K., Imai H., Koumura T., Arai M., Nakagawa Y. Mitochondrial phospholipid hydroperoxide glutathione peroxidase suppresses apoptosis mediated by a mitochondrial death pathway. J. Biol. Chem. 1999;274:29294–29302. doi: 10.1074/jbc.274.41.29294. [DOI] [PubMed] [Google Scholar]
- 38.Wang H. P., Qian S. Y., Schafer F. Q., Domann F. E., Oberley L. W., Buettner G. R. Phospholipid hydroperoxide glutathione peroxidase protects against singlet oxygen-induced cell damage of photodynamic therapy. Free Radical Biol. Med. 2001;30:825–835. doi: 10.1016/s0891-5849(01)00469-5. [DOI] [PubMed] [Google Scholar]
- 39.Nomura K., Imai H., Koumura T., Kobayashi T., Nakagawa Y. Mitochondrial phospholipid hydroperoxide glutathione peroxidase inhibits the release of cytochrome c from mitochondria by suppressing the peroxidation of cardiolipin in hypoglycaemia-induced apoptosis. Biochem. J. 2000;351:183–193. doi: 10.1042/0264-6021:3510183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Petrosillo G., Ruggiero F. M., Paradies G. Role of reactive oxygen species and cardiolipin in the release of cytochrome c from mitochondria. FASEB J. 2003;17:2202–2208. doi: 10.1096/fj.03-0012com. [DOI] [PubMed] [Google Scholar]
- 41.Chan W. H., Yu J. S., Yang S. D. Apoptotic signalling cascade in photosensitized human epidermal carcinoma A431 cells: involvement of singlet oxygen, c-Jun N-terminal kinase, caspase-3 and p21-activated kinase 2. Biochem. J. 2000;351:221–232. doi: 10.1042/0264-6021:3510221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhuang S., Lynch M. C., Kochevar I. E. Caspase-8 mediates caspase-3 activation and cytochrome c release during singlet oxygen-induced apoptosis of HL-60 cells. Exp. Cell Res. 1999;250:203–212. doi: 10.1006/excr.1999.4501. [DOI] [PubMed] [Google Scholar]
- 43.Grether-Beck S., Olaizola-Horn S., Schmitt H., Grewe M., Jahnke A., Johnson J. P., Briviba K., Sies H., Krutmann J. Activation of transcription factor AP-2 mediates UVA radiation- and singlet oxygen-induced expression of the human intercellular adhesion molecule 1 gene. Proc. Natl. Acad. Sci. U.S.A. 1996;93:14586–14591. doi: 10.1073/pnas.93.25.14586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li J., Bombeck C. A., Yang S., Kim Y. M., Billiar T. R. Nitric oxide suppresses apoptosis via interrupting caspase activation and mitochondrial dysfunction in cultured hepatocytes. J. Biol. Chem. 1999;274:17325–17333. doi: 10.1074/jbc.274.24.17325. [DOI] [PubMed] [Google Scholar]
- 45.Borutaite V., Brown G. C. Caspases are reversibly inactivated by hydrogen peroxide. FEBS Lett. 2001;500:114–118. doi: 10.1016/s0014-5793(01)02593-5. [DOI] [PubMed] [Google Scholar]
- 46.Zou H., Li Y., Liu X., Wang X. An APAF-1·cytochrome c multimeric complex is a functional apoptosome that activates procaspase-9. J. Biol. Chem. 1999;274:11549–11556. doi: 10.1074/jbc.274.17.11549. [DOI] [PubMed] [Google Scholar]
- 47.Zech B., Köhl R., von Knethen A., Brüne B. Nitric oxide donors inhibit formation of the Apaf-1/caspase-9 apoptosome and activation of caspases. Biochem. J. 2003;371:1055–1064. doi: 10.1042/BJ20021720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hampton M. B., Morgan P. E., Davies M. J. Inactivation of cellular caspases by peptide-derived tryptophan and tyrosine peroxides. FEBS Lett. 2002;527:289–292. doi: 10.1016/s0014-5793(02)03240-4. [DOI] [PubMed] [Google Scholar]
- 49.Davies M. J. Reactive species formed on proteins exposed to singlet oxygen. Photochem. Photobiol. Sci. 2004;3:17–25. doi: 10.1039/b307576c. [DOI] [PubMed] [Google Scholar]
- 50.Leist M., Jaattela M. Four deaths and a funeral: from caspases to alternative mechanisms. Nat. Rev. Mol. Cell Biol. 2001;2:589–598. doi: 10.1038/35085008. [DOI] [PubMed] [Google Scholar]
- 51.Foghsgaard L., Wissing D., Mauch D., Lademann U., Bastholm L., Boes M., Elling F., Leist M., Jaattela M. Cathepsin B acts as a dominant execution protease in tumor cell apoptosis induced by tumor necrosis factor. J. Cell Biol. 2001;153:999–1010. doi: 10.1083/jcb.153.5.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McCarthy N. J., Whyte M. K., Gilbert C. S., Evan G. I. Inhibition of Ced-3/ICE-related proteases does not prevent cell death induced by oncogenes, DNA damage, or the Bcl-2 homologue Bak. J. Cell Biol. 1997;136:215–227. doi: 10.1083/jcb.136.1.215. [DOI] [PMC free article] [PubMed] [Google Scholar]