Abstract
Pepsin reacts stoicheiometrically with the active-site-directed irreversible inhibitor N-diazoacetyl-l-phenylalanine methyl ester, with concomitant loss of all proteolytic and peptidolytic activity. The reagent esterifies a unique aspartic acid residue in pepsin, which is in the sequence:Ile-Val-Asp-Thr-Gly-Thr-Ser
Full text
PDF


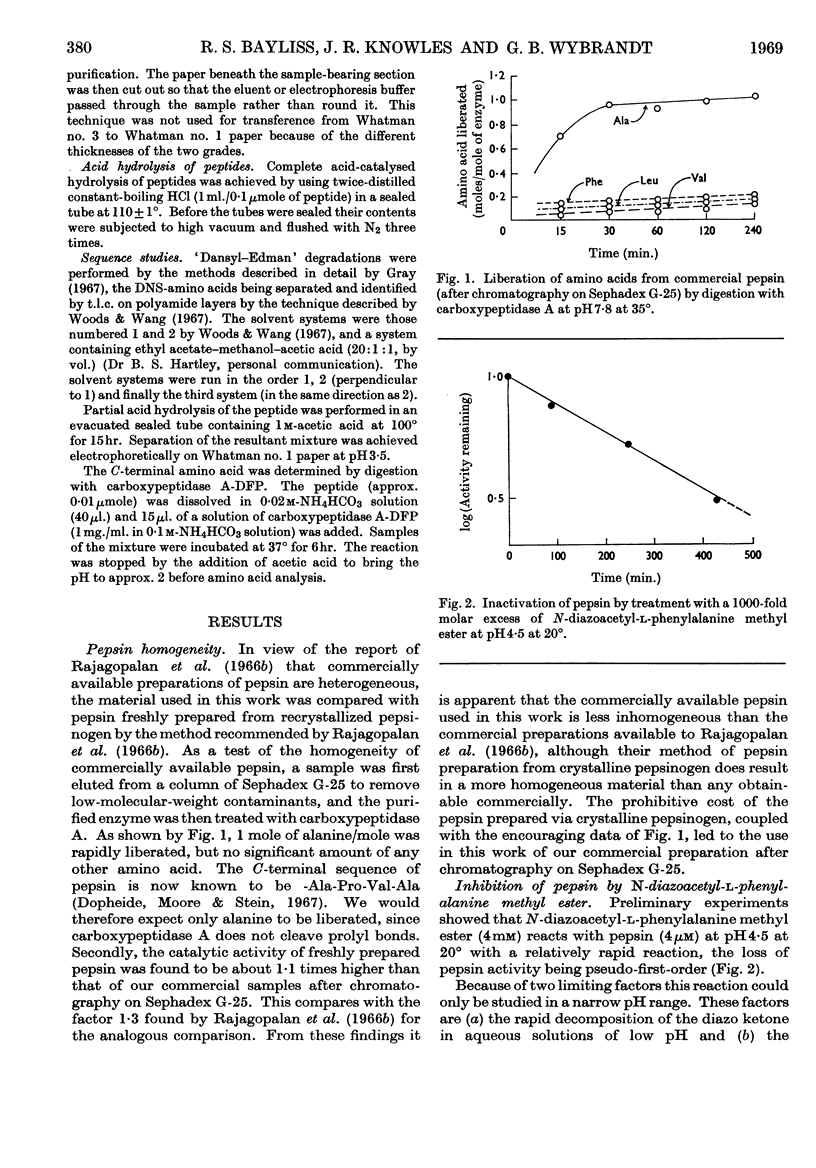
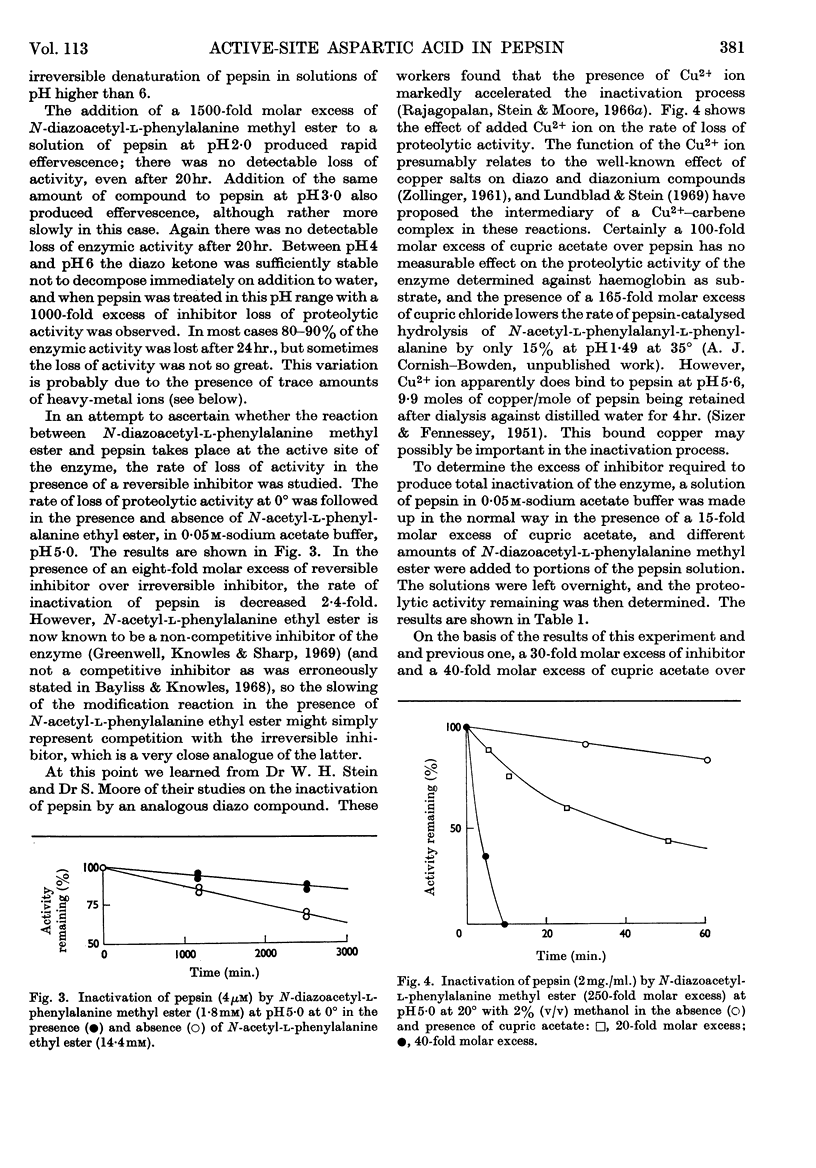
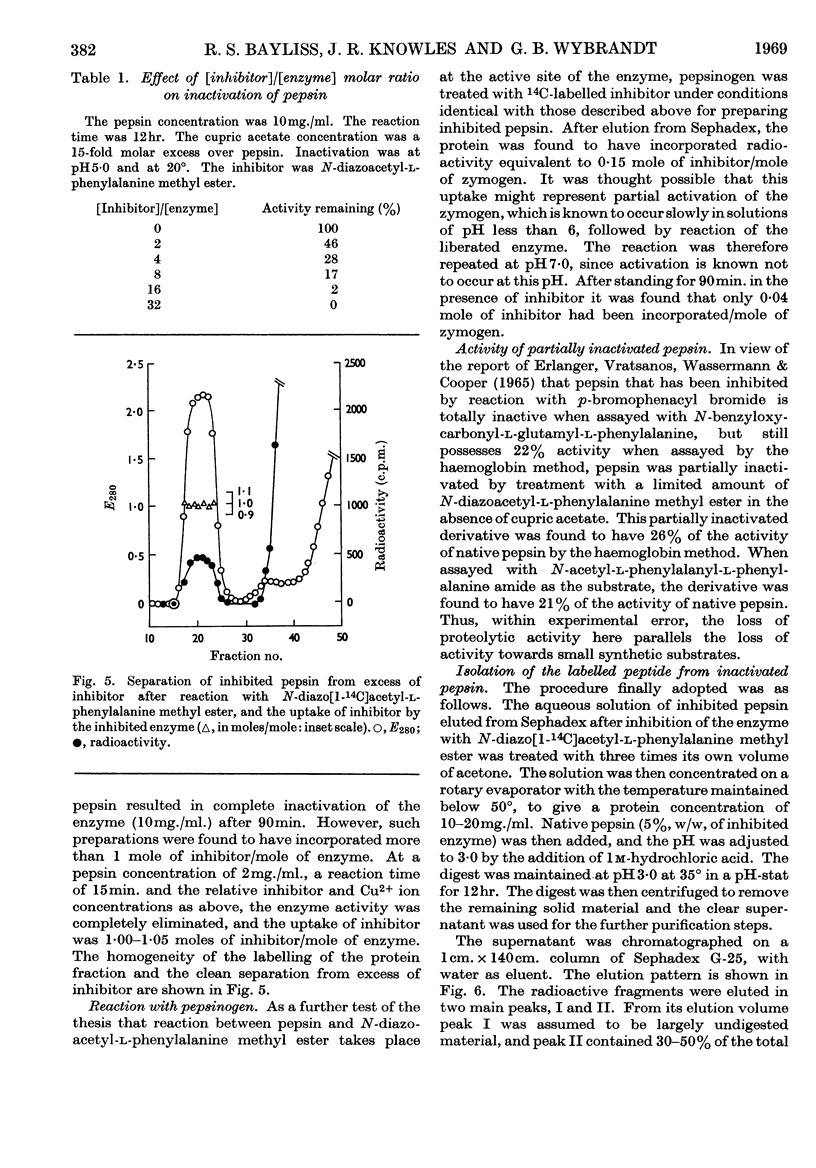
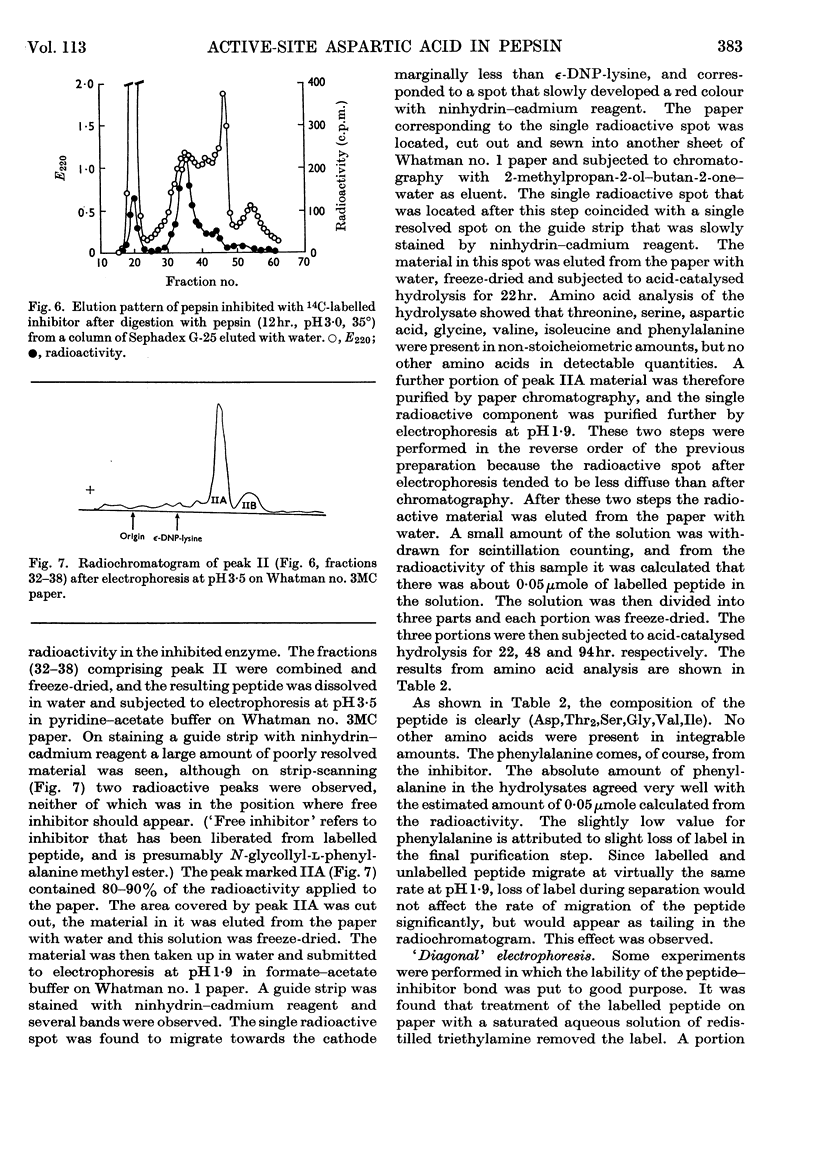


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
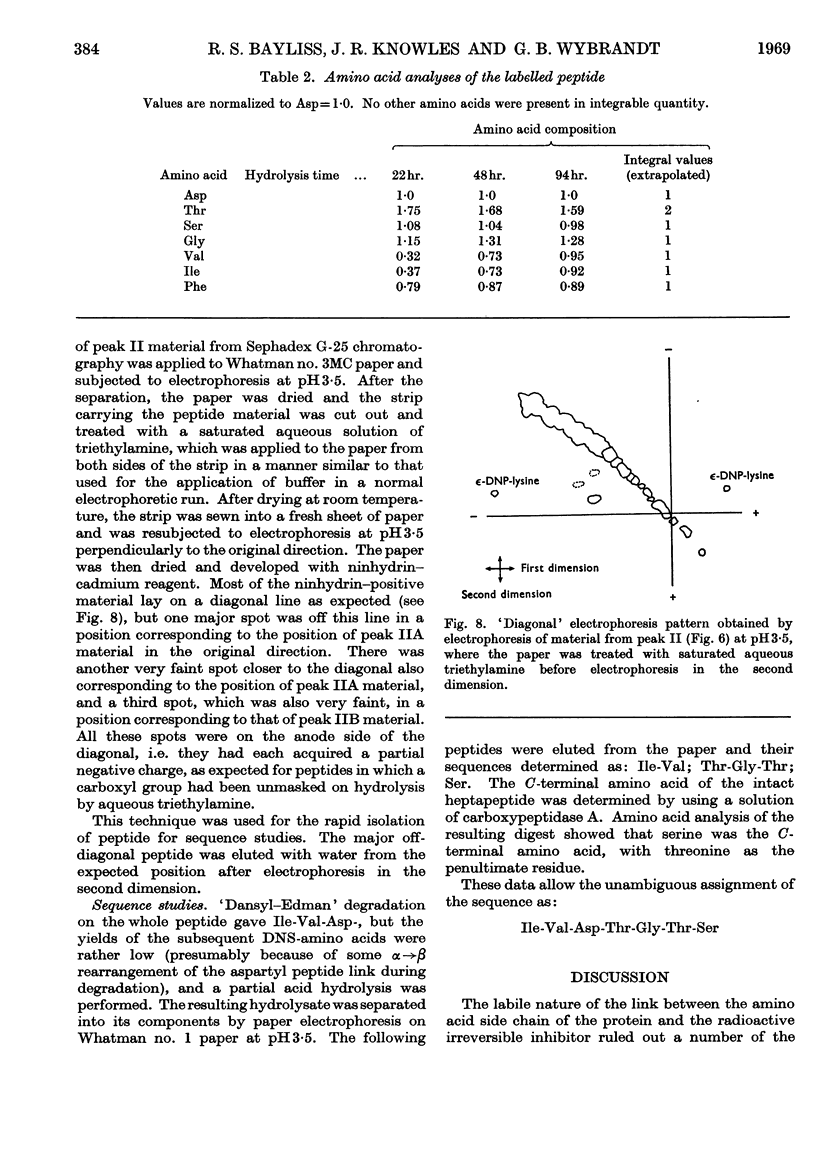
- Brown J. R., Hartley B. S. Location of disulphide bridges by diagonal paper electrophoresis. The disulphide bridges of bovine chymotrypsinogen A. Biochem J. 1966 Oct;101(1):214–228. doi: 10.1042/bj1010214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornish-Bowden A. J., Knowles J. R. The pH-dependence of pepsin-catalysed reactions. Biochem J. 1969 Jun;113(2):353–362. doi: 10.1042/bj1130353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delpierre G. R., Fruton J. S. Inactivation of pepsin by diphenyldiazomethane. Proc Natl Acad Sci U S A. 1965 Oct;54(4):1161–1167. doi: 10.1073/pnas.54.4.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delpierre G. R., Fruton J. S. Specific inactivation of pepsin by a diazo ketone. Proc Natl Acad Sci U S A. 1966 Dec;56(6):1817–1822. doi: 10.1073/pnas.56.6.1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dopheide T. A., Moore S., Stein W. H. The carboxyl-terminal sequence of porcine pepsin. J Biol Chem. 1967 Apr 25;242(8):1833–1837. [PubMed] [Google Scholar]
- Erlanger B. F., Vratsanos S. M., Wassermann N., Cooper A. G. Stereochemical investigation of the active center of pepsin using a new inactivator. Biochem Biophys Res Commun. 1967 Jul 21;28(2):203–208. doi: 10.1016/0006-291x(67)90430-5. [DOI] [PubMed] [Google Scholar]
- Fry K. T., Kim O. K., Kettering C. F., Spona J., Hamilton G. A. A reactive aspartyl residue of pepsin. Biochem Biophys Res Commun. 1968 Mar 12;30(5):489–495. doi: 10.1016/0006-291x(68)90078-8. [DOI] [PubMed] [Google Scholar]
- Greenwell P., Knowles J. R., Sharp H. The inhibition of pepsin-catalysed reactions by products and product analogues. Kinetic evidence for ordered release of products. Biochem J. 1969 Jun;113(2):363–368. doi: 10.1042/bj1130363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HEILMANN J., BARROLLIER J., WATZKE E. Beitrag zur Aminosäurebestimmung auf Papierchromatogrammen. Hoppe Seylers Z Physiol Chem. 1957;309(4-6):219–220. [PubMed] [Google Scholar]
- Hamilton G. A., Spona J., Crowell L. D. The inactivation of pepsin by an equimolar amount of 1-diazo-4-phenylbutanone-2. Biochem Biophys Res Commun. 1967 Jan 23;26(2):193–198. doi: 10.1016/0006-291x(67)90233-1. [DOI] [PubMed] [Google Scholar]
- Inouye K., Fruton J. S. Studies on the specificity of pepsin. Biochemistry. 1967 Jun;6(6):1765–1777. doi: 10.1021/bi00858a027. [DOI] [PubMed] [Google Scholar]
- Knowles J. R., Sharp H., Greenwell P. The pH-dependence of the binding of competitive inhibitors to pepsin. Biochem J. 1969 Jun;113(2):343–351. doi: 10.1042/bj1130343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozlov L. V., Ginodman L. M., Orekhovich V. N. Inaktivatsiia pepsina alifaticheskimi diazokarbonil'nymi soedineniiami. Biokhimiia. 1967 Sep-Oct;32(5):1011–1019. [PubMed] [Google Scholar]
- Lundblad R. L., Stein W. H. On the reaction of diazoacetyl compounds with pepsin. J Biol Chem. 1969 Jan 10;244(1):154–160. [PubMed] [Google Scholar]
- Ong E. B., Perlmann G. E. Specific inactivation of pepsin by benzyloxycarbonyl-L-phenylalanyldiazomethane. Nature. 1967 Sep 30;215(5109):1492–1494. doi: 10.1038/2151492b0. [DOI] [PubMed] [Google Scholar]
- Rajagopalan T. G., Moore S., Stein W. H. Pepsin from pepsinogen. Preparation and properties. J Biol Chem. 1966 Nov 10;241(21):4940–4950. [PubMed] [Google Scholar]
- SIZER E. W., FENNESSEY J. F. The inactivation of invertase by tyrosinase. II. The influence of copper and gold on the oxidation of invertase and pepsin. J Biol Chem. 1951 Jan;188(1):351–359. [PubMed] [Google Scholar]
- Stepanov V. M., Vaganova T. I. Identification of the carboxyl group of pepsin reacting with diazoacetamide derivatives. Biochem Biophys Res Commun. 1968 Jun 10;31(5):825–830. doi: 10.1016/0006-291x(68)90637-2. [DOI] [PubMed] [Google Scholar]
- Woods K. R., Wang K. T. Separation of dansyl-amino acids by polyamide layer chromatography. Biochim Biophys Acta. 1967 Feb 21;133(2):369–370. doi: 10.1016/0005-2795(67)90078-5. [DOI] [PubMed] [Google Scholar]


