Abstract
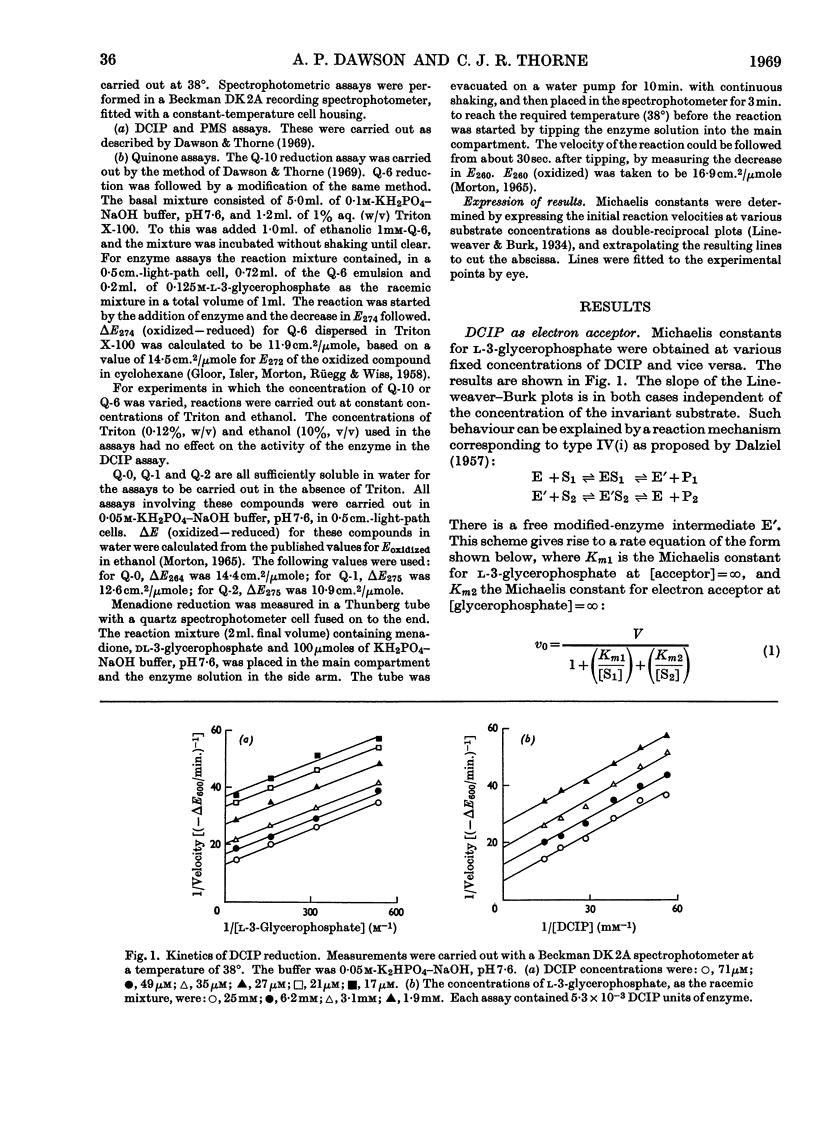
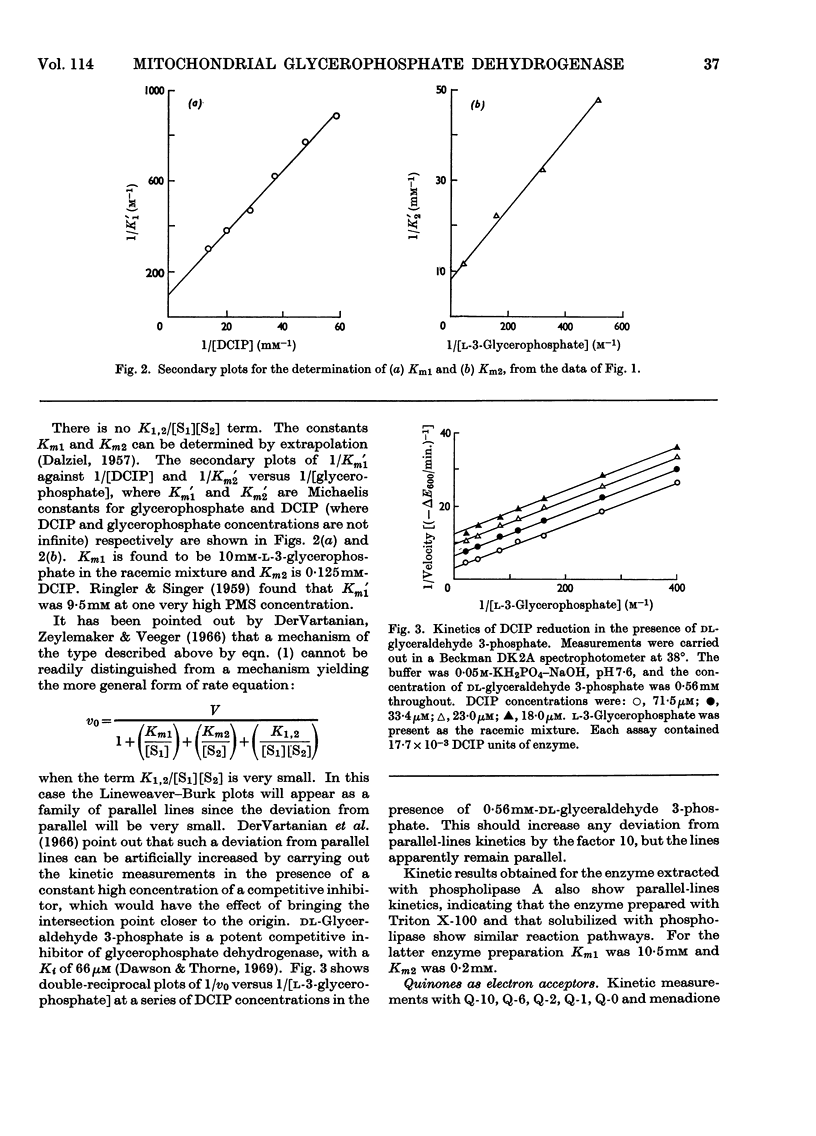
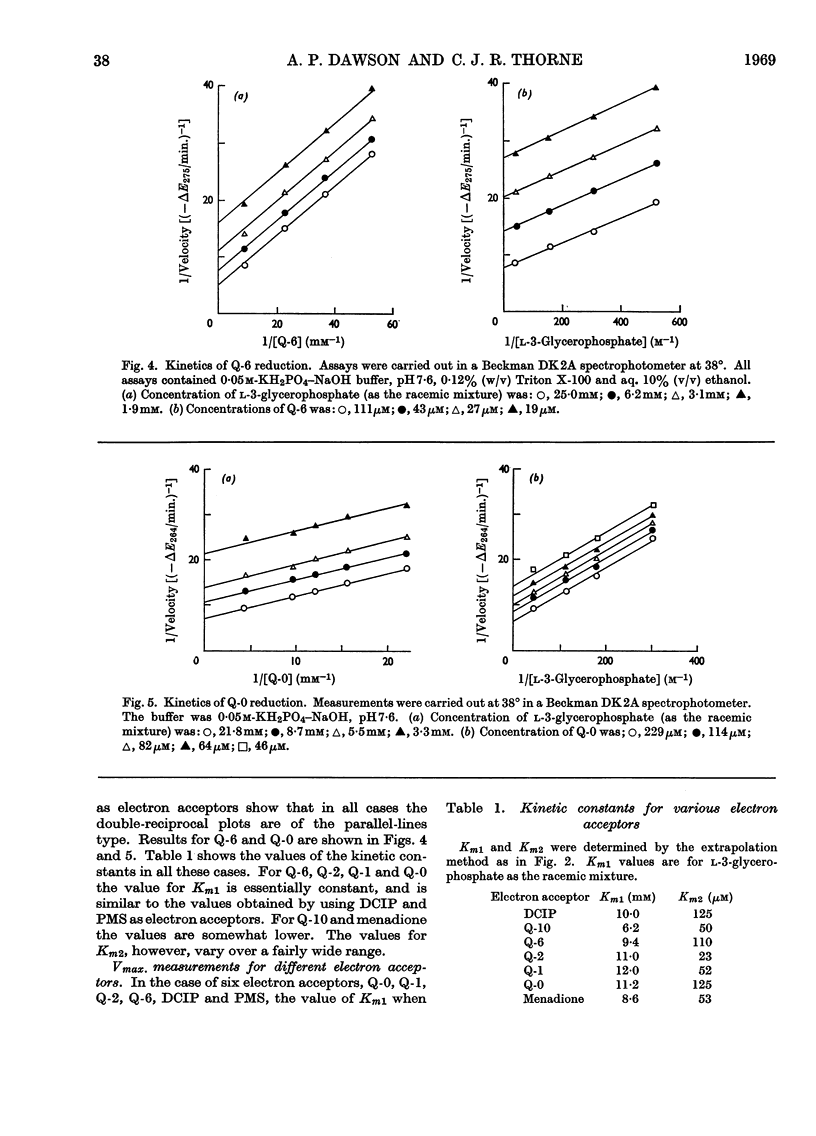
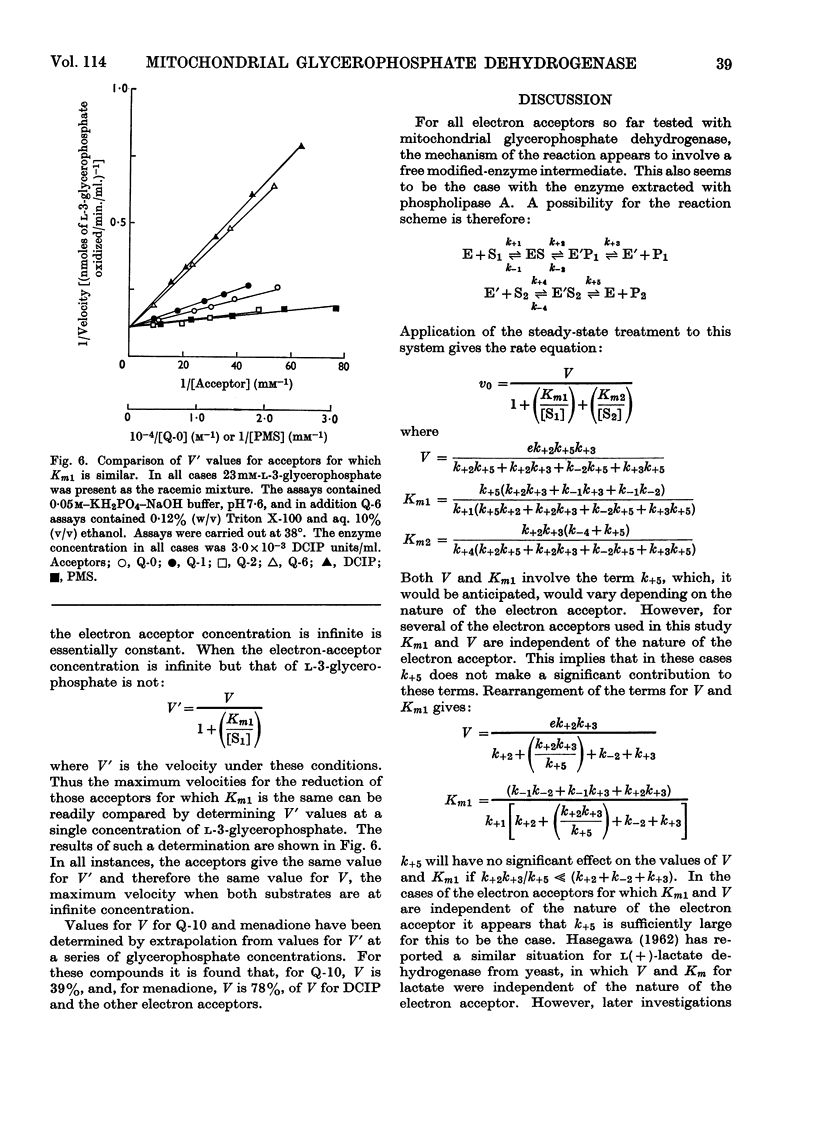
1. The kinetics of the reaction of glycerophosphate dehydrogenase with a variety of electron acceptors have been investigated. 2. In all cases the reaction mechanism appears to involve a free modified-enzyme intermediate. 3. With some electron acceptors, the maximum velocity of the reaction and the Km for glycerophosphate are independent of the nature of the electron acceptor, whereas in other cases this is not so. 4. The reaction mechanism of the enzyme extracted with phospholipase A instead of with Triton X-100 is of a similar type.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dawson A. P., Thorne C. J. Preparation and some properties of L-3-glycerophosphate dehydrogenase from pig brain mitochondria. Biochem J. 1969 Jan;111(1):27–34. doi: 10.1042/bj1110027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green D. E. alpha-Glycerophosphate dehydrogenase. Biochem J. 1936 Apr;30(4):629–644. doi: 10.1042/bj0300629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HASEGAWA H. Kinetic studies on the action of yeast L-lactate dehydrogenase. J Biochem. 1962 Sep;52:207–213. doi: 10.1093/oxfordjournals.jbchem.a127598. [DOI] [PubMed] [Google Scholar]
- HINKSON J. W., MAHLER H. R. Studies on the mechanism of enzyme-catalyzed oxidation-reduction reactions. VI. Kinetic studies with yeast L-lactate dehydrogenase. Biochemistry. 1963 Mar-Apr;2:209–216. doi: 10.1021/bi00902a001. [DOI] [PubMed] [Google Scholar]
- RINGLER R. L., SINGER T. P. Studies on the mitochondrial alpha-glycerophosphate dehydrogenase. I. Reaction of the dehydrogenase with electron acceptors and the respiratory chain. J Biol Chem. 1959 Aug;234(8):2211–2217. [PubMed] [Google Scholar]
- RINGLER R. L. Studies on the mitochondrial alpha-glycerophosphate dehydrogenase. II. Extraction and partial purification of the dehydrogenase from pig brain. J Biol Chem. 1961 Apr;236:1192–1198. [PubMed] [Google Scholar]
- Schurr J. M., McLaren A. D. Enzyme action: comparison on soluble and insoluble substrate. Science. 1966 May 20;152(3725):1064–1066. doi: 10.1126/science.152.3725.1064. [DOI] [PubMed] [Google Scholar]
- TUNG T. C., ANDERSON L., LARDY H. A. Studies on the particulate alpha-glycerophosphate dehydrogenase of muscle. Arch Biochem Biophys. 1952 Sep;40(1):194–204. doi: 10.1016/0003-9861(52)90087-8. [DOI] [PubMed] [Google Scholar]


