Abstract
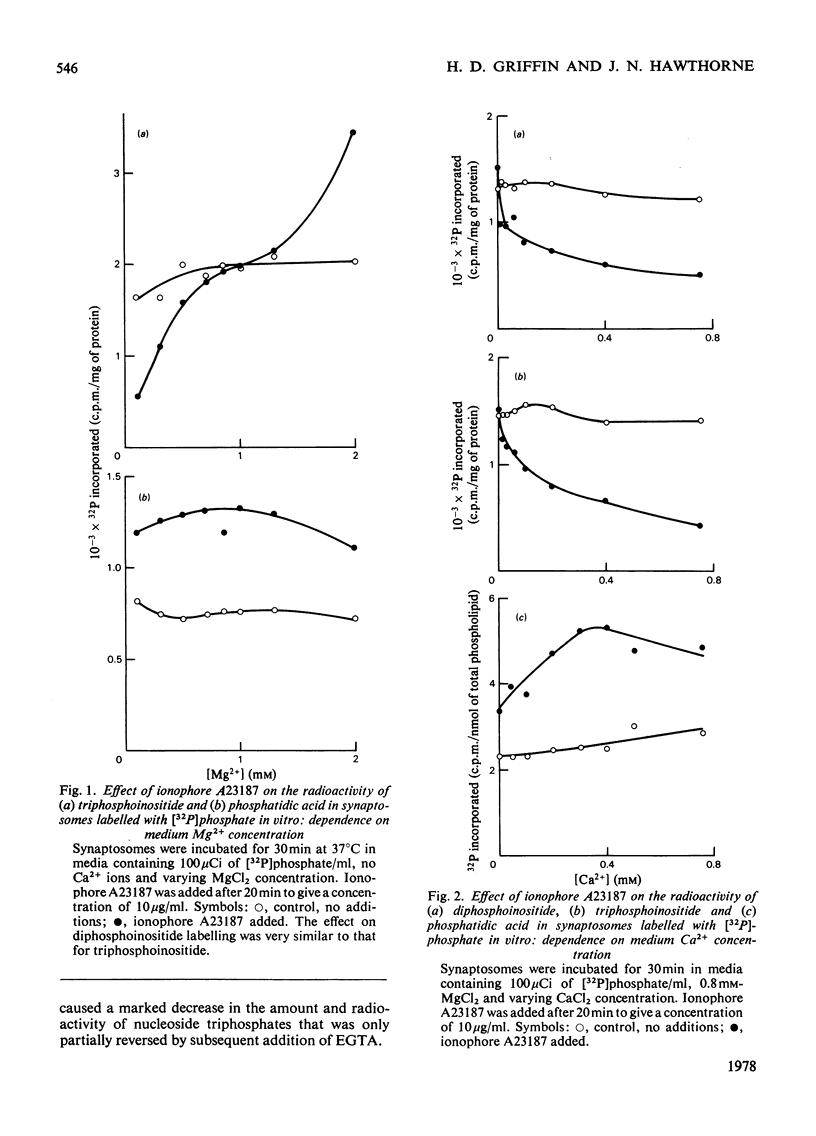
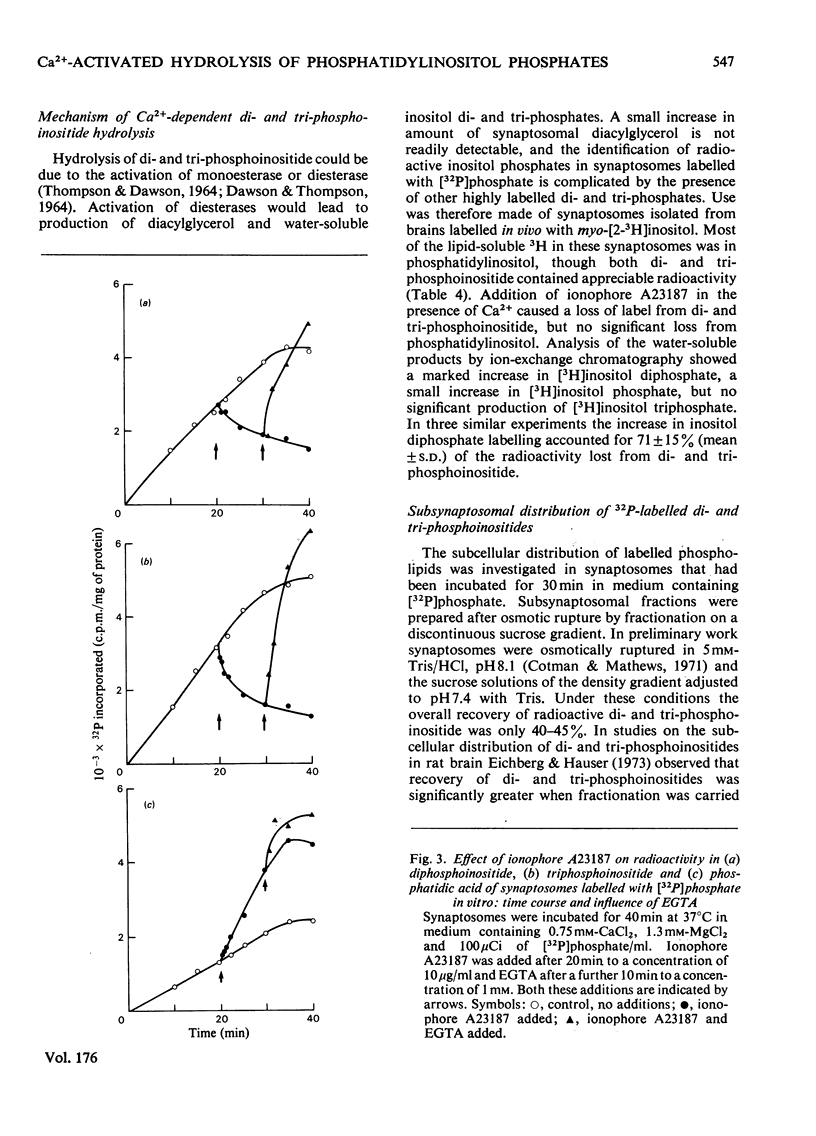
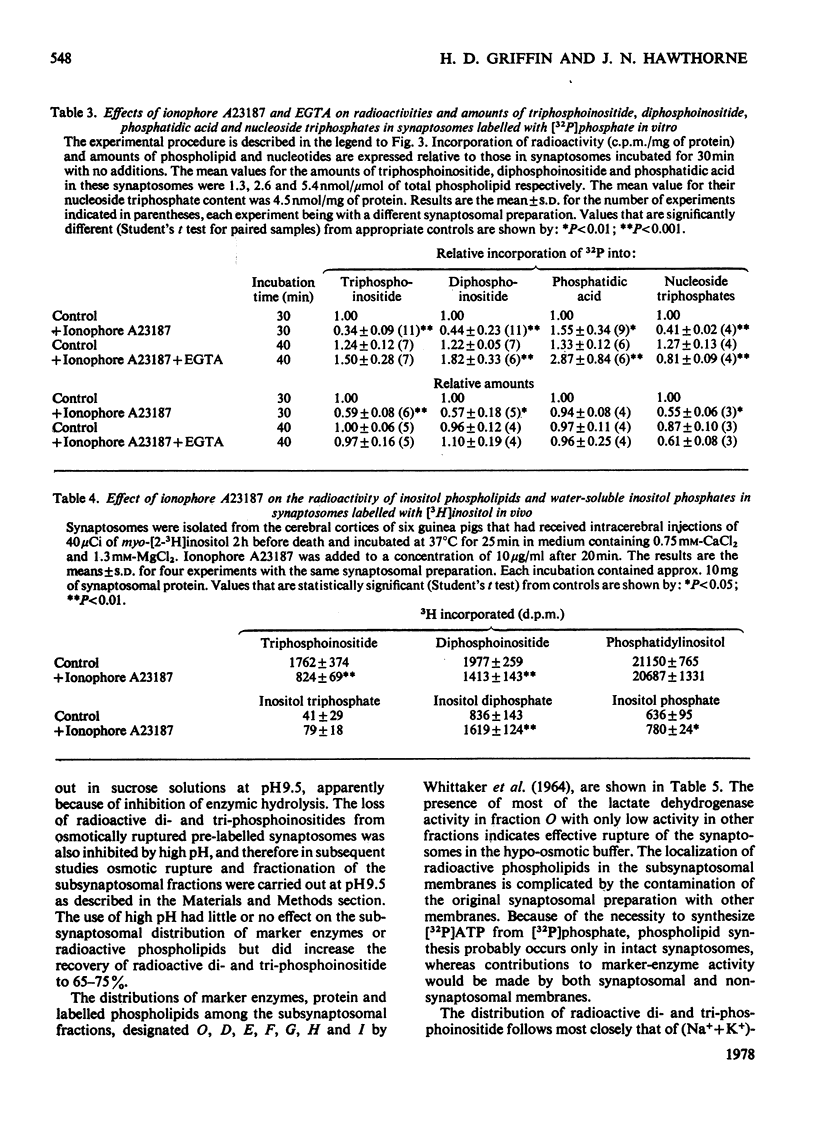
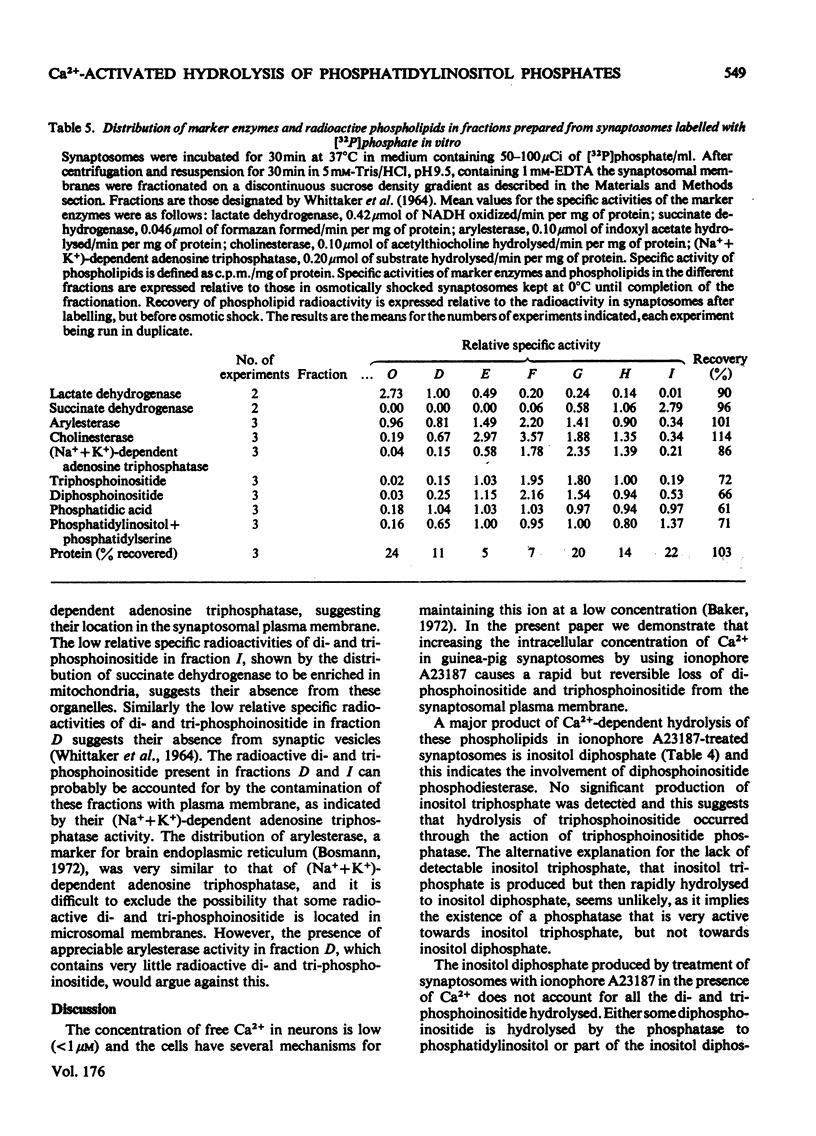
1. Addition of the bivalent ionophore A23187 to synaptosomes isolated from guinea-pig brain cortex and labelled with [32P]phosphate in vitro or in vivo caused a marked loss of radioactivity from phosphatidyl-myo-inositol 4-phosphate (diphosphoinositide) and phosphatidyl-myo-inositol 4,5-bisphosphate (triphosphoinositide) and stimulated labelling of phosphatidate. No change occurred in the labelling of other phospholipids. 2. In conditions that minimized changes in internal Mg2+ concentrations, the effect of ionophore A23187 on labelling of synaptosomal di- and tri-phosphoinositide was dependent on Ca2+ and was apparent at Ca2+ concentrations in the medium as low as 10−5m. 3. An increase in internal Mg2+ concentration stimulated incorporation of [32P]phosphate into di- and tri-phosphoinositide, whereas lowering internal Mg2+ decreased labelling. 4. Increased labelling of phosphatidate was independent of medium Mg2+ concentration and apparently only partly dependent on medium Ca2+ concentration. 5. The loss of label from di- and tri-phosphoinositide caused by ionophore A23187 was accompanied by losses in the amounts of both lipids. 6. Addition of excess of EGTA to synaptosomes treated with ionophore A23187 in the presence of Ca2+ caused a rapid resynthesis of di- and tri-phosphoinositide and a further stimulation of phosphatidate labelling. 7. Addition of ionophore A23187 to synaptosomes labelled in vivo with [3H]inositol caused a significant loss of label from di- and tri-phosphoinositide, but not from phosphatidylinositol. There was a considerable rise in labelling of inositol diphosphate, a small increase in that of inositol phosphate, but no significant production of inositol triphosphate. 8. 32P-labelled di- and tri-phosphoinositides appeared to be located in the synaptosomal plasma membrane. 9. The results indicate that increased Ca2+ influx into synaptosomes markedly activates triphosphoinositide phosphatase and diphosphoinositide phosphodiesterase, but has little or no effect on phosphatidylinositol phosphodiesterase.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdel-Latif A. A., Akhtar R. A., Hawthorne J. N. Acetylcholine increases the breakdown of triphosphoinositide of rabbit iris muscle prelabelled with [32P] phosphate. Biochem J. 1977 Jan 15;162(1):61–73. doi: 10.1042/bj1620061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allan D., Michell R. H. A calcium-activated polyphosphoinositide phosphodiesterase in the plasma membrane of human and rabbit erythrocytes. Biochim Biophys Acta. 1978 Apr 4;508(2):277–286. doi: 10.1016/0005-2736(78)90330-9. [DOI] [PubMed] [Google Scholar]
- Anderson R. E., Feldman L. S., Feldman G. L. Lipids of ocular tissues. II. The phospholipids of mature bovine and rabbit whole retina. Biochim Biophys Acta. 1970 Mar 10;202(2):367–373. [PubMed] [Google Scholar]
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- BROCKERHOFF H., BALLOU C. E. Phosphate incorporation in brain phosphionositides. J Biol Chem. 1962 Jan;237:49–52. [PubMed] [Google Scholar]
- Birnberger A. C., Birnberger K. L., Eliasson S. G., Simpson P. C. Effect of cyanide and electrical stimulation on phosphoinositide metabolism in lobster nerves. J Neurochem. 1971 Jul;18(7):1291–1298. doi: 10.1111/j.1471-4159.1971.tb00228.x. [DOI] [PubMed] [Google Scholar]
- Bosmann H. B. Membrane marker enzymes. Characterization of an arylesterase of guinea pig cerebral cortex utilizing p-nitrophenyl acetate as substrate. Biochim Biophys Acta. 1972 Jul 13;276(1):180–191. doi: 10.1016/0005-2744(72)90019-8. [DOI] [PubMed] [Google Scholar]
- Bradford H. F. Respiration in vitro of synaptosomes from mammalian cerebral cortex. J Neurochem. 1969 May;16(5):675–684. doi: 10.1111/j.1471-4159.1969.tb06444.x. [DOI] [PubMed] [Google Scholar]
- Buckley J. T., Hawthorne J. N. Erythrocyte membrane polyphosphoinositide metabolism and the regulation of calcium binding. J Biol Chem. 1972 Nov 25;247(22):7218–7223. [PubMed] [Google Scholar]
- Burnstock G., Campbell G., Satchell D., Smythe A. Evidence that adenosine triphosphate or a related nucleotide is the transmitter substance released by non-adrenergic inhibitory nerves in the gut. Br J Pharmacol. 1970 Dec;40(4):668–688. doi: 10.1111/j.1476-5381.1970.tb10646.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotman C. W., Matthews D. A. Synaptic plasma membranes from rat brain synaptosomes: isolation and partial characterization. Biochim Biophys Acta. 1971 Dec 3;249(2):380–394. doi: 10.1016/0005-2736(71)90117-9. [DOI] [PubMed] [Google Scholar]
- DAWSON R. M., DITTMER J. C. Evidence for the structure of brain triphosphoinositide from hydrolytic degradation studies. Biochem J. 1961 Dec;81:540–545. doi: 10.1042/bj0810540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson R. M., Eichberg J. Diphosphoinositide and triphosphoinositide in animal tissues. Extraction, estimation and changes post mortem. Biochem J. 1965 Sep;96(3):634–643. doi: 10.1042/bj0960634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson R. M. Metabolism and function of polyphosphoinositides in nervous tissue. Ann N Y Acad Sci. 1969 Oct 17;165(2):774–783. [PubMed] [Google Scholar]
- Dawson R. M., Thompson W. The triphosphoinositide phosphomonoesterase of brain tissue. Biochem J. 1964 May;91(2):244–250. doi: 10.1042/bj0910244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichberg J., Dawson R. M. Polyphosphoinositides in myelin. Biochem J. 1965 Sep;96(3):644–650. doi: 10.1042/bj0960644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichberg J., Hauser G. The subcellular distribution of polyphosphoinositides in myelinated and unmyelinated rat brain. Biochim Biophys Acta. 1973 Nov 29;326(2):210–223. doi: 10.1016/0005-2760(73)90247-6. [DOI] [PubMed] [Google Scholar]
- Flatman P., Lew V. L. Does ionophore A23187 mediate Na transport in the absence of divalent cations? Nature. 1977 Dec 1;270(5636):444–445. doi: 10.1038/270444a0. [DOI] [PubMed] [Google Scholar]
- Flatman P., Lew V. L. Use of ionophore A23187 to measure and to control free and bound cytoplasmic Mg in intact red cells. Nature. 1977 May 26;267(5609):360–362. doi: 10.1038/267360a0. [DOI] [PubMed] [Google Scholar]
- GRAY E. G., WHITTAKER V. P. The isolation of nerve endings from brain: an electron-microscopic study of cell fragments derived by homogenization and centrifugation. J Anat. 1962 Jan;96:79–88. [PMC free article] [PubMed] [Google Scholar]
- Garrett N. E., Garrett R. J., Talwalkar R. T., Lester R. L. Rapid breakdown of diphosphoinositide and triphosphoinositide in erythrocyte membranes. J Cell Physiol. 1976 Jan;87(1):63–69. doi: 10.1002/jcp.1040870109. [DOI] [PubMed] [Google Scholar]
- Glynn I. M., Chappell J. B. A simple method for the preparation of 32-P-labelled adenosine triphosphate of high specific activity. Biochem J. 1964 Jan;90(1):147–149. doi: 10.1042/bj0900147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HENDRICKSON H. S., BALLOU C. E. ION EXCHANGE CHROMATOGRAPHY OF INTACT BRAIN PHOSPHOINOSITIDES ON DIETHYLAMINOETHYL CELLULOSE BY GRADIENT SALT ELUTION IN A MIXED SOLVENT SYSTEM. J Biol Chem. 1964 May;239:1369–1373. [PubMed] [Google Scholar]
- Harwood J. L., Hawthorne J. N. Metabolism of the phosphoinositides in guinea-pig brain synaptosomes. J Neurochem. 1969 Sep;16(9):1377–1387. doi: 10.1111/j.1471-4159.1969.tb05989.x. [DOI] [PubMed] [Google Scholar]
- Hawthorne J. N., White D. A. Myo-inositol lipids. Vitam Horm. 1975;33:529–573. doi: 10.1016/s0083-6729(08)60972-3. [DOI] [PubMed] [Google Scholar]
- Hendrickson H. S., Reinertsen J. L. Phosphoinositide interconversion: a model for control of Na + and K + permeability in the nerve axon membrane. Biochem Biophys Res Commun. 1971 Sep;44(5):1258–1264. doi: 10.1016/s0006-291x(71)80221-8. [DOI] [PubMed] [Google Scholar]
- Hokin L. E. Autoradiographic localization of the acetylcholine-stimulated synthesis of phosphatidylinositol in the superior cervical ganglion. Proc Natl Acad Sci U S A. 1965 Jun;53(6):1369–1376. doi: 10.1073/pnas.53.6.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurwitz L., Suria A. The link between agonist action and response in smooth muscle. Annu Rev Pharmacol. 1971;11:303–326. doi: 10.1146/annurev.pa.11.040171.001511. [DOI] [PubMed] [Google Scholar]
- Kai M., Hawthorne J. N. Physiological significance of polyphosphoinositides in brain. Ann N Y Acad Sci. 1969 Oct 17;165(2):761–773. [PubMed] [Google Scholar]
- Kai M., Salway J. G., Hawthorne J. N. The diphosphoinositide kinase of rat brain. Biochem J. 1968 Feb;106(4):791–801. doi: 10.1042/bj1060791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kai M., White G. L., Hawthorne J. N. The phosphatidylinositol kinase of rat brain. Biochem J. 1966 Nov;101(2):328–337. doi: 10.1042/bj1010328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lang V., Pryhitka G., Buckley J. T. Effect of neomycin and ionophore A23189 on ATP levels and turnover of polyphosphoinositides in human erythrocytes. Can J Biochem. 1977 Sep;55(9):1007–1012. doi: 10.1139/o77-150. [DOI] [PubMed] [Google Scholar]
- Lapetina E. G., Hawthorne J. N. The diglyceride kinase of rat cerebral cortex. Biochem J. 1971 Apr;122(2):171–179. doi: 10.1042/bj1220171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michell R. H. Inositol phospholipids and cell surface receptor function. Biochim Biophys Acta. 1975 Mar 25;415(1):81–47. doi: 10.1016/0304-4157(75)90017-9. [DOI] [PubMed] [Google Scholar]
- Palmer F. B., Rossiter R. J. A simple procedure for the study of inositol phosphatides in cat brain slices. Can J Biochem. 1965 Jun;43(6):671–683. doi: 10.1139/o65-078. [DOI] [PubMed] [Google Scholar]
- Pfeiffer D. R., Reed P. W., Lardy H. A. Ultraviolet and fluorescent spectral properties of the divalent cation ionophore A23187 and its metal ion complexes. Biochemistry. 1974 Sep 10;13(19):4007–4014. doi: 10.1021/bi00716a029. [DOI] [PubMed] [Google Scholar]
- Pickard M. R., Hawthorne J. N. The labelling of nerve ending phospholipids in guinea-pig brain in vivo and the effect of electrical stimulation on phosphatidylinositol metabolism in prelabelled synaptosomes. J Neurochem. 1978 Jan;30(1):145–155. doi: 10.1111/j.1471-4159.1978.tb07045.x. [DOI] [PubMed] [Google Scholar]
- Pumphrey A. M. Incorporation of [32P]orthophosphate into brain-slice phospholipids and their precursors. Effects of electrical stimulation. Biochem J. 1969 Mar;112(1):61–70. doi: 10.1042/bj1120061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed P. W., Lardy H. A. A23187: a divalent cation ionophore. J Biol Chem. 1972 Nov 10;247(21):6970–6977. [PubMed] [Google Scholar]
- Schacht J., Agranoff B. W. Effects of acetylcholine on labeling of phosphatidate and phosphoinositides by ( 32 P) orthophosphate in nerve ending fractions of guinea pig cortex. J Biol Chem. 1972 Feb 10;247(3):771–777. [PubMed] [Google Scholar]
- Sheltawy A., Dawson R. M. The metabolism of polyphosphoinositides in hen brain and sciatic nerve. Biochem J. 1969 Jan;111(2):157–165. doi: 10.1042/bj1110157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shephard E. H., Hübscher G. Phosphatidate biosynthesis in mitochondrial subfractions of rat liver. Biochem J. 1969 Jun;113(2):429–440. doi: 10.1042/bj1130429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson W., Dawson R. M. The triphosphoinositide phosphodiesterase of brain tissue. Biochem J. 1964 May;91(2):237–243. doi: 10.1042/bj0910237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tret'jak A. G., Limarenko I. M., Kossova G. V., Gulak P. V., Kozlov Y. P. Interrelation of phosphoinositide metabolism and ion transport in crab nerve fibres. J Neurochem. 1977 Jan;28(1):199–205. doi: 10.1111/j.1471-4159.1977.tb07727.x. [DOI] [PubMed] [Google Scholar]
- White G. L., Larrabee M. G. Phosphoinositides and other phospholipids in sympathetic ganglia and nerve trunks of rats. Effects of neuronal activity and inositol analogs ( - and -hexachlorocyclohexane (lindane)) on ( 32 P)-labelling, synaptic transmission and axonal conduction. J Neurochem. 1973 Mar;20(3):783–798. doi: 10.1111/j.1471-4159.1973.tb00039.x. [DOI] [PubMed] [Google Scholar]
- White G. L., Schellhase H. U., Hawthorne J. N. Phosphoinositide metabolism in rat superior cervical ganglion, vagus and phrenic nerve: effects of electrical stimulation and various blocking agents. J Neurochem. 1974 Jan;22(1):149–158. doi: 10.1111/j.1471-4159.1974.tb12191.x. [DOI] [PubMed] [Google Scholar]
- Whittaker V. P., Michaelson I. A., Kirkland R. J. The separation of synaptic vesicles from nerve-ending particles ('synaptosomes'). Biochem J. 1964 Feb;90(2):293–303. doi: 10.1042/bj0900293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagihara Y., Bleasdale J. E., Hawthorne J. N. Effects of acetylcholine on the incorporation of (32P)orthophosphate in vitro into the phospholipids of subsynaptosomal, membranes from guinea-pig brain. J Neurochem. 1973 Jul;21(1):173–190. doi: 10.1111/j.1471-4159.1973.tb04237.x. [DOI] [PubMed] [Google Scholar]
- Yagihara Y., Hawthrone J. N. Effects of acetylcholine on the incorporation of ( 32 P) orthophosphate in vitro into the phospholipids of nerve-ending particles from guinea pig brain. J Neurochem. 1972 Feb;19(2):355–367. doi: 10.1111/j.1471-4159.1972.tb01345.x. [DOI] [PubMed] [Google Scholar]


