Abstract
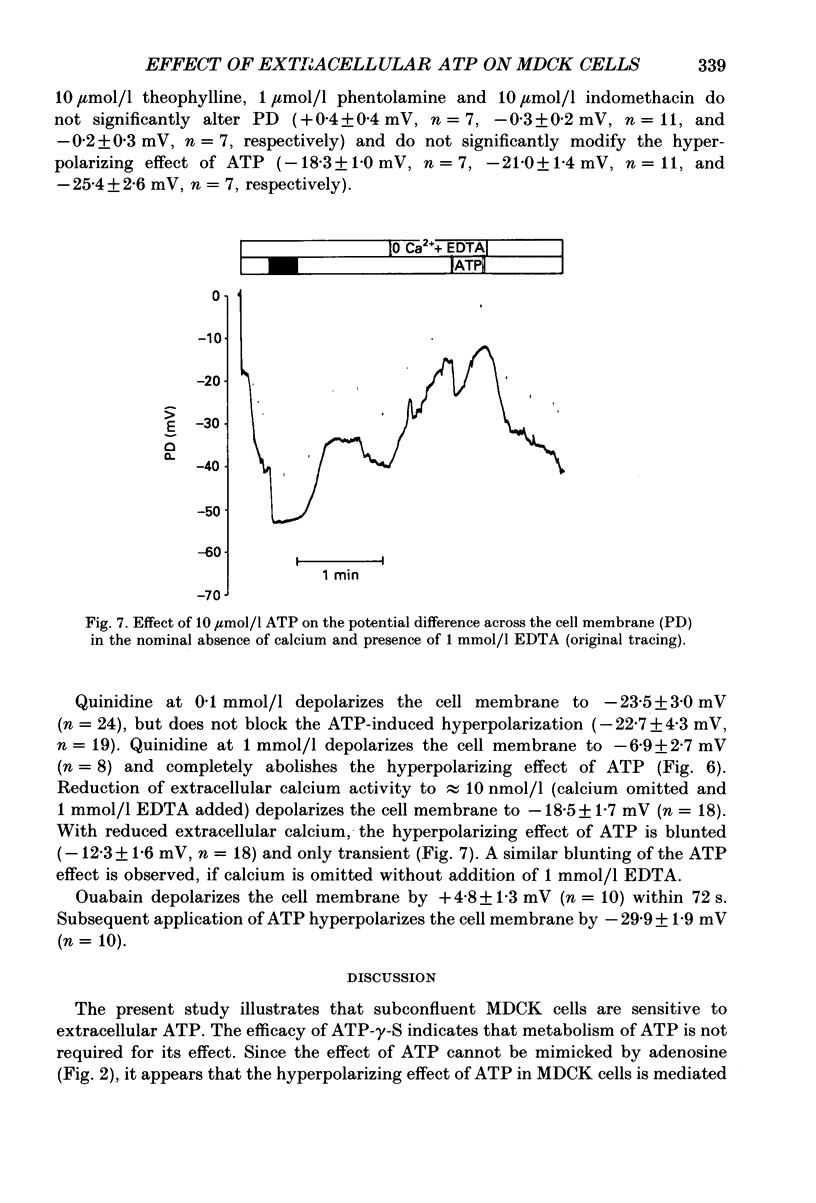
1. The present study has been performed to test for an influence of extracellular ATP on the potential differences across the cell membrane (PD) in subconfluent MDCK cells utilizing conventional microelectrodes. 2. In the absence of ATP, the mean measured PD was -47.5 +/- 0.3 mV (+/- S.E.M., n = 320). Application of 10 mumol/l ATP leads to rapid (less than 2 s) hyperpolarization of the cell membrane by -18.5 +/- 0.4 mV (n = 221), reduction of input resistance by 14 +/- 1 M omega (n = 106) and increase of the sensitivity of PD to alterations of extracellular potassium. 3. The concentration needed for half-maximal effect (K1/2) of ATP is approximately 0.5 mumol/l. ATP-gamma-S (K1/2 approximately 0.4 mumol/l) aand ADP (K1/2 approximately 0.9 similarly effective, whereas up to 1 mmol/l AMP or adenosine does not significantly alter PD. Application of 10 mumol/l theophylline, 1 mumol/l phentolamine and 10 mumol/l indomethacin does not blunt the hyperpolarizing effect of ATP. 4. The ATP-induced hyperpolarization is completely abolished in the presence of 1 mmol/l quinidine but only incompletely by 0.1 mmol/l quinidine or 1 mmol/l barium. In calcium-free extracellular fluid (1 mmol/l EDTA added) PD is 18.5 +/- 1.7 mV (n = 18). With reduced extracellular calcium, the hyperpolarizing effect of ATP is blunted (-12.3 +/- 1.6 mV, n = 18) and only transient. 5. In conclusion, ATP hyperpolarizes MDCK cells by increasing the potassium conductance. The activation of potassium channels requires calcium.
Full text
PDF

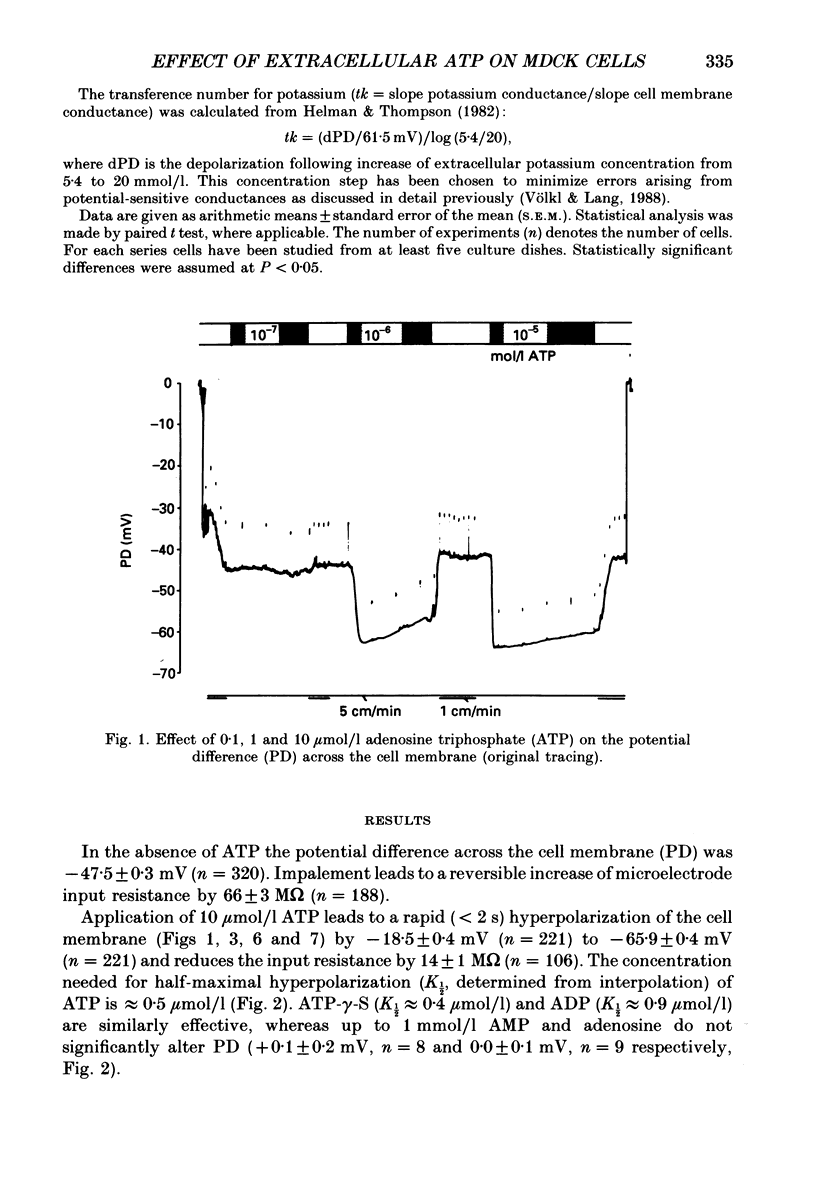
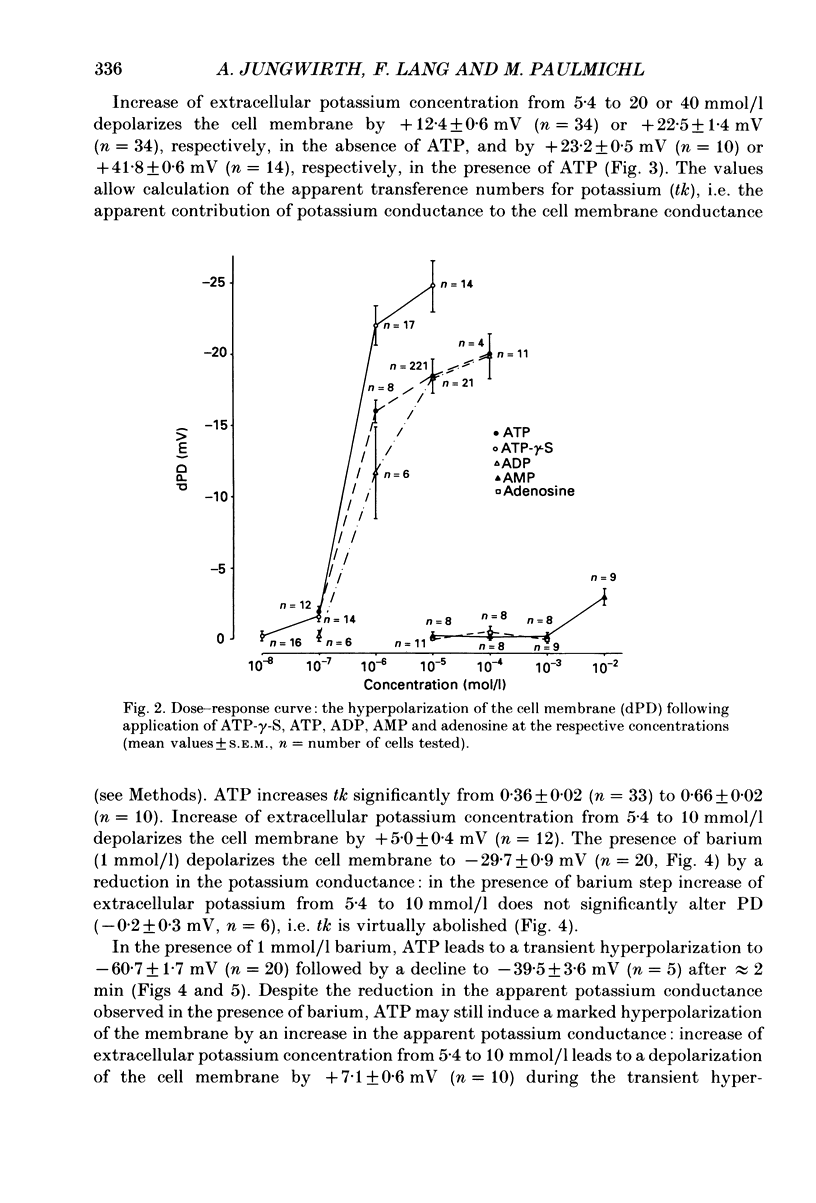
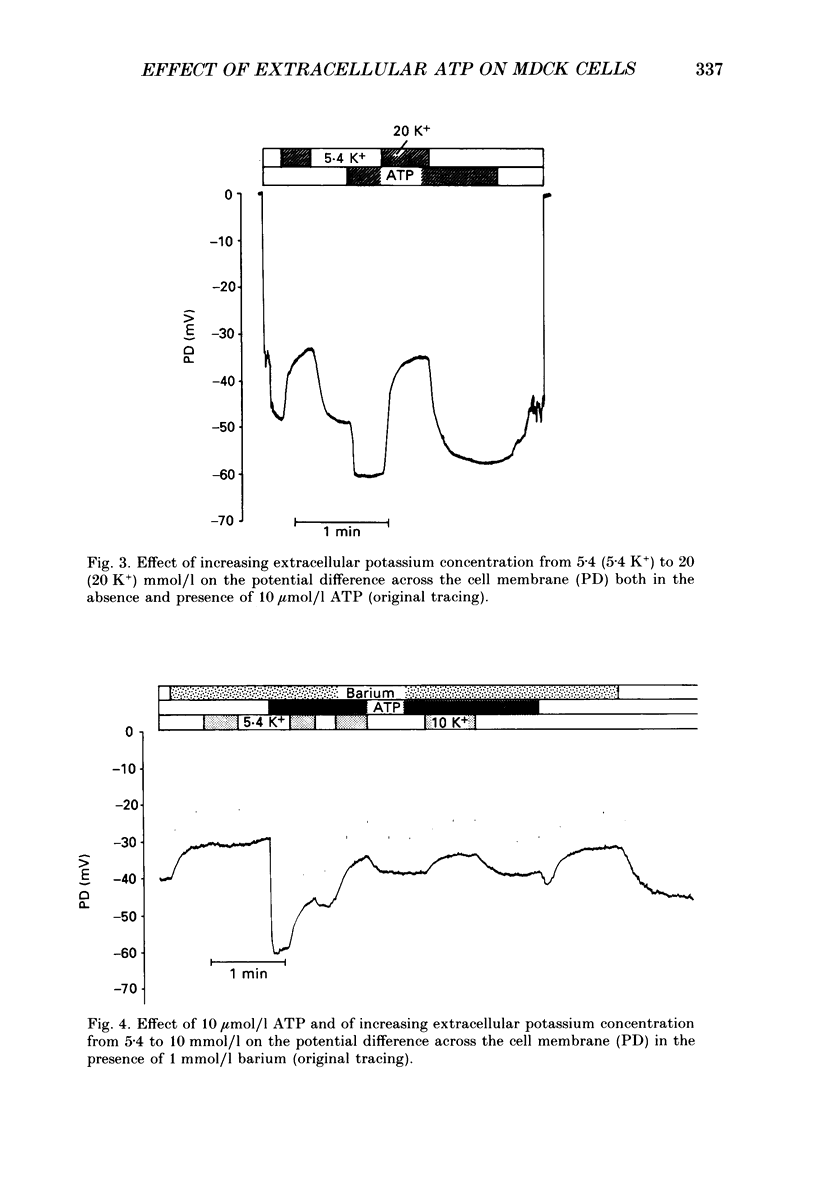
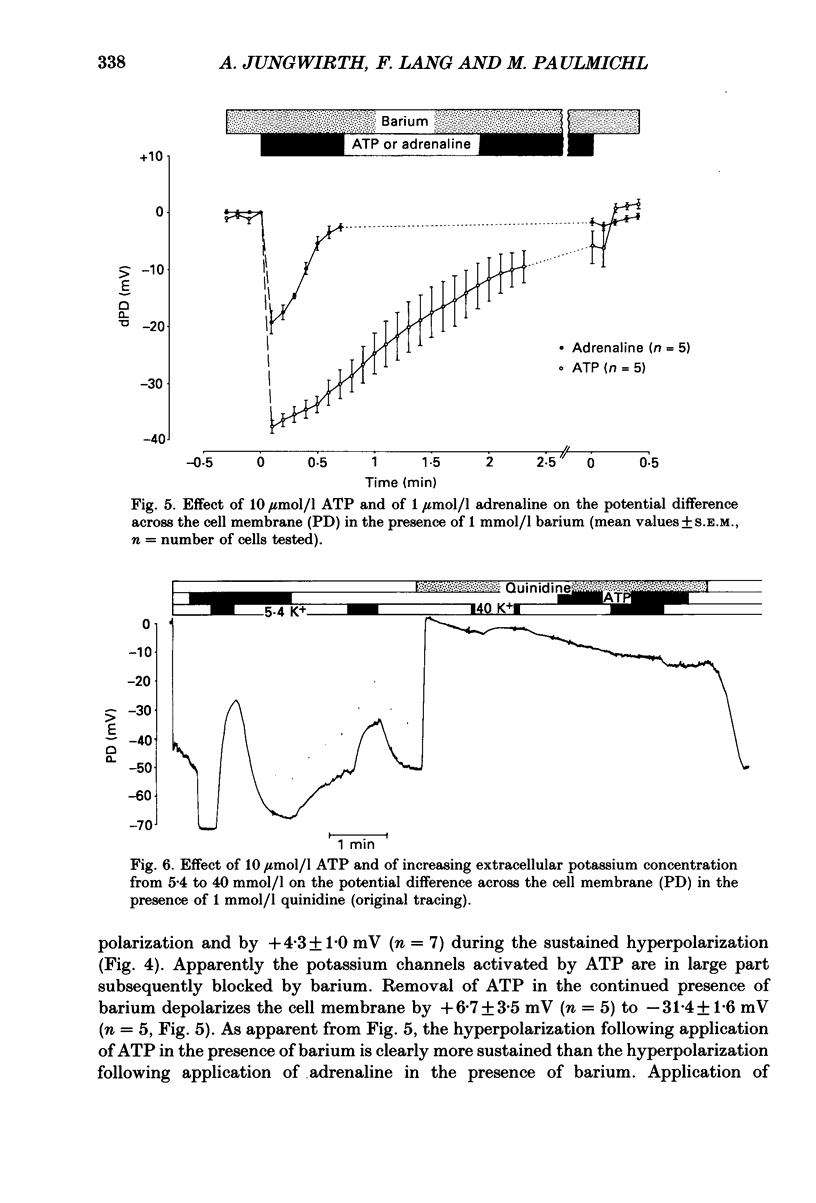




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aiton J. F., Brown C. D., Ogden P., Simmons N. L. K+ transport in "tight' epithelial monolayers of MDCK cells. J Membr Biol. 1982;65(1-2):99–109. doi: 10.1007/BF01870473. [DOI] [PubMed] [Google Scholar]
- Aiton J. F., Chipperfield A. R., Lamb J. F., Ogden P., Simmons N. L. Occurrence of passive furosemide-sensitive transmembrane potassium transport in cultured cells. Biochim Biophys Acta. 1981 Sep 7;646(3):389–398. doi: 10.1016/0005-2736(81)90307-2. [DOI] [PubMed] [Google Scholar]
- Arkhammar P., Nilsson T., Rorsman P., Berggren P. O. Inhibition of ATP-regulated K+ channels precedes depolarization-induced increase in cytoplasmic free Ca2+ concentration in pancreatic beta-cells. J Biol Chem. 1987 Apr 25;262(12):5448–5454. [PubMed] [Google Scholar]
- Benham C. D., Tsien R. W. A novel receptor-operated Ca2+-permeable channel activated by ATP in smooth muscle. Nature. 1987 Jul 16;328(6127):275–278. doi: 10.1038/328275a0. [DOI] [PubMed] [Google Scholar]
- Brown C. D., Simmons N. L. K+ transport in 'tight' epithelial monolayers of MDCK cells. Evidence for a calcium-activated K+ channel. Biochim Biophys Acta. 1982 Aug 25;690(1):95–105. doi: 10.1016/0005-2736(82)90243-7. [DOI] [PubMed] [Google Scholar]
- Burnstock G. Purinergic receptors. J Theor Biol. 1976 Oct 21;62(2):491–503. doi: 10.1016/0022-5193(76)90133-8. [DOI] [PubMed] [Google Scholar]
- Burnstock G. Review lecture. Neurotransmitters and trophic factors in the autonomic nervous system. J Physiol. 1981;313:1–35. doi: 10.1113/jphysiol.1981.sp013648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cereijido M., Ehrenfeld J., Meza I., Martínez-Palomo A. Structural and functional membrane polarity in cultured monolayers of MDCK cells. J Membr Biol. 1980;52(2):147–159. doi: 10.1007/BF01869120. [DOI] [PubMed] [Google Scholar]
- Crooke M. J., Allan E., Pattinson N., Sneyd J. G. Formation of cyclic AMP from exogenous ATP by isolated hepatocytes and adipocytes. Biochim Biophys Acta. 1980 Aug 1;631(1):28–39. doi: 10.1016/0304-4165(80)90050-1. [DOI] [PubMed] [Google Scholar]
- Drury A. N., Szent-Györgyi A. The physiological activity of adenine compounds with especial reference to their action upon the mammalian heart. J Physiol. 1929 Nov 25;68(3):213–237. doi: 10.1113/jphysiol.1929.sp002608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Findlay I., Dunne M. J., Petersen O. H. ATP-sensitive inward rectifier and voltage- and calcium-activated K+ channels in cultured pancreatic islet cells. J Membr Biol. 1985;88(2):165–172. doi: 10.1007/BF01868430. [DOI] [PubMed] [Google Scholar]
- Findlay I. The effects of magnesium upon adenosine triphosphate-sensitive potassium channels in a rat insulin-secreting cell line. J Physiol. 1987 Oct;391:611–629. doi: 10.1113/jphysiol.1987.sp016759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallacher D. V. Are there purinergic receptors on parotid acinar cells? Nature. 1982 Mar 4;296(5852):83–86. doi: 10.1038/296083a0. [DOI] [PubMed] [Google Scholar]
- Gordon J. L. Extracellular ATP: effects, sources and fate. Biochem J. 1986 Jan 15;233(2):309–319. doi: 10.1042/bj2330309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helman S. I., Thompson S. M. Interpretation and use of electrical equivalent circuits in studies of epithelial tissues. Am J Physiol. 1982 Dec;243(6):F519–F531. doi: 10.1152/ajprenal.1982.243.6.F519. [DOI] [PubMed] [Google Scholar]
- Horie M., Irisawa H., Noma A. Voltage-dependent magnesium block of adenosine-triphosphate-sensitive potassium channel in guinea-pig ventricular cells. J Physiol. 1987 Jun;387:251–272. doi: 10.1113/jphysiol.1987.sp016572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohn P. G., Newey H., Smyth D. H. The effect of adenosine triphosphate on the transmural potential in rat small intestine. J Physiol. 1970 May;208(1):203–220. doi: 10.1113/jphysiol.1970.sp009114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolb H. A., Brown C. D., Murer H. Identification of a voltage-dependent anion channel in the apical membrane of a Cl(-)-secretory epithelium (MDCK). Pflugers Arch. 1985 Mar;403(3):262–265. doi: 10.1007/BF00583597. [DOI] [PubMed] [Google Scholar]
- Kolb H. A., Wakelam M. J. Transmitter-like action of ATP on patched membranes of cultured myoblasts and myotubes. Nature. 1983 Jun 16;303(5918):621–623. doi: 10.1038/303621a0. [DOI] [PubMed] [Google Scholar]
- Korman L. Y., Lemp G. F., Jackson M. J., Gardner J. D. Mechanism of action of ATP on intestinal epithelial cells. Cyclic AMP-mediated stimulation of active ion transport. Biochim Biophys Acta. 1982 Sep 13;721(1):47–54. doi: 10.1016/0167-4889(82)90022-2. [DOI] [PubMed] [Google Scholar]
- Lang F., Defregger M., Paulmichl M. Apparent chloride conductance of subconfluent Madin Darby canine kidney cells. Pflugers Arch. 1986 Aug;407(2):158–162. doi: 10.1007/BF00580668. [DOI] [PubMed] [Google Scholar]
- Lang F., Messner G., Rehwald W. Electrophysiology of sodium-coupled transport in proximal renal tubules. Am J Physiol. 1986 Jun;250(6 Pt 2):F953–F962. doi: 10.1152/ajprenal.1986.250.6.F953. [DOI] [PubMed] [Google Scholar]
- MADIN S. H., DARBY N. B., Jr Established kidney cell lines of normal adult bovine and ovine origin. Proc Soc Exp Biol Med. 1958 Jul;98(3):574–576. doi: 10.3181/00379727-98-24111. [DOI] [PubMed] [Google Scholar]
- McMillian M. K., Soltoff S. P., Cantley L. C., Talamo B. R. Extracellular ATP elevates intracellular free calcium in rat parotid acinar cells. Biochem Biophys Res Commun. 1987 Dec 16;149(2):523–530. doi: 10.1016/0006-291x(87)90399-8. [DOI] [PubMed] [Google Scholar]
- Noma A. ATP-regulated K+ channels in cardiac muscle. Nature. 1983 Sep 8;305(5930):147–148. doi: 10.1038/305147a0. [DOI] [PubMed] [Google Scholar]
- Okada Y., Yada T., Ohno-Shosaku T., Oiki S., Ueda S., Machida K. Exogenous ATP induces electrical membrane responses in fibroblasts. Exp Cell Res. 1984 Jun;152(2):552–557. doi: 10.1016/0014-4827(84)90657-8. [DOI] [PubMed] [Google Scholar]
- Paulmichl M., Defregger M., Lang F. Effects of epinephrine on electrical properties of Madin-Darby canine kidney cells. Pflugers Arch. 1986 Apr;406(4):367–371. doi: 10.1007/BF00590938. [DOI] [PubMed] [Google Scholar]
- Paulmichl M., Friedrich F., Lang F. Effects of bradykinin on electrical properties of Madin-Darby canine kidney epithelioid cells. Pflugers Arch. 1987 Apr;408(4):408–413. doi: 10.1007/BF00581137. [DOI] [PubMed] [Google Scholar]
- Paulmichl M., Friedrich F., Lang F. Electrical properties of Madin-Darby-canine-kidney cells. Effects of extracellular sodium and calcium. Pflugers Arch. 1986 Sep;407(3):258–263. doi: 10.1007/BF00585300. [DOI] [PubMed] [Google Scholar]
- Paulmichl M., Gstraunthaler G., Lang F. Electrical properties of Madin-Darby canine kidney cells. Effects of extracellular potassium and bicarbonate. Pflugers Arch. 1985 Sep;405(2):102–107. doi: 10.1007/BF00584529. [DOI] [PubMed] [Google Scholar]
- Rindler M. J., Saier M. H., Jr Evidence for Na+/H+ antiport in cultured dog kidney cells (MDCK). J Biol Chem. 1981 Nov 10;256(21):10820–10825. [PubMed] [Google Scholar]
- Rindler M. J., Taub M., Saier M. H., Jr Uptake of 22Na+ by cultured dog kidney cells (MDCK). J Biol Chem. 1979 Nov 25;254(22):11431–11439. [PubMed] [Google Scholar]
- Romero P. J. Is the Ca2+-sensitive K+ channel under metabolic control in human red cells? Biochim Biophys Acta. 1978 Feb 2;507(1):178–181. doi: 10.1016/0005-2736(78)90385-1. [DOI] [PubMed] [Google Scholar]
- Simmons N. L. Cultured monolayers of MDCK cells: a novel model system for the study of epithelial development and function. Gen Pharmacol. 1982;13(4):287–291. doi: 10.1016/0306-3623(82)90047-7. [DOI] [PubMed] [Google Scholar]
- Simmons N. L. Identification of a purine (P2) receptor linked to ion transport in a cultured renal (MDCK) epithelium. Br J Pharmacol. 1981 Jun;73(2):379–384. doi: 10.1111/j.1476-5381.1981.tb10432.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons N. L. Stimulation of Cl- secretion by exogenous ATP in cultured MDCK epithelial monolayers. Biochim Biophys Acta. 1981 Aug 20;646(2):231–242. doi: 10.1016/0005-2736(81)90329-1. [DOI] [PubMed] [Google Scholar]
- Sturgess N. C., Hales C. N., Ashford M. L. Calcium and ATP regulate the activity of a non-selective cation channel in a rat insulinoma cell line. Pflugers Arch. 1987 Aug;409(6):607–615. doi: 10.1007/BF00584661. [DOI] [PubMed] [Google Scholar]
- Trube G., Hescheler J. Inward-rectifying channels in isolated patches of the heart cell membrane: ATP-dependence and comparison with cell-attached patches. Pflugers Arch. 1984 Jun;401(2):178–184. doi: 10.1007/BF00583879. [DOI] [PubMed] [Google Scholar]
- Völkl H., Lang F. Electrophysiology of cell volume regulation in proximal tubules of the mouse kidney. Pflugers Arch. 1988 May;411(5):514–519. doi: 10.1007/BF00582372. [DOI] [PubMed] [Google Scholar]
- Westcott K. R., Engelhard V. H., Storm D. R. Production of cyclic AMP from extracellular ATP by intact LM cells. Biochim Biophys Acta. 1979 Feb 19;583(1):47–54. doi: 10.1016/0304-4165(79)90308-8. [DOI] [PubMed] [Google Scholar]


