Abstract
Immunotherapy with checkpoint inhibitors has become the cornerstone of systemic treatment for non-oncogene addicted non-small-cell lung cancer. Despite its pivotal role, a significant proportion of patients—approximately 70–85%—either exhibit primary resistance to PD-1 blockade or develop acquired resistance following an initial benefit, even in combination with chemotherapy and/or anti-CTLA-4 agents. The phenomenon of primary and acquired resistance to immunotherapy represents a critical clinical challenge, largely based on our incomplete understanding of the mechanisms of action of immunotherapy, and the resulting lack of accurate predictive biomarkers. Here, we review the definitions and explore the proposed mechanisms of primary and acquired resistance, including those related to the tumor microenvironment, systemic factors, and intrinsic tumor characteristics. We also discuss translational data on adaptive changes within tumor cells and the immune infiltrate following exposure to checkpoint inhibitors. Lastly, we offer a comprehensive overview of current and emerging therapeutic strategies designed to prevent primary resistance and counteract acquired resistance.
Key Points
| Only a minority of patients derives long-term benefit from immune checkpoint inhibitors (ICI), highlighting our incomplete understanding of the biological mechanisms of anti-tumor immune responses. |
| Primary resistance could be the result of inadequate patient selection. Current modalities and timing of ICI-based combination should be reconsidered, to induce a responsive, inflamed tumor microenvironment. |
| Acquired resistance implies adaptive changes in the cancer–immune system crosstalk. For a deeper insight of these changes and the development of tailored treatment strategies, dedicated translational research using longitudinal samples is essential. |
Clinical Context and Definitions of Resistance to Immunotherapy
Immunotherapy with immune checkpoint inhibitors (ICI) has transformed the care and clinical course of numerous cancer types, including non-small-cell lung cancer (NSCLC). To date, treatment with monoclonal antibodies blocking the interaction of the co-inhibitory T-cell receptor PD-1 (programmed cell death 1) with its main ligand PD-L1 represents the backbone of systemic treatment for non-oncogene addicted NSCLC at either advanced and, more recently, earlier stages of the disease [1]. In the advanced-stage setting, PD-1 blockade is delivered on its own or in association with chemotherapy and/or anti-cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) depending on the tumor proportion score of PD-L1, the only recognized predictive factor of response to ICI in NSCLC [1].
The breakthrough of ICI compared with traditional cytotoxic therapies, is the capacity to provide unprecedented durable responses that translate into survival benefit. However, long-term responders represent only a small fraction. In patients with PD-L1 positive (> 1%) tumors treated with pembrolizumab alone, the 5-year overall survival (OS) rate is 15–32%, depending on the PD-L1 tumor proportion score [2, 3]. Similarly, in patients receiving nivolumab and ipilimumab 5-year OS rates were 15% and 20% in patients with PD-L1 negative and positive disease, respectively [4]. When chemotherapy is combined with PD-1 blockade, the proportion of patients alive at 5 years was the 19.4% and the 18.4% in non-squamous and squamous histology respectively [5, 6], thus suggesting that the association of chemotherapy does not provide an additional benefit in terms of durability of response. Of note, in all of the above trials, the proportion of long-survivors increases to above 80% in patients that completed 35 cycles of treatment, thus including responders in the absence of moderate/severe toxicity.
However, the remaining 70–85% of the patients—or possibly more in the real-life scenario—are refractory to immunotherapy with PD-1 blockade or eventually develop acquired resistance after initial benefit, despite the association of chemotherapy and/or anti-CTLA-4 agents. In a retrospective cohort of over 1000 patients, acquired resistance was observed in 61% of the cases, and in half of these (52%), disease progression occurred within 1 year [7]. Considering the risk of immune-related toxicity and the financial burden of immunotherapy, primary and (early) acquired resistance represents a relevant clinical issue and largely reflects our incomplete understanding of the mechanism of action of ICIs.
So far, many criteria for primary and acquired resistance have been proposed [8–12], with the largest consensus being provided by the Society for Immunotherapy of Cancer (SITC), distinguishing three distinct resistance scenarios with PD-1 pathway blockade: primary resistance, secondary resistance, and progression after treatment discontinuation for any reason [12]. More recently, separate criteria have been published by the SITC with regard to resistance to PD-1 pathway blockade in the context of combination therapy with other ICIs and chemotherapy [13, 14]. Irrespective to monotherapy or combination therapy, according to the criteria described in Table 1, primary resistance is considered in cases of disease progression occurring within the first 6 months from the beginning of IC treatment, while secondary resistance indicates a disease progression developing after at least 6 months of ICI, following an initial benefit. In this regard, it is controversial whether to consider stable disease as prior clinical benefit before developing acquired resistance, as in the SITC criteria, owing to the large spectrum of response patterns that fall within the RECIST criteria for stable disease [11]. Indeed, another panel of experts, specifically in the setting of NSCLC, have defined acquired resistance as the occurrence of disease progression after any objective response (excluding stable disease), irrespective of the duration of treatment exposure [11].
Table 1.
SITC criteria for primary and acquired resistance to PD-1 pathway blockade in NSCLC, alone or in combination with other ICI (anti-CTLA-4) or chemotherapy
| Primary resistance | Acquired resistance | |
|---|---|---|
| Immunotherapy alonea | 6 weeks ≤ PD < 6 months | PD ≥ 6 months after PR/CR/SD |
| Double immunotherapya |
PD ≥ two cycles of both drugs but < 6 months PD ≤ 12 weeks after last doseb |
PD > 6 months after PR/CR/SD ≥ 3 months PD > 12 weeks after last doseb |
| Chemo-immunotherapyd | 6–8 weeksc ≤ PD ≤ 6 months | PD > 6 months regardless of best response |
CR, complete response; SD, stable disease; PD, progressive disease; PR, partial response
aConfirmatory scan required at least 4 weeks after PD, if clinically feasible
bAfter halting therapy in the metastatic disease setting
cFor rapidly progressing disease, any exposure is adequate
dNo confirmatory scan required.
Defining resistance after ICI discontinuation, as in cases of immune-related adverse events or completion of a planned treatment regimen, takes into account the time of PD-1 receptor binding to the corresponding monoclonal antibodies, which declines after 2–3 months [15]. Therefore, according to the SITC, if disease progression occurs within 12 weeks after the administration of the last dose, this is considered as primary resistance, whereas in cases of disease progression after 12 weeks, this is considered an acquired phenomenon [8, 11, 12].
As chemo-immunotherapy indication has been recently extended to the perioperative setting, it will also be important to define resistance criteria after neoadjuvant or adjuvant treatment. Even though these criteria are not yet available, both the SITC consensus and the panel in Schoenfeld at al. agree that the extent of pathological response after neoadjuvant therapy, and longitudinal biomarkers—such as circulating cell-free tumor DNA or other immunological factors—upon neoadjuvant or adjuvant treatment are promising and would need dedicated prospective studies [11, 14].
Mechanisms of Resistance to Immunotherapy Depending on the Anti-tumor Immunity
Primary Resistance
From a biological standpoint, primary resistance is meant as an a priori inability of the host immune system to mount an effective anti-tumor response upon ICI [8, 9]. This can occur due to a disruption in the sequence of events regulating a successful anti-tumor immune response, including: T-cell priming through optimal tumor antigen presentation, the differentiation of long-term effector CD8+ T cells from stem-like progenitor antigen-specific cells, the expansion and migration of these and other effector cells, their accumulation at cancer sites, and effective tumor cell killing [9, 16]. To identify and develop strategies to prevent or overcome resistance mechanisms, a deeper understanding of the factors at play in the tumor microenvironment is essential.
T-cell Phenotypes and Interaction with Dendritic Cells
Immunotherapy with PD-1 blockade operates by amplifying an existing immune response, mediating a proliferative burst of exhausted CD8+ T cells, which develop upon persistent antigen-stimulation, as in the case of chronic infection and cancer [17, 18]. Exhausted CD8+ T cells are long-lived CD8+ T cells with reduced effector functions (capacity to proliferate, produce cytotoxic cytokines, and direct cytotoxicity). A hallmark of CD8+ T-cell exhaustion is the overexpression of PD-1, a negative regulator of T-cell receptor signaling, and of other markers such as TIM-3, CTLA-4, LAG-3, TIGIT, and CD39. Tumor infiltration by exhausted CD8+ T cells has long been regarded as a constraint for anti-tumor immunity and response to PD-1 blockade [9]. However, it has been recently clarified that CD8+ T-cell exhaustion is not a single state, but rather a progressive process comprising different functional states, recapitulating their development and behavior upon PD-1 based immunotherapies [18, 19]. Among those, the self-renewing progenitor subset of TCF-1+ PD-1+ CD8+ T cells is responsible for the effector response generated by PD-1/PD-L1 blockade [17, 19, 20]. When stimulated by PD-1/PD-L1 blocking agents, these progenitors of exhausted cells increase their differentiation and self-renewal, providing a proliferative burst of effector CD8+ T cells with high migratory and cytotoxic functions [17, 19]. In humans, accumulating evidence is confirming the association between the density of TCF-1+ PD-1+ CD8+ T cells in the tumor microenvironment and favorable outcomes to immunotherapy across many different tumor types, including NSCLC [21–24]. Therefore, in their absence, the CD8+ T-cell response to PD-1 pathway blockade cannot be elicited, configurating a scenario of primary resistance. The reason why certain tumors are infiltrated by stem-like TCF-1+ PD-1+ CD8+ T cells while others are not is unknown, even though preclinical/translational evidence indicate that their expansion and differentiation relies on co-stimulation, where dendritic cells play a key role [25–29].
Dendritic cells are professional antigen-presenting cells essential for optimal anti-tumor T-cell priming and activation in the tumor-draining lymph nodes, and to sustain the differentiation of effector CD8+ T cells at tumor sites [27, 30]. The aberrant production of many soluble factors in the tumor microenvironment can suppress intratumor dendritic cell responses, and thereby, CD8+ T-cell activation and effector differentiation [30]. Among these factors, IL-6, prostaglandin E2, and granulocyte colony stimulating factor were found to block the development of the dendritic cell precursors, while IL-10, vascular endothelial growth factor (VEGF), transforming growth factor beta (TGF-b), IL-6, and IL-4 inhibited the maturation of conventional dendritic cells [30]. Furthermore, other immune cells, like CD4+ T regulatory cells have also been shown to interact with, and potentially inhibit, type 1 conventional dendritic cells in the tumor-draining lymph nodes [31] and intratumor CCR7+ dendritic cells [21]. On this matter, in a recent translational work [21], multiplex RNA-based imaging, protein-based imaging, and spatial transcriptomics were used to investigate the intratumor immune infiltrate in pre-immunotherapy tissue from patients with NSCLC. Here, patients reporting an objective response to later immunotherapy presented “stem-immunity hubs” in their tumors, which were characterized by aggregates of the above-mentioned stem-like TCF-1+ PD-1+ CD8+ T cells and macrophages with an interferon-signature, and other aggregates of CCR7+ dendritic cells interacting with regulatory CD4+ T cells. These hubs resemble tertiary lymphoid structures, which are ectopic lymphoid structures that develop in chronically inflamed tissue and have also been associated with immunotherapy responses [32, 33]. However, they are distinct, owing to the lack of B-cell follicles and because they are characterized by a CXCR3-related interferon-gamma (IFN-γ) response [21].
Soluble Signals in the Tumor Microenvironment
To generate a successful immune reinvigoration after PD-1-directed immunotherapy a permissive tumor immune microenvironment characterized by a baseline inflammatory IFN-γ signature is needed [16, 21]. IFN-γ is produced primarily by natural killer cells, T cells, dendritic cells, and macrophages, and plays a critical role in the antitumor immune response by promoting the recruitment, activation, maturation, and effector function of immune cells, as well as promoting antigen presentation and apoptosis in tumors cells [34]. The binding of IFN-γ to its heterodimer receptor IFN-γR1/IFN-γR2 triggers the activation of intracellular Janus-activated kinases (JAK1 and JAK2). This leads to the phosphorylation of the intracellular domains of IFN-γR1/IFN-γR2 that can then interact with the signal transducer and activator of transcription factors (STAT), that ultimately regulates the transcription and expression of various IFN-γ-related genes, including PD-L1 [35, 36]. By interacting with PD-1+ T cells, this phenomenon inhibits antitumor T-cell responses but may also render these cells susceptible to anti-PD1/-L1 blockade.
Despite the limitations related to its spatial and temporal heterogeneity, PD-L1 expression in the tumor microenvironment is also a surrogate marker to estimate the level of inflammation at the tumor site [16, 37–39]. PD-L1 can be innately expressed on tumor cells (discussed below), especially in the surrounding healthy tissue, and also be induced by IFN-γ exposure as an adaptive protective mechanism to preserve tissue integrity upon chronic inflammation [16, 39]. In the exploratory translational analysis from the phase I atezolizumab trial, its combined expression on tumor cells and in the tumor microenvironment was a better predictor of response, compared with the sole expression on tumor cells [38]. However, it still needs to be elucidated if this is also true for anti-PD-1 agents.
An important factor in primary resistance to immunotherapy is the presence of soluble and metabolic factors that make the tumor “immune desert/excluded”; hindering T-cell infiltration and their anti-tumor functions [16]. Among the soluble factors, TGF-b is regarded as one of the most potent immunosuppressive cytokines which are of vital importance to the maintenance of immune homeostasis and self-tolerance [40, 41]. This pleiotropic cytokine can be broadly produced by tumors cells, immune cells (especially macrophages), and stromal cells. In the tumor microenvironment TGF-b is a master regulator, with broad immunosuppressive effects on CD8+ T cells, blocking their infiltration and effector differentiation; on CD4+ T cells, where it drives the differentiation into T regulatory cells and Th17 cells; and on tumor cells themselves, where TGF-b is deeply involved in epithelial-to-mesenchymal transition [41]. In the development of immune excluded/desert tumors, TGF-b, along with mechanical forces, mediates the differentiation of fibroblasts in cancer-associated myofibroblasts that are responsible for the dense network of matrix fibers organized circumferentially around immune excluded tumors [16, 42]. This TGF-b-dependent fibrosis has been associated with poor response to immunotherapy [43, 44]. In myeloid cells, beyond the inhibition of dendritic cell maturation mentioned above, TGF-b contributes to the polarization of macrophages and neutrophils in acquiring a tumor-promoting phenotype, the so-called myeloid-derived suppressor cells [40, 41].
Macrophage Polarity
With the limitations related to the heterogeneous nomenclature and the type of characterization, myeloid-derived suppressor cells usually comprise a monocyte-derived lineage, consisting of M2 tumor-associated macrophages and a polymorphonuclear-derived lineage, consisting of tumor-promoting neutrophils [45]. M2 macrophages (CD163+/CD206+/CD204+) infiltrate the tumor microenvironment at later disease stages, as they are thought to differentiate from M1 macrophages (CD68+), which instead dominate the early stages of solid tumor development [45]. As highly plastic cells, M1 and M2 macrophages represent the extremities of the same spectrum, where M1 macrophages present a pro-inflammatory signature (CXCL9 and CXCL10) and are able to produce nitric oxide and TNF alfa, whereas, upon chronic inflammation, M2 macrophages produce the anti-inflammatory cytokines IL-10 and IL-34, and angiogenic factors, such as vascular endothelial growth factor (VEGF), ultimately contributing to the formation of a hyper-dense vascular network that promotes tumor cell growth [45, 46]. M2 macrophages have been found to have a negative prognostic role in NSCLC [47], and their tumor density (CD163+ macrophages) was associated with worse outcomes after immunotherapy in a retrospective analysis on pre-immunotherapy tissue from 152 NSCLC cases [48]. However, using transcriptional signatures, this study and others, highlighted how the M1/M2 classification can be too simplistic to describe a continuum of biological processes. Recently, the polarity of macrophages was redefined based on CXCL9 (pro-inflammatory) or SPP1 (tissue repair gene) expression, with prognostic and potential immunotherapy predictive value [49]. Macrophages have also been proposed to play a role in hyperprogressive disease, a rare form of primary resistance to immunotherapy, characterized by an accelerated progression and clinical deterioration early after ICI [50]. Baseline NSCLC biopsies of patients developing hyperprogressive disease were enriched in CD163+ PD-L1+ M2 macrophages in the study by LoRusso et al. [51]. With the limitation of using immune-deficient mice, the proposed mechanism relies on dysfunctional macrophage activation that favors tumor growth upon interaction between the Fc receptor on macrophages and the Fc fraction of the therapeutic anti-PD-1 antibody [51]. Similarly, FGF2, an M2 marker was found to up upregulated in melanoma and NSCLC tumors in patients who developed disease hyperprogression [52, 53].
Neutrophils
While the translational evidence for the role of macrophages influencing response to immunotherapy is limited, a consistent body of literature has explored the contribution of neutrophils, mostly in the blood [54–56]. Indeed, as macrophages are not present in the blood, their investigation is limited by tissue availability, notoriously scarce in NSCLC clinical practice, whereas neutrophils are abundant in NSCLC tissue and blood. A high neutrophil tumor content, especially when associated with low CD8+ T-cell infiltration has shown negative predictive value in a small cohort of patients with NSCLC treated with immunotherapy [57].
In a large retrospective evaluation of patients with NSCLC, renal cell carcinoma, and melanoma, treated with immunotherapy with anti-PD1 ± anti-CTLA-4 within clinical trials (n = 1344), elevated baseline serum IL-8 was associated with high neutrophil intratumor infiltration and a worse prognosis after immunotherapy [58]. IL-8 is a strong chemotactic agent for neutrophils, and contributes to neo-angiogenesis and epithelial–mesenchymal transition [58].
In the blood, other studies have shown that high neutrophil content, and in particular high neutrophil-to-lymphocyte ratio (NLR), is negatively associated with response to immunotherapy in patients with NSCLC [54, 55]. Even in cases of high PD-L1 expression (> 50%), a NLR > 4 was shown to be an independent negative prognostic factor in a large retrospective cohort of patients with NSCLC receiving single-agent anti-PD-1/PD-L1. The negative prognostic ability of NLR was further reinforced by the association with poor performance status and pre-ICI steroid treatment, analyzed as a composite 3-class prognostic classification tool [59].
Very recently, blood NLR, as a continuous variable, has been integrated in a six-feature “logistic regression-based immunotherapy-response score”, which is available online [60], to predict immunotherapy objective responses across a variety of primary tumors, including NSCLC. An important limitation of these studies, is that neutrophils were assessed as a whole entity, even though accumulating evidence has shown functionally different neutrophil subsets, including an IFN-stimulated one [61, 62], associated with favorable outcomes after immunotherapy in patients with NSCLC in preliminary findings [63].
A greater understanding of whether myeloid cells are the primary drivers of resistance—versus merely being associated with poor response—will be critical for our capacity to appropriately select patients for treatment with ICI. In fact, the fundamental question that needs to be addressed is why some tumors present this baseline inflamed tumor microenvironment while others do not, and instead present an immunosuppressive tumor infiltration. The reason is likely multifactorial and depends on the type of tumors, systemic characteristics of the host, and environmental factors. This is an area of research that is generating significant interest and is currently under extensive investigation.
Acquired Resistance
Translational evidence elucidating immune mechanisms of acquired resistance in NSCLC are scarce. Early findings in this context indicate that the immune landscape characterizing primary immunotherapy resistance from acquired immunotherapy resistance can be different. Furthermore, the available data suggest that the immune mechanisms of acquired resistance can be varied.
Ricciuti et al. [64] analyzed 82 matched tumor biopsies of patients with NSCLC before and after immunotherapy, showing an immune desertification at the time of progression, with an overall decrease in T cells and PD-1/PD-L1 engagement. In contrast, at about the same time, Memon et al. [7] showed that tumor progression after the initial benefit of PD-1 blockade presents features of chronic IFN-γ stimulation. Genomic and transcriptional analyses were carried out in 29 patients with available tissue samples at the time of progression. Compared with patients with primary resistance, non-surprisingly, acquired resistance was associated with higher baseline PD-L1 tumor expression, and longer post-progression OS. Principal component analysis on microarray-based whole-transcriptome analyses on pre and post progression tumor samples (n = 13), revealed that these sample differed in terms of immune-related genes. Surprisingly, using a bioinformatic deconvolution tool (CIBERSORT), an increased CD8+ T-cell infiltration was found in post-progression samples. Similarly, post-treatment samples were transcriptionally enriched in IFN-γ signature, and upregulation of the GZMA, CXCL9 and B2M genes, all hallmarks of a sustained immune response to PD-1 blockade. These paired samples were further segregated into stable or upregulated IFN-γ signatures, after treatment. The use of whole transcriptomics for analysis did not make it possible to discriminate if the upregulated IFN-γ signature is tumor or immune-related. This distinction is functionally relevant, as shown in syngeneic tumor-bearing mice [65 , 66], where upregulation of the IFN signature in immune cells disable inhibitory pathways and allows tumor control of low immunogenic tumors by natural killer cells/innate-lymphoid cells. On the contrary, increased IFN-signaling in tumor cells drives T-cell exhaustion. While early upon tumor development/PD-1 blockade, IFN-γ signaling is necessary for T-cell priming and activation, and to elicit a successful innate immune response, chronic IFN-γ exposure induces a transcriptional and epigenetic program in tumor cells, making them resistant to T-cell killing and generating an adaptive and innate immune exhaustion and dysfunction [66–68].
Supporting the notion that acquired resistance does not imply an immune desertification but instead supplements a persistently active immune response, Pai et al. [69] reported an increased T-cell infiltration in terms of exhausted CD8+ and regulatory CD4+ T cells in tumor lesions resected from patients with NSCLC (oligo)progressing to PD-1 pathway blockade.
In another small report on seven patients with NSCLC [70], pre- and post-progression tumor biopsies were analyzed with spatial mass cytometry, whole-exome sequencing, and RNA sequencing. Despite high inter-patient heterogeneity, two different patterns of acquired resistance were noted: one associated with increased infiltration of PD-1+ CD8+ T cells, and another one where CD8+ T-cell infiltration decreased or remained stable. Matched (bulk) transcriptomics was performed in two patients, one with reduced and one with increased T-cell infiltration. In both cases, a decreased IFN type I and II was noted, along with upregulation of genes involved in extracellular matrix remodeling. From the spatial mass cytometry, no major changes in the myeloid compartment were found across the study population, even though this could be related to the limited antibody panel used. In this study, it was also proposed that several progressing tumors presented a decrease or loss in TCF-1+ CD8+ T cells. These cells were interpreted as progenitor stem-like cells, however the co-expression of PD-1 was not assessed, making it difficult to distinguish naïve from memory CD8+ T cells [18, 20].
A common limitation of the studies above is the lack of a control arm of pre- and post-treatment biopsies in responding patients, so that it cannot be excluded that the immune changes observed are a result of PD-1 blockade exposure rather than resistance-driven alterations. These hypotheses-generating findings would need broader translational confirmation.
Despite the paucity of data, it should be considered that numerous indirect evidence support the notion that the efficacy of PD-1 pathway blockade on reinvigorating (pre-existing) antigen-specific CD8+ T-cell effector functions is intrinsically transient. This was shown mostly in the chronic infection model of T-cell exhaustion, but also in cancer [17, 71–73]. In a translational study on circulating PD-1+ CD8+ T cells longitudinally analyzed after immunotherapy (alone or in association with anti-CTLA-4 or chemotherapy), the expansion of circulating PD-1+ CD8+ T cells, associated with clinical response, was observed early after treatment initiation, but in most cases it started to wane after cycle two or three [71, 73]. Remarkably, clinical response and OS were longer in patients in whom the expansion of PD-1+ CD8+ T cells was longer. Interestingly, these patients were more often treated with PD-1-based combination therapies than PD-1-based monotherapies [73]. CTLA-4 or chemotherapy combination, may indirectly change the differentiation rate of stem-like PD-1+ CD8+ T cells, which is only transitorily increased by PD-1 blockade [17, 72].
Tumor-Intrinsic Characteristics of Resistance
Primary Resistance
PD-L1 and Tumor Mutational Burden
PD-L1 expression by tumor cells still represents the only biomarker used in the clinic to guide the treatment of patients with non-oncogene addicted NSCLC. A direct association exists between PD-L1 expression and the benefit from PD-1-based ICI, with higher expression being associated with higher chances of durable response and prolonged OS in NSCLC [5, 6]. Conversely, PD-L1 expression in < 1% of tumors cells has consistently been associated with worse outcomes on immunotherapy or chemo-immunotherapy. PD-L1 expression, however, presents several limitations, such as spatial and temporal heterogeneity [74], and a lack of robustness to accurately determine which patients will benefit from ICI, as a significant proportion of patients with PD-L1 high tumors do not respond to immune checkpoints, while some patients with PD-L1 negative cancers still derive significant benefit.
Beyond PD-L1 expression, several other tumor-intrinsic factors have been linked with response to ICI. Tumor-intrinsic resistance factors thus refer to the genetic, transcriptional, or functional characteristics of the tumor cells themselves that can influence the response to ICI. Some tumors intrinsic factors influence indeed the establishment of the anti-tumor immune response, by affecting tumor antigenicity, antigen presentation, and T-cell recruitment and activation. Tumor-intrinsic factors driving resistance to ICI will be covered below.
Tumor neoantigens represent direct targets for the anti-tumor immune response. Higher tumor mutational burden (TMB) has been shown to correlate with neoantigen burden, and T-cell clonality and density in NSCLC [75]. Several studies demonstrated an association between the response to immunotherapy and TMB, measured by targeted next-generation-sequencing panels [76]. However, despite the rationale and encouraging preliminary data, TMB has failed to serve as an accurate and reliable biomarker of response to ICI. Specifically, in the Checkmate 227 (tissue-based TMB) and NEPTUNE (blood-based TMB) trials [77, 78], high TMB was unable to identify patients who derived significant survival benefits from double ICI compared with chemotherapy in a prospective validation setting. These negative findings indicate that the performance of TMB as a tissue and blood biomarker still requires optimization. Refined versions of TMB, incorporating the detection of genetic alterations at low allele frequency in blood [79] or epigenetic alterations in tissue [80], have been proposed and now warrant prospective validation.
Genetic Mutations/Rearrangements
The association between neoantigen load and the efficacy of immunotherapy is further supported by the higher efficacy of ICI across various cancers with deficient DNA mismatch repair machinery or with polymerase epsilon mutations [81–83]. In NSCLC, as discussed below, higher neoantigen loads are associated with tobacco exposure, and smoking history is associated with better responses to ICI [84, 85]. A low neo-antigenicity thus represents a possible primary resistance mechanism to ICI. An example is oncogene addicted NSCLC, which are frequently found in non-smokers and represent a distinct subgroup of NSCLC with low TMB and lower T-cell clonality [75]. Oncogene addicted NSCLC has indeed been consistently associated with poorer outcomes of ICI, with the possible exceptions of BRAF, V600E, and MET ex14 skipping mutations among smokers [86, 87].
Various other genomic alterations have been associated with intrinsic resistance to ICI. Inactivating mutations of the serine/threonine kinase 11 (STK11), also known as liver kinase B1 (LKB1), have been described as a driver of primary intrinsic resistance to immunotherapy in NSCLC [88, 89]. This effect is observed only in KRAS mutant tumors, where the co-occurrence of KRAS and STK11 mutations is associated with distinct immunophenotypes [90]. STK11 functions as a tumor suppressor gene, regulating cellular metabolism, energy balance, and cellular stress responses to nutrient deficiency [91]. The underlying mechanism by which STK11 mutations are driving resistance to ICI remains poorly understood. One postulated mechanism is the enhanced production and secretion of lactate by STK11-deficient tumor cells that could be associated with increased M2 macrophage polarization and hypofunctional T cells in the tumor microenvironment [92]. Inactivating mutations of KEAP1 represents another driver of intrinsic resistance to immunotherapy in NSCLC. Like STK11 mutations, studies have reported that KEAP1 mutations, especially when co-mutant with KRAS, are associated with poor response to ICI [90]. KEAP1 is a tumor suppressor gene that under normal cellular circumstances promotes the ubiquitination of NRF2 by the CUL3-RBX1 complex and leads to its proteasomal degradation [93]. Under a situation of oxidative stress, a change in the conformation of KEAP1 alleviates this process of NRF2 regulation. NRF2 then acts at restoring the redox cellular balance through its transcriptional regulation of diverse proteins implicated in the cellular antioxidant response [94]. Inactivating mutations of KEAP1, through the upregulation of NRF2, are thought to play a role in the antitumor immune response by interfering with the transcription of cytokines and type I IFN-inducing cGAS/STING signaling [95]. KEAP1 tumors are indeed associated with a low density of tumor-infiltrating lymphocytes. Furthermore, the metabolic changes induced by KEAP1 loss and NRF2 activation might also impact the tumor microenvironment. Other oncogenic signaling pathways may play a role in primary resistance to ICI. Epidermal growth factor receptor (EGFR) mutations in NSCLC, as previously described, lead to a cold tumor microenvironment, through reduced neoantigens and other mechanisms that can promote immune evasion, such as the increased amphiregulin expression (96) that enhances T regulatory cell activation [97]. BRAF mutations and MAP-kinase pathway activation have also been shown to favor cancer immune evasion in melanoma, through an increase expression of IL-6 and IL-10 [98]. PTEN loss and the resulting activation of the PIK3 signaling pathway has been associated with poorer responses to anti-PD1 therapy in patients with melanoma [99]. In in vivo models, PTEN loss resulted in an enhanced production of immunosuppressive cytokines and a reduction in T-cell infiltration and T-cell anti-tumor activity [100]. Wnt/β-catenin oncogenic signaling represents another pathway that may hinder anti-tumor responses. In metastatic melanoma, WntT/β-catenin activation correlates with an immunosuppressive microenvironment [101], through the reduced tumor secretion of the cytokine CCL4, which normally promotes the recruitment and activation of immune cells. Among other genomic alterations, the loss of 9p21.3, which contains CDKN2A/B and MTAP genes, has also been linked to resistance to immune checkpoint inhibitors [102]. The postulated mechanisms rely on the downregulation of T-cell recruitment, expansion, and activation through a decrease in the production of several cytokines, such as XCL13, CXCL9, XCL2, and CCL5, and the upregulation of immunosuppressive factors: PVR, TGF-b 1, CD73, VEGFA, and B7-H3.
Downregulation of Interferon-γ Signaling and Antigen Presentation
Resistance to IFN-γ signaling may represent another mechanism of intrinsic primary resistance of cancer cells to immunotherapy [34]. Beyond PD-L1 expression discussed above, alterations in the IFN-γ signaling pathway in cancer cells, such as inactivating JAK1 and JAK2 mutations, have been reported as potential mechanisms of resistance to immune checkpoint inhibitors [103–105]. While there is substantial evidence supporting IFN-γ's role in anti-tumor immunity, numerous studies have reported that IFN-γ may promote tumor growth in certain conditions [106]. As discussed above, the type of cells with an IFN-γ signature, as well as the magnitude and time of exposure to IFN, that makes a difference in the type of immune response elicited [65–67].
Another mechanism by which tumor cells can escape T-cell-mediated killing is by reducing the expression of major histocompatibility complex (MHC) molecules on their surface. Downregulation of beta-2-microglobulin has been described as a mechanism of resistance to immune checkpoint inhibitors, through mutations of the beta-2-microglobulin gene [9, 107]. This leads to inefficient antigen presentation by the MHC complex. These mutations have been shown to arise in tumor evolution under the pressure of the immune system, even without immunotherapy treatment [108].
Acquired Resistance
Some of the mechanisms of resistance, similar to the intrinsic tumor mechanisms described above, can be acquired by the tumor under the pressure of PD-1-based immunotherapy. In their analysis of 82 patients with matched pre- and post-immunotherapy biopsy, Ricciuti et al. found that common genomic alterations associated with resistance to immune checkpoint inhibitors emerged at treatment progression, such as inactivating mutations in STK11, KEAP1, JAK1/2 and B2M, or activating mutations in MAPK, PI3K/Akt/mTOR, and Wnt/β-catenin pathways [64]. Interestingly, a decrease in MHC class-1 expression at the time of acquired resistance to immune checkpoint inhibitors was also found. Similar findings in the acquisition of genomic alterations upon progression on immune checkpoint inhibitors were also described by Memon et al. [7].
Systemic Factors
In its complexity, the immune system is regulated by several intrinsic host-related factors that can influence the capacity to recognize antigens and the type of adaptive and innate immune response generated.
Sexual Dimorphism
Sexual dimorphism in the immune system is a well-established phenomenon, even if the underpinnings are not fully understood. The greater susceptibility of women to autoimmune diseases could be accompanied by enhanced immune surveillance against various tumor types [109]. In fact, females overall have a lower risk and better survival than males in multiple types of cancer that are unrelated to reproductive function [109]. These sex-based differences in immune responses are likely influenced by a combination of genetic, hormonal, and environmental factors. Interestingly, both estrogen and androgen receptors are present on immune cells [109]. In murine models, the activity of the androgen receptors promotes neutrophil production and restrains T-cell proliferation [110]. Moreover, 17β-estradiol has been shown to regulate the expansion of T regulatory cells, at least in part through the regulation of the FOXP3 gene, which is X-linked [109].
In this context, sex has also been reported to influence ICI response. Most studies conclude that female sex confers a primary resistance to immunotherapy, but others, either do not show any difference, or prove the opposite [111]. According to recent translational evidence, the mechanisms of immune dysfunction in NSCLC can vary across sexes [112]. The tumor microenvironment in females more often presents features of chronic inflammation, characterized by greater T-cell exhaustion and higher abundance of myeloid immune-suppressive cells, cancer-associated fibroblasts, and regulatory T cells. In contrast, males more often present a T-cell—excluded tumor microenvironment, probably as a consequence of impaired neoantigen presentation. In two metanalyses from NSCLC clinical trials [113, 114], the same authors suggest that females derive a higher magnitude of benefit from chemo-immunotherapy compared with chemotherapy, whereas the magnitude of ICI as single agents seems to be higher in males. However, it must be considered that many clinical trials demonstrated the efficacy of ICI in female cancers, such as cervical cancer, endometrial cancer, and breast cancer [115–117]. Dissecting the predictive role of gender in cancer immunotherapy is limited by many societal and biological aspects. First, females are often underrepresented in clinical trials. Second, as mentioned above, females have a lower incidence and better prognosis in several cancer types [109]. Healthcare access and daily habits also differ between males and females and should also be taken into account [113, 118].
Intestinal Microbiota and Metabolism
The intestinal microbiota is an important environmental component that regulates the immune system. Several studies in melanoma have assessed the impact of gut microbiota on tumor immune control. A significant impact of certain gut bacteria on tumor growth and response to checkpoint inhibitors was first seen in mice [119]. These data were confirmed in human patients with melanoma, who also showed improved tumor control and higher response rates for those harboring a highly diverse gut microbiota, rich in Faecalibacterium as opposed to a low diversity rich in Bacteroidales [120]. Modification of the tumor microenvironment and increased T-cell efficiency are likely underlying mechanisms according to the authors. A study with 16 patients with melanoma went a step further and described a sensitization of tumor cells to PD-L1 therapy after fecal transplant (from responders to non-responders) in six patients [121]. It could explain why Routy et al. described a negative impact of antibiotics during immunotherapy through dysbiosis [122]. The authors explain that the microbiome alterations induced by antibiotics reduced response rates to PD1 blockade. Fecal transplantation of antibiotic-naïve responder mice to antibiotic-treated mice improved PD1-blockade sensitivity. Decreased progression-free survival (PFS) and OS were also observed in patients with renal cell carcinoma and non-small cell lung cancer treated with PD1-blockade who received antibiotics compared with patients who did not [123, 124].
Obesity has also been found to negatively impact the immune system, possibly explaining the worse prognosis in obese patients with cancer [125]. However, when focusing on the efficacy of checkpoint inhibitors in obese and non-obese patients, results proved the opposite, with better outcomes for obese patients [126]. Some evidence suggests that obesity itself is not associated with immunotherapy resistance, but rather lipid metabolism [127]. Cell membranes and intracellular signaling pathways require lipids, including cholesterol. Low cellular cholesterol levels reduce CD8+ T-cell signaling. Increasing cellular cholesterol levels could improve CD8+ T-cell function. Avasimibe, an ACAT1 inhibitor that was studied for atherosclerosis, increases plasma membrane cholesterol, upregulating CD8+ T-cell signaling and function. In mouse models of lung cancer and melanoma, avasimibe showed anti-tumor activity that was increased by the association with PD1-blockade [128]. As mentioned above, a bad lipid profile seems to induce resistance to immunotherapy through a double mechanism. On the one hand, oxidized low-density lipoprotein decreases CD8+ T-cell function and on the other, it increases heme oxygenase 1 in tumor cells, conferring resistance to reactive oxygen species and thus, to apoptosis. The latter could be the target of a new drug to be combined with checkpoint inhibitors to bypass this resistance mechanism [127]. Maintaining a favorable lipid profile might also be important to improve immunotherapy efficacy.
HLA Genotype
Human leukocyte antigen class I (HLA-I) is involved in cell interactions required for immune checkpoints. There are many different subtypes of HLA-I, and each of us harbors two copies. One can either be HLA-I homozygote or heterozygote. It is postulated that HLA-I heterozygosity confers a better response to immunotherapy because a wider panel of tumor antigens can be presented and elicit a targeted immune response. The correlation is even stronger when HLA-I heterozygosity is associated with a high tumor mutational burden. Apart from the hetero- or homozygosity, the response to immune checkpoint inhibitors is influenced by HLA-I supertypes; HLA-B44 correlates with a better response, whereas HLA-B62 a worse one [129] (Fig. 1)
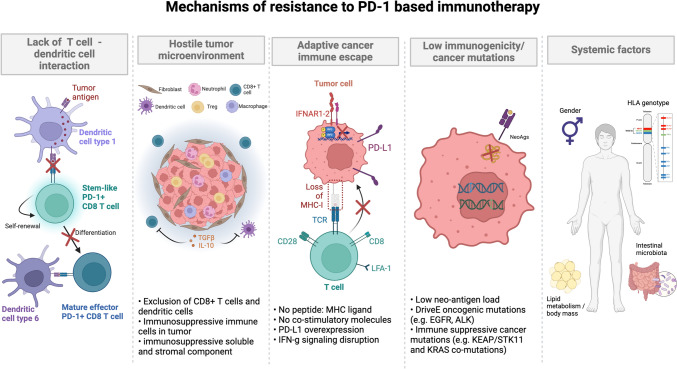
Fig. 1.
Graphic summary of the mechanisms of resistance to immunotherapy strategies based on PD-1 pathway blockade. Mechanisms of resistance include a lack of T cell–dendritic cell interaction; a hostile tumor microenvironment; adaptive cancer immune escape; low immunogenicity/cancer mutations; and systemic factors. HLA, human leukocyte antigen; IFNAR1-2, interferon-a receptor 1–2; IFN-g, interferon-g; LFA-1, lymphocyte function-associated antigen 1; MHC-I, major histocompatibility complex I; PD-1, programmed death-1; PD-L1, programmed death-ligand 1; TCR, T-cell receptor; TGF-b, transforming growth factor-b. Figure generated with BioRender®
Treatment Strategies to Prevent/Overcome Primary Resistance
Primary resistance to ICI is defined as a lack of initial tumor response and no clinical benefit. As discussed previously, a significant proportion of patients exhibit primary resistance to these treatments. Developing strategies to overcome this resistance is essential to improving therapeutic outcomes. Various approaches have been investigated over recent years. The most notable endeavor in the past few years has been focused on combination therapies aimed at increasing the proportion of patients responding to PD-1-based immunotherapy. A summary of published and ongoing trials is provided in Tables 2 and 3.
Table 2.
Published trials in the setting of primary resistance
| Trial name/code | Phase | Treatment strategy | Number of patients | ORR | DCR | Median PFS | Median OS |
|---|---|---|---|---|---|---|---|
| Impower 150 [130] | III | Carboplatin, paclitaxel, atezolizumab (ACP), ACP plus bevacizumab (ABCP), carboplatin, paclitaxel, bevacizumab (BCP). | 1040 | 63.5% (ABCP) versus 48% (BCP) | 85% (ABCP) versus 82.7% (BCP) | 8.3 (ABCP) versus 6.8 months (BCP), p < 0.001 | 19.5 (ABCP) versus 14.7 months (BCP), HR = 0.80, and 19 months (ACP) versus BCP group, HR = 0.84 |
| NCT03083041[131] | II | Camrelizumab and apatinib | 25 | 40% | 92% | 11 months | NR |
| LEAP-007 [132] | III | Pembrolizumab and lenvatinib versus placebo | 623 | 40.5% versus 27.7% | – | 6.6 versus 4.2 months, p = 0.006 | 14.1 versus 16.4 months, p = 0.79 |
| NCT02119650 | II | Ruxolitinib or placebo plus cisplatin, pemetrexed | 63 | 30.8% versus 35.1% | 41% versus 48.6% | – | 7.5 versus 5.9 months, p = 0.76 |
| KEYNOTE 598 [140] | III | Pembrolizumab plus ipilimumab or placebo | 568 | 45.4% in each group | 70% versus 71% | 8.2 vs 8.4 months | 21.4 versus 21.9 months, p = 0.74 |
| POSEIDON [141] | III |
CT plus durvalumab, tremelimumab (CTDT), or CT plus durvalumab (CTD) , or CT |
1013 | 38.8% versus 41.5% versus 24.4% | – | 6.5 (CTDT) versus 4.8 months (CT) and 5.5 (CTD) versus 4.8 months (CT) | 14 months (CTDT) versus 11.6 months (CT), and 13.3 months (CTD) versus 11.6 months (CT) |
CT, chemotherapy; DCR, disease control rate; NR, not reached; ORR, objective response rate; OS, overall survival; PFS: progression-free survival.
Table 3.
Ongoing trials in the setting of primary resistance
| Trial name/code | Phase | Treatment strategy | Expected number of patients |
|---|---|---|---|
| HARMONi-2 | III | Ivonescimab, pembrolizumab | 398 |
| NCT04515979 | II | Vactosertib | 55 |
| NCT03425006 | II | Pembrolizumab, itacitinib | 23 |
| NCT04623775 | II | Relatlimab, nivolumab | 468 |
| NCT06162572 | I | Cemiplimab, S095018 (anti-TIM3 antibody), S095024 (anti-CD73 antibody), or S095029 (anti-NKG2A antibody) | 176 |
| NCT04294810/SKYSCRAPER-02 | III | Tiragolumab or placebo, atezolizumab | 620 |
| NCT04619797/SKYSCRAPER-06 | II/III | Tiragolumab or placebo, atezolizumab and carboplatin/cisplatin-pemetrexed | 542 |
Targeting the Tumor Microenvironment
Anti-angiogenic therapy has been the first anti-tumor strategy targeting the tumor microenvironment, and its combination with first-line chemo-immunotherapy is already in use, based on the positive results of the Impower 150 clinical trial. Here, the combination of atezolizumab, bevacizumab, and doublet platinum-based chemotherapy demonstrated an improvement in outcomes compared with the bevacizumab and chemotherapy arm [130]. Ensuing studies have tested other approaches based on newer anti-angiogenics, with inconsistent results. In a small phase II trial, the combination of camrelizumab (anti-PD1) plus low dose apatinib, an oral tyrosine kinase inhibitor targeting the VEGF receptor-2, demonstrated notable efficacy as a first-line treatment for advanced non-squamous NSCLC, regardless of PD-L1 expression. The objective response rate (ORR) was 40%, with a median PFS of 9.6 months [131]. Lenvatinib plus pembrolizumab did not show any benefit compared with pembrolizumab alone for PD-L1 ≥ 1% NSCLC in the LEAP-007 trial [132]. Ivonescimab is a bispecific antibody targeting VEGF and PD-1 under investigation in patients with positive PD-L1 (> 1%) NSCLC in the phase III HARMONi-2 trial. In a recent press release, it was reported that ivonescimab had an improved PFS compared with pembrolizumab [133].
As the presence of TGF-β in the tumor microenvironment is associated with immune suppression (Sect. 2.1), inhibitory agents have been developed and tested in clinical trials. Vasctosertib, a novel TGF-β1 type I receptor kinase inhibitor is currently being investigated in combination with pembrolizumab as a first-line treatment for NSCLC with a PD-L1 score ≥ 1% in an ongoing phase II study that is still recruiting (NCT04515979).
As it has become increasingly recognized that the IFN-signature is fundamental in regulating the balance between immune stimulation and immune suppression, agents modulating IFN-γ signaling were recently tested. As mentioned in Sect. 2.1, the intracellular activation of JAK1 and JAK2 upregulated the expression of various IFN-γ-related genes. JAK inhibitors are immuno-modulating drugs already approved for several hematological and rheumatological conditions, including graft-versus-host disease [134]. They have been previously tested as single agents or in combination with chemotherapy for several malignancies, with negative results [135–137]. A small phase II academic trial on 21 patients is assessing if the temporary administration of itacitinib, a JAK-1 inhibitor, can enhance the effectiveness of anti PD-1 treatment in patients with PD-L1 high NSCLC (NCT03425006). As the opposing effects of IFN-γ rely on a temporal component that activates immune responses early but inhibits these responses at later times, itacitinib was given from the third cycle of pembrolizumab until the fifth cycle. The preclinical findings, clinical and translational results were recently published by Mathew et al. [138], who observed durable and high tumor responses accompanied by reduced inflammatory signals. In responding patients, a circulating subset of CD8+ T cells, resembling the stem-like progenitor, was identified, with signs of enhanced plasticity. Notably, preliminary favorable results of the delayed administration of JAK inhibitors to PD-1 blockade have been simultaneously reported for non-Hodgkin lymphoma [139]. These preliminary results would need validation in phase III trials, To the best of our knowledge, currently no ongoing trial is currently testing JAK inhibitors in non-oncogene addicted NSCLC.
Targeting T-cell Co-stimulation and Co-inhibition
In recent years, the discovery and understanding of additional immune checkpoints (e.g., TIGIT, LAG-3, and TIM-3) have enabled the development of numerous promising investigational monoclonal antibodies, either alone or in combination with anti-PD1, anti-PD-L1, or anti-CTLA-4 therapies. These approaches aim to modulate various aspects of the immune response and overcome primary resistance to immunotherapy.
Since the combination of ipilimumab and nivolumab has demonstrated a survival benefit compared with chemotherapy alone in the CheckMate 227 trial [4], ipilimumab plus pembrolizumab were recently tested, compared with pembrolizumab alone, in NSCLC with PD-L1 > 50%, with negative results on both PFS and OS [140]. The phase III POSEIDON study investigated tremelimumab, another anti-CTLA-4, plus durvalumab and chemotherapy; durvalumab plus chemotherapy; versus chemotherapy alone. Chemotherapy plus durvalumab improved PFS versus chemotherapy alone, but not OS, which was the co-primary endpoint. The addition of tremelimumab to durvalumab and chemotherapy improved both PFS and OS compared with chemotherapy alone (secondary endpoint). Notably, the study was not designed to compare the immunotherapy-containing arms [141]. More recent evidence suggests that some specific subgroups associated with poor response to anti-PD1/L1 therapies, such as patients with KEAP1 and/or STK11 inactivating mutations, might derive more benefit from the addition of an anti-CTLA-4. In a post-hoc exploratory analysis of the POSEIDON trial, Skoulidis et al. showed that patients with KEAP1 and/or STK11 mutations seemed to derive some benefit from the addition of CTLA-4 blockade to anti-PD-L1 and chemotherapy [142]. In lung cancer mouse models, they further demonstrated that STK11 and KEAP1 mutations were associated with an enrichment of CD4+ T cells and myeloid cells. Compared with anti-PD1 alone, the addition of an anti-CTLA-4 led to significantly better tumor regression in models with STK11 and KEAP1 mutations, supposedly owing to a CD4 T-cell-mediated activation and a shift in the myeloid compartment toward potentially tumoricidal iNOS+ cells.
TIGIT (T-cell immunoreceptor with Ig and ITIM domains) is an immune checkpoint receptor that inhibits T cell and natural killer cell activity [143]. SKYSCRAPER-01 is an ongoing randomized, double-blinded phase III study evaluating the anti-TIGIT tiragolumab combined with atezolizumab versus atezolizumab alone, as a first-line therapy for high PD-L1 locally advanced or metastatic NSCLC. Preliminary data from interim analyses and press releases indicate that its co-primary end point of PFS has not been met. The data for OS are immature but the estimated median is numerically better in the combination arm (22.9 months versus 16.7 months), with a hazard ratio of 0.81 [144].
Furthermore, in the phase II-III SKYSCRAPER-06 trial, the combination of tiragolumab with atezolizumab and chemotherapy showed reduced efficacy in both PFS and OS compared with pembrolizumab and chemotherapy in non-squamous NSCLC, according to a recent press release [145].
LAG-3 (Lymphocyte-Activation Gene-3) is an immune checkpoint receptor expressed on the surface of T cells, regulatory T cells, and natural killer cells. Relatlimab, a LAG-3-blocking antibody, in combination with nivolumab, demonstrated a greater benefit compared with nivolumab alone in untreated metastatic or unresectable melanoma [146]. A randomized phase II study investigating the addition of relatlimab to nivolumab-chemotherapy versus nivolumab-chemotherapy as the first-line treatment for metastatic NSCLC is currently underway (NCT04623775).
TIM-3 (T cell immunoglobulin and mucin-domain-containing-3) is a transmembrane protein mostly expressed on T cells, but it is also present on NK cells, macrophages and on cancer cells of various malignancies including NSCLC [147, 148]. Its overexpression on CD8+ T cells is a well-known marker of exhaustion and has been associated with a poor prognosis [149]. While other anti-TIM-3 agents have been tested in the second line setting, upon progression to immunotherapy (see Sect. 6.2), the combination of S095018 (anti-TIM3) and cemiplimab (anti-PD1) for treatment-naïve NSCLC is open but not yet recruiting [150].
As discussed above, co-stimulatory signals, such as those mediated by CD28 and 4-1BB, are crucial for the activation and proliferation of CD8+ T cells. The manipulation of these signals through therapeutic agents can potentiate the immune system’s capacity to recognize and destroy tumor cells thus potentially improving the ability to overcome immunotherapy resistance. 4-1BB (or CD137) is a co-stimulatory receptor expressed primarily on activated T cells, NK cells, and dendritic cells. Upon binding to its ligand, 4-1BBL, 4-1BB provides critical signals that enhance T-cell proliferation, survival, and effector functions [151]. In immunotherapy-naïve patients, urelumab, a 4-1BB agonist, in combination with pembrolizumab, showed disappointing activity in advanced NSCLC [152]. The accrual is complete, but the results have not yet been published. ICOS (CD278) is another co-stimulatory receptor of the CD28 superfamily that is expressed on the surface of activated T lymphocytes. A phase II study investigated the combination of pimivalimab (anti-PD1) and vopratelimab (JTX-2011), an ICOS agonist, in adult subjects with metastatic NSCLC who were immunotherapy naïve. The development of pimivalimab has been discontinued and the results have not been published (NCT04549025). Numerous studies have also been conducted with OX40 (CD134) agonists, a member of the TNF receptor family, either as monotherapy or in combination with anti-PD1 or anti-CTLA-4 agents, also yielding modest results.
These negative data consistently indicate our incomplete understanding of the anti-tumor immune response, at steady-state and upon PD-1-based immunotherapies, and highlight the need to reconsider the current modalities to manipulate T-cell co-stimulation and co-inhibition.
Treatment Strategies to Overcome Acquired Resistance
Several efforts have been made to overcome acquired resistance to checkpoint inhibitors, with in general poor results so far. In Tables 4 and 5, an overview of recently published or ongoing trials in the setting of acquired resistance is provided. Most of the study drug(s) were compared with docetaxel, the current standard of care upon failure of chemo-immunotherapy.
Table 4.
Published trials in the setting of acquired resistance
| Trial name/code | Phase | Treatment strategy | Number of patients | ORR (CR) | DCR | Median PFS | Median OS |
|---|---|---|---|---|---|---|---|
| SAPPHIRE [153] | III | Nivolumab + sitravatinib versus docetaxel | 577 | 15.6% versus 17.2% | CBR 75.5% versus 64.5%, p = 0.004 | 4.4 months versus 5.4 months, HR: 1.08, p = 0.452 | 12.2 months versus 10.6 months, HR: 0.86, p = 0.144 |
| CONTACT-01 [154] | III | Atezolizumab + cabozantinib versus docetaxel | 366 | 11.8% (0%) versus 13.3% (0%) | 77.4% versus 68.3% | 4.6 months versus 4.0 months, HR: 0.74, NS | 10.7 months versus 10.5 months, HR: 0.88, p = 0.37 |
| LEAP-008 [155] | III | Pembrolizumab + lenvatinib versus docetaxel | 422 | 21.6% versus 10.4% | – | 5.6 months versus 4.2 months HR: 0.89, p = 0.16 | 11.3 months versus 12 months, HR: 0.98, p = 0.43 |
| Lung-MAP S1800A [159] | II randomized | Pembrolizumab + ramucirumab versus SoC (docetaxel-ramu, docetaxel, gemcitabine, pemetrexed) | 136 | 22% versus 28% | 75% versus 73% | 4.5 months versus 5.2 months, HR: 0.86, p = 0.25 | 14.5 months versus 11.6 months, HR: 0.69, p = 0.05 |
| Lung-MAP S1800D [162] | II/III | Pembrolizumab + N-803 (IL-15 superagonist) versus SoC (chemotherapy) | 71 | 11.4% (2.8%) versus 13.9% (0%) | – | Not reported, HR: 1.29, p = 0.33 |
1 year-OS 44% versus 25%, HR: 0.73, p = 0.32 |
| BGBC008/NCT03184571 [157] | II | Bemcentinib (AXL inhibitor) + pembrolizumab | 90 non squamous (cohort C: AR) | 11.1% [21.9% if AXL > 5] | – | 6.2 months | 13.0 months (14.8 months if AXL exp > 5) |
| NCT01307267 | I/II | Utolimumab (41BB agonist) | 20 (2 AR) | 0% (AR: not evaluable) | 50% | 3.6 months | |
| ATALANTE-1 [163] | III | OSE2101 versus SoC chemotherapy (docetaxel or pemetrexed) | 118 | 7.7% vs 18.4% | DCR 6 months 25% versus 24% | 2.7 months versus 3 months, HR: 1.28, p = 0.29 |
11.1 months versus 7.5 months 1 year-OS: 44.4% versus 27.5%, HR: 0.59, p = 0.017 |
| NCT03215810 | I | TILS | 16 (2 AR) | 46% | 92% | – | – |
| TILS Lifileucel [165] | II | Lifileucel (LN-145) | 28 (21 AR) | 21.4% (3.6%) | 64.3% | – | – |
| NCT02517398 | I | Bintrafusp alfa | 45 with AR | 2.2% | 26.7% | 6 months—PFS: 13.2% | 10 months |
| NCT03525782 | I | CAR-T cells anti-MUC1 | 20 | 0% | 55% | – | – |
AR acquired resistance; SoC standard of care
Table 5.
Ongoing trials in the setting of acquired resistance
| Trial name/code | Phase | Treatment strategy | Expected number of patients |
|---|---|---|---|
| SAFFRON-301/NCT04921358 | III | Tislelizumab-sitravatinib versus docetaxel | 420 |
| PRAGMATIC-LUNG/ NCT05633602 | III | Pembrolizumab-ramucirumab versus SoC chemotherapy | 700 |
| NCT04496674 | I | CC-1 (bispecific antibody PSMAxCD3) in squamous lung cancer | 10–72 |
| NCT06060613 | I/II | Membrane bound IL-15 expressing tumor-infiltrating lymphocytes (OBX-115) + acetazolamide | 52 |
| NCT05060796 | I | CAR-T anti EGFR | 11 |
Targeting the Tumor Microenvironment
Upon progression to first-line strategies based on PD-1/PD-L1 blocking therapies, targeting the tumor microenvironment has been regarded as the most promising strategy, however, results were mostly disappointing in three recently published phase III studies.
Sitravatinib is a double inhibitor of TAM receptors (TYRO3, AXL, and MERTK) involved in M2 macrophage polarization, and VEGRF2. Its combination with nivolumab was compared with docetaxel in patients progressing to chemo-immunotherapy in the phase III SAPPHIRE trial. Despite the good safety profile, no significant improvement in OS was found [153]. Another trial testing its association to tislelitumab is ongoing (NCT04921358).
Similarly, the associations of other multi-angiogenetics such as cabozantinib or lenvatinib respectively with atezolizumab or pembrolizumab failed in the same setting [154, 155].
TGF-β has been a target for the development of new molecules also upon failure of PD-1 based immunotherapy. Bintrafusp alfa, a first-in-class bifunctional fusion protein composed of the extracellular domain of TGF- b receptor II with an anti-PD-L1, was developed and tested in a phase I trial, with low objective response rate and over 20% of severe toxicity [156].
Despite these disappointing results, preliminary evidence from at least two studies showed potential benefit. Bemcentimab is a selective inhibitor of AXL, belonging to family of TAM receptors on macrophages and other innate immunity cells. This antibody was combined with pembrolizumab in an open label single-arm study. This trial enrolled three cohorts, one of which involving patients progressing after chemo-immunotherapy, and showed an interesting survival results for patients whose tumors had AXL expression > 5 or KRAS mutations [157, 158]. The second phase II randomized study showed a survival benefit for ramucirumab and pembrolizumab over docetaxel or other investigator-chosen chemotherapies [159]. A phase III trial testing the same combination is ongoing (Pragmatica-Lung, NCT05633602).
It is worth mentioning a small ongoing phase I-II study, testing the addition of dupilumab, an anti-IL-4Rα monoclonal antibody, in patients with NSCLC refractory or resistant to single-agent PD-1 based immunotherapy, which is continued upon disease progression [160]. Dupilumab, which is already used for various atopic conditions was found to inhibit monocyte-derived macrophage development by blocking basophil-derived IL-4. In a translational preliminary report on six patients treated, one achieved a deep partial response, while in three patients undergoing longitudinal biopsies, tumor remodeling with expanded CD8+ T cells and LAMP3 dendritic cells were observed post-treatment [36 days] [160].
Targeting T-cell Co-stimulation and Co-inhibition
Enhancing co-stimulatory signals is another possible strategy to overcome acquired resistance, but interesting results in this field are lacking.
One phase I/II study with single-agent utolimumab, a 4-1BB/CD137 agonist, showed good tolerability but disappointing efficacy (DCR 50% with ORR 0%, mPFS 3.6 months) in 20 patients with NSCLC progressing under PD-1 based immunotherapy. Based on supplementary material, only two patients with NSCLC treated with utolimumab met the criteria for acquired resistance and the response to treatment was not evaluable [161].
A recently published abstract from a randomized phase II/III study showed a potential benefit for the combination of N-803, an IL-15 superagonist, and pembrolizumab versus standard of care chemotherapy in 71 patients with acquired resistance [162]. Although OS was not statistically different between the two groups (HR: 0.73, p = 0.32), a numerical OS difference was seen at 1 year (44% versus 25%), warranting further research and biomarker analysis.
In the context of acquired resistance, blockade of other co-inhibitory receptors, in particular with TIM-3, raised considerable enthusiasm. Following the positive results of the phase I trial [150], cobolimab, and other TIM-3 agents are underway in phase I-II trials, mostly in association with PD-1 blockade.
Beyond Checkpoint Blockade
Other interesting strategies to overcome immunotherapy resistance are cellular and vaccine therapies.
The latter approach is supported by the data of the ATALANTE-1 study, which tested the OSE2101 cancer vaccine versus chemotherapy in a randomized phase III trial [163]. Although a statistical survival benefit was met for patients’ subgroups with acquired resistance, this was an outcome defined after multiple protocol adaptions, which have not made it a practice-changing trial.
Tumor-infiltrating lymphocyte-based therapies are an interesting approach in the context of acquired resistance. A previous phase I publication demonstrated the feasibility and promising efficacy of this treatment in selected patients progressing under nivolumab, even if they were mostly anti-PD-1 refractory [164]. In a recent phase II study, of 28 patients treated, 21 had acquired resistance to immunotherapy. In this study, lifileucel showed an overall response rate of 21.4% with 79.2% of patients experiencing a reduction in tumor burden [165].
Finally CAR-T cells are still a matter of interest in solid tumors, with some ongoing trials with different targets such as mesothelin, prostate stem cell antigen (PSCA), MUC1, or EGFR in patients with NSCLC [166, 167].
Concluding Remarks
Treatment with ICI has introduced a paradigm change for cancer care, showing that instructing the immune system to recognize and selectively eliminate cancer cells can provide long-term responses with limited impact on the quality of life of patients. As indicated by the translational and clinical evidence discussed here, the pursuit of a universal and durable anti-tumor control upon ICI is an intricate and prolonged endeavor.
Primary resistance is a consequence of inadequate patient selection. Understanding why certain tumors possess a baseline IFN-stimulated microenvironment, while others do not, would enable the design of strategies to convert an immunosuppressive tumor infiltrate into an inflamed, ICI-responsive one. Acquired resistance, instead, arises from various events, particularly adaptive changes in the tumor microenvironment. To tackle this, dedicated research is needed. Longitudinal assessments of clinical samples are necessary to dissect tumor heterogeneity and evolution, as well as to understand the stromal and immune remodeling induced by ICIs.
At present, the recent efforts in preventing primary resistance and overcoming the acquired one, have shown limited efficacy in clinical trials, suggesting that the therapeutic potential of ICIs has reached a plateau, at least in the realm of NSCLC. However, the numerous knowledge gaps in ICI biology indicate considerable room for improvement. In particular, to timely detect primary and acquired resistance, interesting perspectives regarding the use of early biomarkers of response, such as circulating tumor DNA for tumor burden kinetics, and IFN-γ signatures [168]. These tools offer additional advantages in exploring tumor evolution and examining immune remodeling under therapeutic pressure, respectively.
Moreover, the early identification of resistance would enable response-directed tailored combination strategies [169]. Growing attention has been dedicated to the immunological effects of conventional treatments such as surgery, chemotherapy, and radiotherapy given in combination with ICI. Accordingly, it has been appreciated that the modalities and timing of these combination therapies should be reconsidered to unfold their optimal anti-tumor potential [170].
In conclusion, we believe that a more in-depth understanding of cancer–immune crosstalk, at steady-state and during ICI intervention, will enable a shift from empirical combination therapies to the design of clinical trials testing more rational, biomarker-based immunotherapy strategies.
Declarations
Funding
Open access funding provided by University of Geneva. No external funding was used in the preparation of this manuscript.
Conflicts of interest
A.M. received financial support to attend scientific meetings for registration travel and accommodation from Johnson&Johnson, Roche and PharmaMar. M.B. received financial support to attend scientific meetings for registration travel and accommodation from Bayer, MSD, AstraZeneca and PharmaMar. D.F. received financial support to attend scientific meetings for registration travel and accommodation from MSD. A.A. declares receiving institutional fees from the following companies: BMS, AstraZeneca, Boehringer-Ingelheim, Roche, MSD, Pfizer, Eli Lilly, Merck, PharmaMar, and Novartis. A.A. also reports participation in the Speaker Bureau for Eli Lilly and AstraZeneca. M.W. and F.K. declare that they have no conflicts of interest that might be relevant to the contents of this manuscript.
Ethics approval, consent to participate, consent for publication, availability of data and material, code availability
Not applicable.
References
- 1.Hendriks LE, Kerr KM, Menis J, Mok TS, Nestle U, Passaro A, et al. Non-oncogene-addicted metastatic non-small-cell lung cancer: ESMO clinical practice guideline for diagnosis, treatment and follow-up. Ann Oncol. 2023;34(4):358–76. [DOI] [PubMed] [Google Scholar]
- 2.Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, et al. Five-year outcomes with pembrolizumab versus chemotherapy for metastatic non–small-cell lung cancer with PD-L1 tumor proportion score ≥ 50%. JCO. 2021;39(21):2339–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.De Castro G, Kudaba I, Wu YL, Lopes G, Kowalski DM, Turna HZ, et al. Five-year outcomes with pembrolizumab versus chemotherapy as first-line therapy in patients with non–small-cell lung cancer and programmed death ligand-1 tumor proportion score ≥ 1% in the KEYNOTE-042 Study. JCO. 2023;41(11):1986–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brahmer JR, Lee JS, Ciuleanu TE, Bernabe Caro R, Nishio M, Urban L, et al. Five-year survival outcomes with nivolumab plus ipilimumab versus chemotherapy as first-line treatment for metastatic non-small-cell lung cancer in CheckMate 227. JCO. 2023;41(6):1200–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Novello S, Kowalski DM, Luft A, Gümüş M, Vicente D, Mazières J, et al. Pembrolizumab plus chemotherapy in squamous non-small-cell lung cancer: 5-year update of the phase III KEYNOTE-407 study. JCO. 2023;41(11):1999–2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garassino MC, Gadgeel S, Speranza G, Felip E, Esteban E, Dómine M, et al. Pembrolizumab plus pemetrexed and platinum in nonsquamous non-small-cell lung cancer: 5-year outcomes from the phase 3 KEYNOTE-189 study. JCO. 2023;41(11):1992–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Memon D, Schoenfeld AJ, Ye D, Fromm G, Rizvi H, Zhang X, et al. Clinical and molecular features of acquired resistance to immunotherapy in non-small cell lung cancer. Cancer Cell. 2024;42(2):209-224.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Passaro A, Brahmer J, Antonia S, Mok T, Peters S. Managing resistance to immune checkpoint inhibitors in lung cancer: treatment and novel strategies. JCO. 2022;40(6):598–610. [DOI] [PubMed] [Google Scholar]
- 9.Sharma P, Hu-Lieskovan S, Wargo JA, Ribas A. Primary, adaptive, and acquired resistance to cancer immunotherapy. Cell. 2017;168(4):707–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frisone D, Friedlaender A, Addeo A, Tsantoulis P. The landscape of immunotherapy resistance in NSCLC. Front Oncol. 2022;12: 817548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schoenfeld AJ, Antonia SJ, Awad MM, Felip E, Gainor J, Gettinger SN, et al. Clinical definition of acquired resistance to immunotherapy in patients with metastatic non-small-cell lung cancer. Ann Oncol. 2021;32(12):1597–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kluger HM, Tawbi HA, Ascierto ML, Bowden M, Callahan MK, Cha E, et al. Defining tumor resistance to PD-1 pathway blockade: recommendations from the first meeting of the SITC Immunotherapy Resistance Taskforce. J Immunother Cancer. 2020;8(1): e000398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kluger H, Barrett JC, Gainor JF, Hamid O, Hurwitz M, LaVallee T, et al. Society for Immunotherapy of Cancer (SITC) consensus definitions for resistance to combinations of immune checkpoint inhibitors. J Immunother Cancer. 2023;11(3): e005921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rizvi N, Ademuyiwa FO, Cao ZA, Chen HX, Ferris RL, Goldberg SB, et al. Society for Immunotherapy of Cancer (SITC) consensus definitions for resistance to combinations of immune checkpoint inhibitors with chemotherapy. J Immunother Cancer. 2023;11(3): e005920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brahmer JR, Drake CG, Wollner I, Powderly JD, Picus J, Sharfman WH, et al. Phase i study of single-agent anti–programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. JCO. 2010;28(19):3167–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mellman I, Chen DS, Powles T, Turley SJ. The cancer-immunity cycle: indication, genotype, and immunotype. Immunity. 2023;56(10):2188–205. [DOI] [PubMed] [Google Scholar]
- 17.Im SJ, Hashimoto M, Gerner MY, Lee J, Kissick HT, Burger MC, et al. Defining CD8+ T cells that provide the proliferative burst after PD-1 therapy. Nature. 2016;537(7620):417–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gebhardt T, Park SL, Parish IA. Stem-like exhausted and memory CD8+ T cells in cancer. Nat Rev Cancer. 2023;23(11):780–98. [DOI] [PubMed] [Google Scholar]
- 19.Siddiqui I, Schaeuble K, Chennupati V, Fuertes Marraco SA, Calderon-Copete S, Pais Ferreira D, et al. Intratumoral Tcf1+PD-1+CD8+ T Cells with stem-like properties promote tumor control in response to vaccination and checkpoint blockade immunotherapy. Immunity. 2019;50(1):195-211.e10. [DOI] [PubMed] [Google Scholar]
- 20.Im SJ, Obeng RC, Nasti TH, McManus D, Kamphorst AO, Gunisetty S, et al. Characteristics and anatomic location of PD-1 + TCF1 + stem-like CD8 T cells in chronic viral infection and cancer. Proc Natl Acad Sci USA. 2023;120(41): e2221985120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen JH, Nieman LT, Spurrell M, Jorgji V, Elmelech L, Richieri P, et al. Human lung cancer harbors spatially organized stem-immunity hubs associated with response to immunotherapy. Nat Immunol. 2024;25(4):644–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koh J, Kim S, Woo YD, Song SG, Yim J, Han B, et al. TCF1+PD-1+ tumour-infiltrating lymphocytes predict a favorable response and prolonged survival after immune checkpoint inhibitor therapy for non-small-cell lung cancer. Eur J Cancer. 2022;174:10–20. [DOI] [PubMed] [Google Scholar]
- 23.Miller BC, Sen DR, Al Abosy R, Bi K, Virkud YV, LaFleur MW, et al. Subsets of exhausted CD8+ T cells differentially mediate tumor control and respond to checkpoint blockade. Nat Immunol. 2019;20(3):326–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sade-Feldman M, Yizhak K, Bjorgaard SL, Ray JP, De Boer CG, Jenkins RW, et al. Defining T cell states associated with response to checkpoint immunotherapy in melanoma. Cell. 2018;175(4):998-1013.e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mayoux M, Roller A, Pulko V, Sammicheli S, Chen S, Sum E, et al. Dendritic cells dictate responses to PD-L1 blockade cancer immunotherapy. Sci Transl Med. 2020;12(534):eaav7431. [DOI] [PubMed] [Google Scholar]
- 26.Jansen CS, Prokhnevska N, Master VA, Sanda MG, Carlisle JW, Bilen MA, et al. An intra-tumoral niche maintains and differentiates stem-like CD8 T cells. Nature. 2019;576(7787):465–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Di Pilato M, Kfuri-Rubens R, Pruessmann JN, Ozga AJ, Messemaker M, Cadilha BL, et al. CXCR6 positions cytotoxic T cells to receive critical survival signals in the tumor microenvironment. Cell. 2021;184(17):4512-4530.e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schenkel JM, Herbst RH, Canner D, Li A, Hillman M, Shanahan SL, et al. Conventional type I dendritic cells maintain a reservoir of proliferative tumor-antigen specific TCF-1+ CD8+ T cells in tumor-draining lymph nodes. Immunity. 2021;54(10):2338-2353.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Humblin E, Korpas I, Lu J, Filipescu D, Van Der Heide V, Goldstein S, et al. Sustained CD28 costimulation is required for self-renewal and differentiation of TCF-1+ PD-1+ CD8 T cells. Sci Immunol. 2023;8(86):eadg0878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pittet MJ, Di Pilato M, Garris C, Mempel TR. Dendritic cells as shepherds of T cell immunity in cancer. Immunity. 2023;56(10):2218–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zagorulya M, Yim L, Morgan DM, Edwards A, Torres-Mejia E, Momin N, et al. Tissue-specific abundance of interferon-gamma drives regulatory T cells to restrain DC1-mediated priming of cytotoxic T cells against lung cancer. Immunity. 2023;56(2):386-405.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vanhersecke L, Brunet M, Guégan JP, Rey C, Bougouin A, Cousin S, et al. Mature tertiary lymphoid structures predict immune checkpoint inhibitor efficacy in solid tumors independently of PD-L1 expression. Nat Cancer. 2021;2(8):794–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brunet M, Crombé A, Cousin S, Vanhersecke L, Le Loarer F, Bessede A, et al. Mature tertiary lymphoid structure is a specific biomarker of cancer immunotherapy and does not predict outcome to chemotherapy in non-small-cell lung cancer. Ann Oncol. 2022;33(10):1084–5. [DOI] [PubMed] [Google Scholar]
- 34.Han J, Wu M, Liu Z. Dysregulation in IFN-γ signaling and response: the barricade to tumor immunotherapy. Front Immunol. 2023;14:1190333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Abiko K, Matsumura N, Hamanishi J, Horikawa N, Murakami R, Yamaguchi K, et al. IFN-γ from lymphocytes induces PD-L1 expression and promotes progression of ovarian cancer. Br J Cancer. 2015;112(9):1501–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Spranger S, Spaapen RM, Zha Y, Williams J, Meng Y, Ha TT, et al. Up-regulation of PD-L1, IDO, and Tregs in the melanoma tumor microenvironment is driven by CD8 + T cells. Sci Transl Med [Internet]. 2013 Aug 28 [cited 2024 Jul 13];5(200). 10.1126/scitranslmed.3006504 [DOI] [PMC free article] [PubMed]
- 37.Liberini V, Mariniello A, Righi L, Capozza M, Delcuratolo MD, Terreno E, et al. NSCLC biomarkers to predict response to immunotherapy with checkpoint inhibitors (ICI): from the cells to in vivo images. Cancers. 2021;13(18):4543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Herbst RS, Soria JC, Kowanetz M, Fine GD, Hamid O, Gordon MS, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515(7528):563–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Garcia-Diaz A, Shin DS, Moreno BH, Saco J, Escuin-Ordinas H, Rodriguez GA, et al. Interferon receptor signaling pathways regulating PD-L1 and PD-L2 expression. Cell Rep. 2017;19(6):1189–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Derynck R, Turley SJ, Akhurst RJ. TGFβ biology in cancer progression and immunotherapy. Nat Rev Clin Oncol. 2021;18(1):9–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Deng Z, Fan T, Xiao C, Tian H, Zheng Y, Li C, et al. TGF-β signaling in health, disease, and therapeutics. Sig Transduct Target Ther. 2024;9(1):61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Plikus MV, Wang X, Sinha S, Forte E, Thompson SM, Herzog EL, et al. Fibroblasts: origins, definitions, and functions in health and disease. Cell. 2021;184(15):3852–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mariathasan S, Turley SJ, Nickles D, Castiglioni A, Yuen K, Wang Y, et al. TGFβ attenuates tumour response to PD-L1 blockade by contributing to exclusion of T cells. Nature. 2018;554(7693):544–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Herzog BH, Baer JM, Borcherding N, Kingston NL, Belle JI, Knolhoff BL, et al. Tumor-associated fibrosis impairs immune surveillance and response to immune checkpoint blockade in non-small cell lung cancer. Sci Transl Med. 2023;15(699):eadh8005. [DOI] [PubMed] [Google Scholar]
- 45.Barry ST, Gabrilovich DI, Sansom OJ, Campbell AD, Morton JP. Therapeutic targeting of tumour myeloid cells. Nat Rev Cancer. 2023;23(4):216–37. [DOI] [PubMed] [Google Scholar]
- 46.Yu Z, Zou J, Xu F. Tumor-associated macrophages affect the treatment of lung cancer. Heliyon. 2024;10(7): e29332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jackute J, Zemaitis M, Pranys D, Sitkauskiene B, Miliauskas S, Vaitkiene S, et al. Distribution of M1 and M2 macrophages in tumor islets and stroma in relation to prognosis of non-small cell lung cancer. BMC Immunol. 2018;19(1):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Larroquette M, Guegan JP, Besse B, Cousin S, Brunet M, Le Moulec S, et al. Spatial transcriptomics of macrophage infiltration in non-small cell lung cancer reveals determinants of sensitivity and resistance to anti-PD1/PD-L1 antibodies. J Immunother Cancer. 2022;10(5): e003890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bill R, Wirapati P, Messemaker M, Roh W, Zitti B, Duval F, et al. CXCL9:SPP1 macrophage polarity identifies a network of cellular programs that control human cancers. Science. 2023;381(6657):515–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Adashek JJ, Subbiah IM, Matos I, Garralda E, Menta AK, Ganeshan DM, et al. Hyperprogression and immunotherapy: fact, fiction, or alternative fact? Trends Cancer. 2020;6(3):181–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lo Russo G, Moro M, Sommariva M, Cancila V, Boeri M, Centonze G, et al. Antibody-Fc/FcR interaction on macrophages as a mechanism for hyperprogressive disease in non-small cell lung cancer subsequent to PD-1/PD-L1 blockade. Clin Cancer Res. 2019;25(3):989–99. [DOI] [PubMed] [Google Scholar]
- 52.Shen L, Li Y, Zhao H. Fibroblast growth factor signaling in macrophage polarization: impact on health and diseases. Front Immunol. 2024;15:1390453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li G, Choi JE, Kryczek I, Sun Y, Liao P, Li S, et al. Intersection of immune and oncometabolic pathways drives cancer hyperprogression during immunotherapy. Cancer Cell. 2023;41(2):304-322.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Valero C, Lee M, Hoen D, Weiss K, Kelly DW, Adusumilli PS, et al. Pretreatment neutrophil-to-lymphocyte ratio and mutational burden as biomarkers of tumor response to immune checkpoint inhibitors. Nat Commun. 2021;12(1):729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mezquita L, Auclin E, Ferrara R, Charrier M, Remon J, Planchard D, et al. Association of the lung immune prognostic index with immune checkpoint inhibitor outcomes in patients with advanced non-small cell lung cancer. JAMA Oncol. 2018;4(3):351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mezquita L, Preeshagul I, Auclin E, Saravia D, Hendriks L, Rizvi H, et al. Predicting immunotherapy outcomes under therapy in patients with advanced NSCLC using dNLR and its early dynamics. Eur J Cancer. 2021;151:211–20. [DOI] [PubMed] [Google Scholar]
- 57.Kargl J, Zhu X, Zhang H, Yang GHY, Friesen TJ, Shipley M, et al. Neutrophil content predicts lymphocyte depletion and anti-PD1 treatment failure in NSCLC. JCI Insight. 2019;4(24): e130850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schalper KA, Carleton M, Zhou M, Chen T, Feng Y, Huang SP, et al. Elevated serum interleukin-8 is associated with enhanced intratumor neutrophils and reduced clinical benefit of immune-checkpoint inhibitors. Nat Med. 2020;26(5):688–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Banna GL, Cortellini A, Cortinovis DL, Tiseo M, Aerts JGJV, Barbieri F, et al. The lung immuno-oncology prognostic score (LIPS-3): a prognostic classification of patients receiving first-line pembrolizumab for PD-L1 ≥ 50% advanced non-small-cell lung cancer. ESMO Open. 2021;6(2): 100078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chang TG, Cao Y, Sfreddo HJ, Dhruba SR, Lee SH, Valero C, et al. LORIS robustly predicts patient outcomes with immune checkpoint blockade therapy using common clinical, pathologic and genomic features. Nat Cancer [Internet]. 2024 Jun 3 [cited 2024 Jul 1]. https://www.nature.com/articles/s43018-024-00772-7. [DOI] [PMC free article] [PubMed]
- 61.Zilionis R, Engblom C, Pfirschke C, Savova V, Zemmour D, Saatcioglu HD, et al. Single-cell transcriptomics of human and mouse lung cancers reveals conserved myeloid populations across individuals and species. Immunity. 2019;50(5):1317-1334.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Salcher S, Sturm G, Horvath L, Untergasser G, Kuempers C, Fotakis G, et al. High-resolution single-cell atlas reveals diversity and plasticity of tissue-resident neutrophils in non-small cell lung cancer. Cancer Cell. 2022;40(12):1503-1520.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Benguigui M, Cooper TJ, Kalkar P, Schif-Zuck S, Halaban R, Bacchiocchi A, et al. Interferon-stimulated neutrophils as a predictor of immunotherapy response. Cancer Cell. 2024;42(2):253-265.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ricciuti B, Lamberti G, Puchala SR, Mahadevan NR, Lin JR, Alessi JV, et al. Genomic and immunophenotypic landscape of acquired resistance to pd-(l)1 blockade in non–small-cell lung cancer. JCO. 2024;42(11):1311–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Qiu J, Xu B, Ye D, Ren D, Wang S, Benci JL, et al. Cancer cells resistant to immune checkpoint blockade acquire interferon-associated epigenetic memory to sustain T cell dysfunction. Nat Cancer [Internet]. 2023 Jan 16 [cited 2024 Jul 2]. https://www.nature.com/articles/s43018-022-00490-y. [DOI] [PubMed]
- 66.Benci JL, Johnson LR, Choa R, Xu Y, Qiu J, Zhou Z, et al. Opposing functions of interferon coordinate adaptive and innate immune responses to cancer immune checkpoint blockade. Cell. 2019;178(4):933-948.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Benci JL, Xu B, Qiu Y, Wu TJ, Dada H, Twyman-Saint Victor C, et al. Tumor interferon signaling regulates a multigenic resistance program to immune checkpoint blockade. Cell. 2016;167(6):1540-1554.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Minn AJ, Wherry EJ. Combination cancer therapies with immune checkpoint blockade: convergence on interferon signaling. Cell. 2016;165(2):272–5. [DOI] [PubMed] [Google Scholar]
- 69.Pai JA, Hellmann MD, Sauter JL, Mattar M, Rizvi H, Woo HJ, et al. Lineage tracing reveals clonal progenitors and long-term persistence of tumor-specific T cells during immune checkpoint blockade. Cancer Cell. 2023;41(4):776-790.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hiltbrunner S, Cords L, Kasser S, Freiberger SN, Kreutzer S, Toussaint NC, et al. Acquired resistance to anti-PD1 therapy in patients with NSCLC associates with immunosuppressive T cell phenotype. Nat Commun. 2023;14(1):5154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kamphorst AO, Pillai RN, Yang S, Nasti TH, Akondy RS, Wieland A, et al. Proliferation of PD-1+ CD8 T cells in peripheral blood after PD-1–targeted therapy in lung cancer patients. Proc Natl Acad Sci USA. 2017;114(19):4993–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gill AL, Wang PH, Lee J, Hudson WH, Ando S, Araki K, et al. PD-1 blockade increases the self-renewal of stem-like CD8 T cells to compensate for their accelerated differentiation into effectors. Sci Immunol. 2023;8(86):eadg0539. 10.1126/sciimmunol.adg0539. [DOI] [PMC free article] [PubMed]
- 73.Obeng RC, Nasti TH, Martens K, Li P, Mariniello A, Chang DY, et al. Characterisation of clinical response and transcriptional profiling of proliferating CD8 T cells in the blood of cancer patients after PD-1 monotherapy or combination therapy. bmjonc. 2024;3(1):e000328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wang M, Wang S, Trapani JA, Neeson PJ. Challenges of PD-L1 testing in non-small cell lung cancer and beyond. J Thorac Dis. 2020;12(8):4541–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Reuben A, Zhang J, Chiou SH, Gittelman RM, Li J, Lee WC, et al. Comprehensive T cell repertoire characterization of non-small cell lung cancer. Nat Commun. 2020;11(1):603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Marabelle A, Fakih M, Lopez J, Shah M, Shapira-Frommer R, Nakagawa K, et al. Association of tumour mutational burden with outcomes in patients with advanced solid tumours treated with pembrolizumab: prospective biomarker analysis of the multicohort, open-label, phase 2 KEYNOTE-158 study. Lancet Oncol. 2020;21(10):1353–65. [DOI] [PubMed] [Google Scholar]
- 77.De Castro G, Rizvi NA, Schmid P, Syrigos K, Martin C, Yamamoto N, et al. NEPTUNE: phase 3 study of first-line durvalumab plus tremelimumab in patients with metastatic NSCLC. J Thorac Oncol. 2023;18(1):106–19. [DOI] [PubMed] [Google Scholar]
- 78.Hellmann MD, Paz-Ares L, Bernabe Caro R, Zurawski B, Kim SW, Carcereny Costa E, et al. Nivolumab plus ipilimumab in advanced non-small-cell lung cancer. N Engl J Med. 2019;381(21):2020–31. [DOI] [PubMed] [Google Scholar]
- 79.Wang Z, Duan J, Wang G, Zhao J, Xu J, Han J, et al. Allele frequency-adjusted blood-based tumor mutational burden as a predictor of overall survival for patients with NSCLC Treated With PD-(L)1 inhibitors. J Thorac Oncol. 2020;15(4):556–67. [DOI] [PubMed] [Google Scholar]
- 80.Cai L, Bai H, Duan J, Wang Z, Gao S, Wang D, et al. Epigenetic alterations are associated with tumor mutation burden in non-small cell lung cancer. J Immunother Cancer. 2019;7(1):198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Marabelle A, Le DT, Ascierto PA, Di Giacomo AM, De Jesus-Acosta A, Delord JP, et al. Efficacy of pembrolizumab in patients with noncolorectal high microsatellite instability/mismatch repair-deficient cancer: results from the phase II KEYNOTE-158 study. JCO. 2020;38(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ambrosini M, Rousseau B, Manca P, Artz O, Marabelle A, André T, et al. Immune checkpoint inhibitors for POLE or POLD1 proofreading-deficient metastatic colorectal cancer. Ann Oncol. 2024;35(7):643–55. [DOI] [PubMed] [Google Scholar]
- 83.André T, Shiu KK, Kim TW, Jensen BV, Jensen LH, Punt C, et al. Pembrolizumab in microsatellite-instability-high advanced colorectal cancer. N Engl J Med. 2020;383(23):2207–18. [DOI] [PubMed] [Google Scholar]
- 84.El-Osta H, Jafri S. Predictors for clinical benefit of immune checkpoint inhibitors in advanced non-small-cell lung cancer: a meta-analysis. Immunotherapy. 2019;11(3):189–99. [DOI] [PubMed] [Google Scholar]
- 85.Wang GZ, Zhang L, Zhao XC, Gao SH, Qu LW, Yu H, et al. The aryl hydrocarbon receptor mediates tobacco-induced PD-L1 expression and is associated with response to immunotherapy. Nat Commun. 2019;10(1):1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lau SCM, Perdrizet K, Fung AS, Mata DGMM, Weiss J, Holzapfel N, et al. Programmed cell death protein 1 inhibitors and MET targeted therapies in NSCLC with MET exon 14 skipping mutations: efficacy and toxicity as sequential therapies. JTO Clin Res Rep. 2023;4(10): 100562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Negrao MV, Skoulidis F, Montesion M, Schulze K, Bara I, Shen V, et al. Oncogene-specific differences in tumor mutational burden, PD-L1 expression, and outcomes from immunotherapy in non-small cell lung cancer. J Immunother Cancer. 2021;9(8): e002891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Skoulidis F, Byers LA, Diao L, Papadimitrakopoulou VA, Tong P, Izzo J, et al. Co-occurring genomic alterations define major subsets of KRAS -mutant lung adenocarcinoma with distinct biology, immune profiles, and therapeutic vulnerabilities. Cancer Discov. 2015;5(8):860–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Skoulidis F, Goldberg ME, Greenawalt DM, Hellmann MD, Awad MM, Gainor JF, et al. STK11/LKB1 mutations and PD-1 inhibitor resistance in KRAS-mutant lung adenocarcinoma. Cancer Discov. 2018;8(7):822–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ricciuti B, Arbour KC, Lin JJ, Vajdi A, Vokes N, Hong L, et al. Diminished efficacy of programmed death-(ligand)1 inhibition in STK11- and KEAP1-mutant lung adenocarcinoma is affected by KRAS mutation status. J Thorac Oncol. 2022;17(3):399–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Alexander A, Walker CL. The role of LKB1 and AMPK in cellular responses to stress and damage. FEBS Lett. 2011;585(7):952–7. [DOI] [PubMed] [Google Scholar]
- 92.Qian Y, Galan-Cobo A, Guijarro I, Dang M, Molkentine D, Poteete A, et al. MCT4-dependent lactate secretion suppresses antitumor immunity in LKB1-deficient lung adenocarcinoma. Cancer Cell. 2023;41(7):1363-1380.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhang DD, Lo SC, Cross JV, Templeton DJ, Hannink M. Keap1 is a redox-regulated substrate adaptor protein for a Cul3-dependent ubiquitin ligase complex. Mol Cell Biol. 2004;24(24):10941–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Taguchi K, Motohashi H, Yamamoto M. Molecular mechanisms of the Keap1-Nrf2 pathway in stress response and cancer evolution: molecular mechanisms of the Keap1-Nrf2 pathway. Genes Cells. 2011;16(2):123–40. [DOI] [PubMed] [Google Scholar]
- 95.Scalera S, Mazzotta M, Cortile C, Krasniqi E, De Maria R, Cappuzzo F, et al. KEAP1-mutant NSCLC: the catastrophic failure of a cell-protecting hub. J Thorac Oncol. 2022;17(6):751–7. [DOI] [PubMed] [Google Scholar]
- 96.Yonesaka K, Zejnullahu K, Lindeman N, Homes AJ, Jackman DM, Zhao F, et al. Autocrine production of amphiregulin predicts sensitivity to both gefitinib and cetuximab in EGFR wild-type cancers. Clin Cancer Res. 2008;14(21):6963–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zaiss DMW, van Loosdregt J, Gorlani A, Bekker CPJ, Gröne A, Sibilia M, et al. Amphiregulin enhances regulatory T cell-suppressive function via the epidermal growth factor receptor. Immunity. 2013;38(2):275–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sumimoto H, Imabayashi F, Iwata T, Kawakami Y. The BRAF–MAPK signaling pathway is essential for cancer-immune evasion in human melanoma cells. J Exp Med. 2006;203(7):1651–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lin Z, Huang L, Li SL, Gu J, Cui X, Zhou Y. PTEN loss correlates with T cell exclusion across human cancers. BMC Cancer. 2021;21(1):429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Peng W, Chen JQ, Liu C, Malu S, Creasy C, Tetzlaff MT, et al. Loss of PTEN promotes resistance to T cell-mediated immunotherapy. Cancer Discov. 2016;6(2):202–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Spranger S, Bao R, Gajewski TF. Melanoma-intrinsic β-catenin signalling prevents anti-tumour immunity. Nature. 2015;523(7559):231–5. [DOI] [PubMed] [Google Scholar]
- 102.Han G, Yang G, Hao D, Lu Y, Thein K, Simpson BS, et al. 9p21 loss confers a cold tumor immune microenvironment and primary resistance to immune checkpoint therapy. Nat Commun. 2021;12(1):5606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Shin DS, Zaretsky JM, Escuin-Ordinas H, Garcia-Diaz A, Hu-Lieskovan S, Kalbasi A, et al. Primary resistance to PD-1 blockade mediated by JAK1/2 mutations. Cancer Discov. 2017;7(2):188–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zaretsky JM, Garcia-Diaz A, Shin DS, Escuin-Ordinas H, Hugo W, Hu-Lieskovan S, et al. Mutations associated with acquired resistance to PD-1 blockade in melanoma. N Engl J Med. 2016;375(9):819–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gao J, Shi LZ, Zhao H, Chen J, Xiong L, He Q, et al. Loss of IFN-γ pathway genes in tumor cells as a mechanism of resistance to anti-CTLA-4 therapy. Cell. 2016;167(2):397-404.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Mojic M, Takeda K, Hayakawa Y. The dark side of IFN-γ: its role in promoting cancer immunoevasion. IJMS. 2017;19(1):89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.D’Urso CM, Wang ZG, Cao Y, Tatake R, Zeff RA, Ferrone S. Lack of HLA class I antigen expression by cultured melanoma cells FO-1 due to a defect in B2m gene expression. J Clin Investig. 1991;87(1):284–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sucker A, Zhao F, Real B, Heeke C, Bielefeld N, Maβen S, et al. Genetic evolution of T-cell resistance in the course of melanoma progression. Clin Cancer Res. 2014;20(24):6593–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Clocchiatti A, Cora E, Zhang Y, Dotto GP. Sexual dimorphism in cancer. Nat Rev Cancer. 2016;16(5):330–9. [DOI] [PubMed] [Google Scholar]
- 110.Lai JJ, Lai KP, Zeng W, Chuang KH, Altuwaijri S, Chang C. Androgen receptor influences on body defense system via modulation of innate and adaptive immune systems: lessons from conditional AR knockout mice. Am J Pathol. 2012;181(5):1504–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Huang Y, Zhao JJ, Soon YY, Kee A, Tay SH, Aminkeng F, et al. Factors predictive of primary resistance to immune checkpoint inhibitors in patients with advanced non-small cell lung cancer. Cancers. 2023;15(10):2733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Conforti F, Pala L, Pagan E, Bagnardi V, De Pas T, Queirolo P, et al. Sex-based dimorphism of anticancer immune response and molecular mechanisms of immune evasion. Clin Cancer Res. 2021;27(15):4311–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Conforti F, Pala L, Pagan E, Corti C, Bagnardi V, Queirolo P, et al. Sex-based differences in response to anti-PD-1 or PD-L1 treatment in patients with non-small-cell lung cancer expressing high PD-L1 levels. A systematic review and meta-analysis of randomized clinical trials. ESMO Open. 2021;6(5):100251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Conforti F, Pala L, Bagnardi V, Viale G, De Pas T, Pagan E, et al. Sex-based heterogeneity in response to lung cancer immunotherapy: a systematic review and meta-analysis. JNCI J Natl Cancer Inst. 2019;111(8):772–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Colombo N, Dubot C, Lorusso D, Caceres MV, Hasegawa K, Shapira-Frommer R, et al. Pembrolizumab for persistent, recurrent, or metastatic cervical cancer. N Engl J Med. 2021;385(20):1856–67. [DOI] [PubMed] [Google Scholar]
- 116.Eskander RN, Sill MW, Beffa L, Moore RG, Hope JM, Musa FB, et al. Pembrolizumab plus chemotherapy in advanced endometrial cancer. N Engl J Med. 2023;388(23):2159–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Schmid P, Cortes J, Dent R, Pusztai L, McArthur H, Kümmel S, et al. Event-free survival with pembrolizumab in early triple-negative breast cancer. N Engl J Med. 2022;386(6):556–67. [DOI] [PubMed] [Google Scholar]
- 118.Wu Y, Ju Q, Jia K, Yu J, Shi H, Wu H, et al. Correlation between sex and efficacy of immune checkpoint inhibitors (PD-1 and CTLA-4 inhibitors). Int J Cancer. 2018;143(1):45–51. [DOI] [PubMed] [Google Scholar]
- 119.Sivan A, Corrales L, Hubert N, Williams JB, Aquino-Michaels K, Earley ZM, et al. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science. 2015;350(6264):1084–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Gopalakrishnan V, Spencer CN, Nezi L, Reuben A, Andrews MC, Karpinets TV, et al. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science. 2018;359(6371):97–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Davar D, Dzutsev AK, McCulloch JA, Rodrigues RR, Chauvin JM, Morrison RM, et al. Fecal microbiota transplant overcomes resistance to anti-PD-1 therapy in melanoma patients. Science. 2021;371(6529):595–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Routy B, Le Chatelier E, Derosa L, Duong CPM, Alou MT, Daillère R, et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science. 2018;359(6371):91–7. [DOI] [PubMed] [Google Scholar]
- 123.Derosa L, Hellmann MD, Spaziano M, Halpenny D, Fidelle M, Rizvi H, et al. Negative association of antibiotics on clinical activity of immune checkpoint inhibitors in patients with advanced renal cell and non-small-cell lung cancer. Ann Oncol. 2018;29(6):1437–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Hakozaki T, Richard C, Elkrief A, Hosomi Y, Benlaïfaoui M, Mimpen I, et al. The gut microbiome associates with immune checkpoint inhibition outcomes in patients with advanced non-small cell lung cancer. Cancer Immunol Res. 2020;8(10):1243–50. [DOI] [PubMed] [Google Scholar]
- 125.Fang S, Wang Y, Dang Y, Gagel A, Ross MI, Gershenwald JE, et al. Association between body mass index, C-reactive protein levels, and melanoma patient outcomes. J Investig Dermatol. 2017;137(8):1792–5. [DOI] [PubMed] [Google Scholar]
- 126.McQuade JL, Daniel CR, Hess KR, Mak C, Wang DY, Rai RR, et al. Association of body-mass index and outcomes in patients with metastatic melanoma treated with targeted therapy, immunotherapy, or chemotherapy: a retrospective, multicohort analysis. Lancet Oncol. 2018;19(3):310–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Khojandi N, Kuehm LM, Piening A, Donlin MJ, Hsueh EC, Schwartz TL, et al. Oxidized lipoproteins promote resistance to cancer immunotherapy independent of patient obesity. Cancer Immunol Res. 2021;9(2):214–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wu W, Shi X, Xu C. Regulation of T cell signalling by membrane lipids. Nat Rev Immunol. 2016;16(11):690–701. [DOI] [PubMed] [Google Scholar]
- 129.Chowell D, Morris LGT, Grigg CM, Weber JK, Samstein RM, Makarov V, et al. Patient HLA class I genotype influences cancer response to checkpoint blockade immunotherapy. Science. 2018;359(6375):582–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Socinski MA, Jotte RM, Cappuzzo F, Orlandi F, Stroyakovskiy D, Nogami N, et al. Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N Engl J Med. 2018;378(24):2288–301. [DOI] [PubMed] [Google Scholar]
- 131.Ren S, He J, Fang Y, Chen G, Ma Z, Chen J, et al. Camrelizumab plus apatinib in treatment-naive patients with advanced nonsquamous NSCLC: a multicenter, open-label, single-arm, phase 2 trial. JTO Clin Res Rep. 2022;3(5): 100312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Yang JCH, Han B, De La Mora JE, Lee JS, Koralewski P, Karadurmus N, et al. Pembrolizumab with or without lenvatinib for first-line metastatic NSCLC with programmed cell death-ligand 1 tumor proportion score of at least 1% (LEAP-007): a randomized, double-blind, phase 3 trial. J Thorac Oncol. 2024;19(6):941–53. [DOI] [PubMed] [Google Scholar]
- 133.Ivonescimab monotherapy decisively beats pembrolizumab monotherapy head-to-head, achieves statistically significant superiority in PFS in first-line treatment of patients with PD-L1 positive NSCLC in China. News release. Summit Therapeutics. May 30, 2024. https://www.businesswire.com/news/home/20240530536730/en. Accessed 14 July 2024.
- 134.Shawky AM, Almalki FA, Abdalla AN, Abdelazeem AH, Gouda AM. A comprehensive overview of globally approved JAK inhibitors. Pharmaceutics. 2022;14(5):1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Fogelman D, Cubillo A, García-Alfonso P, Mirón MLL, Nemunaitis J, Flora D, et al. Randomized, double-blind, phase two study of ruxolitinib plus regorafenib in patients with relapsed/refractory metastatic colorectal cancer. Cancer Med. 2018;7(11):5382–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Hurwitz HI, Uppal N, Wagner SA, Bendell JC, Beck JT, Wade SM, et al. Randomized, double-blind, phase ii study of ruxolitinib or placebo in combination with capecitabine in patients with metastatic pancreatic cancer for whom therapy with gemcitabine has failed. JCO. 2015;33(34):4039–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Giaccone G, Sanborn RE, Waqar SN, Martinez-Marti A, Ponce S, Zhen H, et al. A placebo-controlled phase II study of ruxolitinib in combination with pemetrexed and cisplatin for first-line treatment of patients with advanced nonsquamous non-small-cell lung cancer and systemic inflammation. Clin Lung Cancer. 2018;19(5):e567–74. [DOI] [PubMed] [Google Scholar]
- 138.Mathew D, Marmarelis ME, Foley C, Bauml JM, Ye D, Ghinnagow R, et al. Combined JAK inhibition and PD-1 immunotherapy for non-small cell lung cancer patients. Science. 2024;384(6702):eadf1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Zak J, Pratumchai I, Marro BS, Marquardt KL, Zavareh RB, Lairson LL, et al. JAK inhibition enhances checkpoint blockade immunotherapy in patients with Hodgkin lymphoma. Science. 2024;384(6702):eade8520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Boyer M, Şendur MAN, Rodríguez-Abreu D, Park K, Lee DH, Çiçin I, et al. Pembrolizumab plus ipilimumab or placebo for metastatic non-small-cell lung cancer with PD-L1 tumor proportion score ≥ 50%: randomized, double-blind phase III KEYNOTE-598 study. JCO. 2021;39(21):2327–38. [DOI] [PubMed] [Google Scholar]
- 141.Johnson ML, Cho BC, Luft A, Alatorre-Alexander J, Geater SL, Laktionov K, et al. Durvalumab with or without tremelimumab in combination with chemotherapy as first-line therapy for metastatic non-small-cell lung cancer: the phase III POSEIDON study. JCO. 2023;41(6):1213–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Skoulidis F, Araujo HA, Do MT, Qian Y, Sun X, Cobo AG, et al. CTLA4 blockade abrogates KEAP1/STK11-related resistance to PD-(L)1 inhibitors. Nature. 2024;635(8038):462–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Hasan MF, Croom-Perez TJ, Oyer JL, Dieffenthaller TA, Robles-Carrillo LD, Eloriaga JE, et al. TIGIT expression on activated nk cells correlates with greater anti-tumor activity but promotes functional decline upon lung cancer exposure: implications for adoptive cell therapy and TIGIT-targeted therapies. Cancers. 2023;15(10):2712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Genentech: Press Releases|Tuesday, Aug 22, 2023. https://www.gene.com/media/press-releases/14998/2023-08-22/genentech-provides-update-on-phase-iii-s. Accessed 9 July 2024.
- 145.Genentech: Press Releases|Wednesday, Jul 3, 2024. https://www.gene.com/media/press-releases/15029/2024-07-03/genentech-provides-update-on-phase-iiiii. Accessed 14 July 2024.
- 146.Tawbi HA, Schadendorf D, Lipson EJ, Ascierto PA, Matamala L, Castillo Gutiérrez E, et al. Relatlimab and nivolumab versus nivolumab in untreated advanced melanoma. N Engl J Med. 2022;386(1):24–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Cai L, Li Y, Tan J, Xu L, Li Y. Targeting LAG-3, TIM-3, and TIGIT for cancer immunotherapy. J Hematol Oncol. 2023;16(1):101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Friedlaender A, Addeo A, Banna G. New emerging targets in cancer immunotherapy: the role of TIM3. ESMO Open. 2019;4(Suppl 3): e000497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Zhuang X, Zhang X, Xia X, Zhang C, Liang X, Gao L, et al. Ectopic expression of TIM-3 in lung cancers. Am J Clin Pathol. 2012;137(6):978–85. [DOI] [PubMed] [Google Scholar]
- 150.Falchook GS, Ribas A, Davar D, Eroglu Z, Wang JS, Luke JJ, et al. Phase 1 trial of TIM-3 inhibitor cobolimab monotherapy and in combination with PD-1 inhibitors nivolumab or dostarlimab (AMBER). JCO. 2022;40(16_suppl):2504–2504. [Google Scholar]
- 151.Singh R, Kim YH, Lee SJ, Eom HS, Choi BK. 4-1BB immunotherapy: advances and hurdles. Exp Mol Med. 2024;56(1):32–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Lundqvist A, Van Hoef V, Zhang X, Wennerberg E, Lorent J, Witt K, et al. 31st Annual Meeting and Associated Programs of the Society for Immunotherapy of Cancer (SITC 2016): part one: National Harbor, MD, USA. 9–13 November 2016. J Immunother Cancer. 2016;4(S1):82. s40425-016-0172–7.
- 153.Borghaei H, De Marinis F, Dumoulin D, Reynolds C, Theelen WSME, Percent I, et al. SAPPHIRE: phase III study of sitravatinib plus nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. Ann Oncol. 2024;35(1):66–76. [DOI] [PubMed] [Google Scholar]
- 154.Neal J, Pavlakis N, Kim SW, Goto Y, Lim SM, Mountzios G, et al. CONTACT-01: a randomized phase iii trial of atezolizumab + cabozantinib versus docetaxel for metastatic non-small cell lung cancer after a checkpoint inhibitor and chemotherapy. JCO. 2024;42(20):2393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Leighl N, Paz-Ares L, Rodrigues Abreu DS, et al. Phase 3 LEAP-008 study of lenvatinib plus pembrolizumab versus docetaxel for metastatic non-small cell lung cancer (NSCLC) that progressed on a PD-(L)1 inhibitor and platinum-containing chemotherapy. Ann Oncol. 2023;20(suppl 1):100535. 10.1016/iotech/iotech100535. [Google Scholar]
- 156.Barlesi F, Isambert N, Felip E, Cho BC, Lee DH, Peguero J, et al. Bintrafusp alfa, a bifunctional fusion protein targeting TGF-β and PD-L1, in patients with non-small cell lung cancer resistant or refractory to immune checkpoint inhibitors. Oncologist. 2023;28(3):258–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Felip E, Krebs MG, Carcereny E, Smeland KB, Arriola E, Llacer Perez C, et al. 1440P Final top-line results of the BGBC008 phase II, multicenter study of bemcentinib and pembrolizumab (bem+pembro) in second-line (2L) advanced non-squamous (NS) non-small cell lung cancer (NSCLC) (NCT03184571). Ann Oncol. 2023;34:S819. [Google Scholar]
- 158.Li H, Liu Z, Liu L, Zhang H, Han C, Girard L, et al. AXL targeting restores PD-1 blockade sensitivity of STK11/LKB1 mutant NSCLC through expansion of TCF1+ CD8 T cells. Cell Rep Med. 2022;3(3): 100554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Reckamp KL, Redman MW, Dragnev KH, Minichiello K, Villaruz LC, Faller B, et al. Phase II randomized study of ramucirumab and pembrolizumab versus standard of care in advanced non-small-cell lung cancer previously treated with immunotherapy—lung-MAP S1800A. JCO. 2022;40(21):2295–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.LaMarche NM, Hegde S, Park MD, Maier BB, Troncoso L, Le Berichel J, et al. An IL-4 signalling axis in bone marrow drives pro-tumorigenic myelopoiesis. Nature. 2024;625(7993):166–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Hong DS, Gopal AK, Shoushtari AN, Patel SP, He AR, Doi T, et al. Utomilumab in patients with immune checkpoint inhibitor-refractory melanoma and non-small-cell lung cancer. Front Immunol. 2022;13: 897991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Wrangle JM, Redman MW, Husain H, Reckamp KL, Stinchcombe T, Edelman MJ, et al. Lung-MAP S1800D: A phase II/III study of N-803 (ALT-803) plus pembrolizumab versus standard of care in participants with stage IV or recurrent non-small cell lung cancer (NSCLC) previously treated with anti-PD-1 or anti-PD-L1 therapy. JCO. 2024;42(16_suppl):2619–2619. [Google Scholar]
- 163.Besse B, Felip E, Garcia Campelo R, Cobo M, Mascaux C, Madroszyk A, et al. Randomized open-label controlled study of cancer vaccine OSE2101 versus chemotherapy in HLA-A2-positive patients with advanced non-small-cell lung cancer with resistance to immunotherapy: ATALANTE-1. Ann Oncol. 2023;34(10):920–33. [DOI] [PubMed] [Google Scholar]
- 164.Creelan BC, Wang C, Teer JK, Toloza EM, Yao J, Kim S, et al. Tumor-infiltrating lymphocyte treatment for anti-PD-1-resistant metastatic lung cancer: a phase 1 trial. Nat Med. 2021;27(8):1410–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Schoenfeld AJ, Lee SM, Doger De Spéville B, Gettinger SN, Häfliger S, Sukari A, et al. Lifileucel, an autologous tumor-infiltrating lymphocyte monotherapy, in patients with advanced non-small cell lung cancer resistant to immune checkpoint inhibitors. Cancer Discov. 2024;OF1–14. [DOI] [PMC free article] [PubMed]
- 166.Ma H, Das J, Prendergast C, De Jong D, Braumuller B, Paily J, et al. Advances in CAR T cell therapy for non-small cell lung cancer. CIMB. 2023;45(11):9019–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Lin Y, Chen S, Zhong S, An H, Yin H, McGowan E. Phase I clinical trial of PD-1 knockout anti-MUC1 CAR-T cells in the treatment of patients with non-small cell lung cancer. Ann Oncol. 2019;30:xi12. [Google Scholar]
- 168.Sivapalan L, Murray JC, Canzoniero JV, Landon B, Jackson J, Scott S, et al. Liquid biopsy approaches to capture tumor evolution and clinical outcomes during cancer immunotherapy. J Immunother Cancer. 2023;11(1): e005924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Anagnostou V, Ho C, Nicholas G, Juergens RA, Sacher A, Fung AS, et al. ctDNA response after pembrolizumab in non-small cell lung cancer: phase 2 adaptive trial results. Nat Med. 2023;29(10):2559–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Zemek RM, Anagnostou V, Pires Da Silva I, Long GV, Lesterhuis WJ. Exploiting temporal aspects of cancer immunotherapy. Nat Rev Cancer. 2024;24(7):480–97. [DOI] [PubMed] [Google Scholar]