Abstract
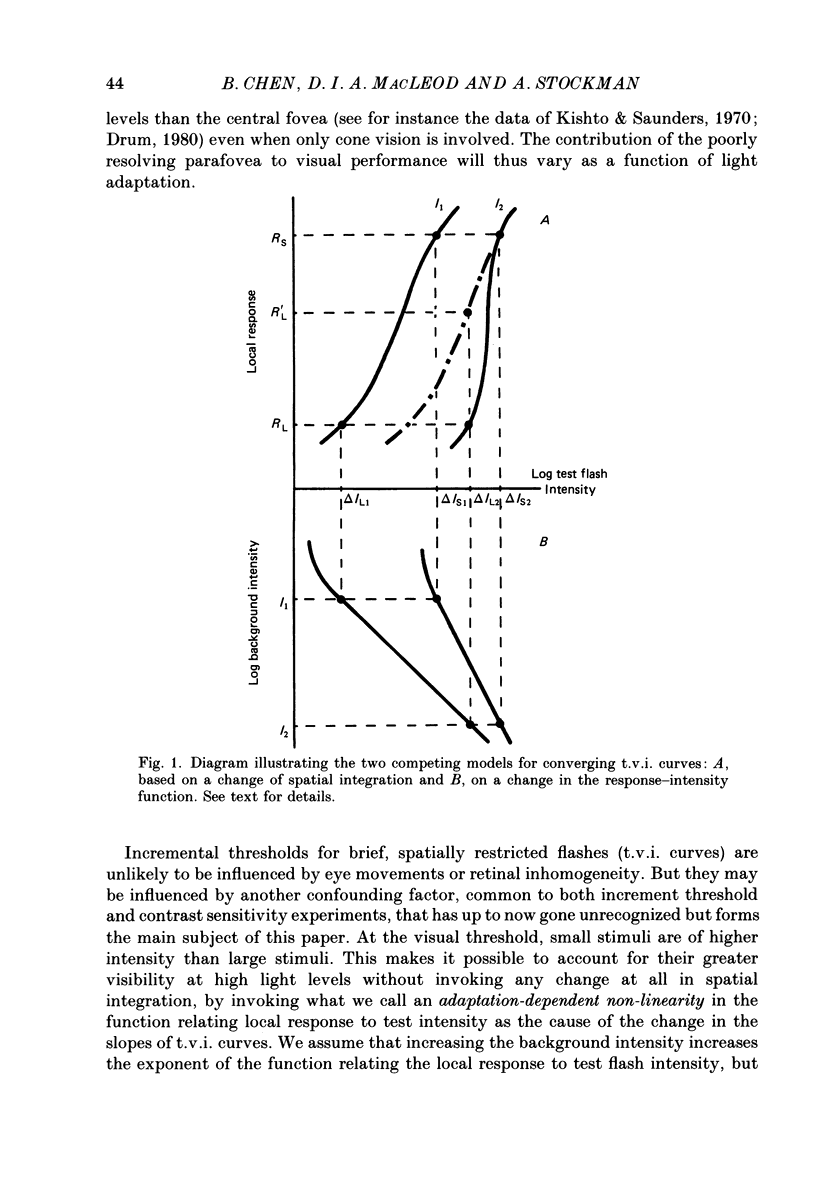
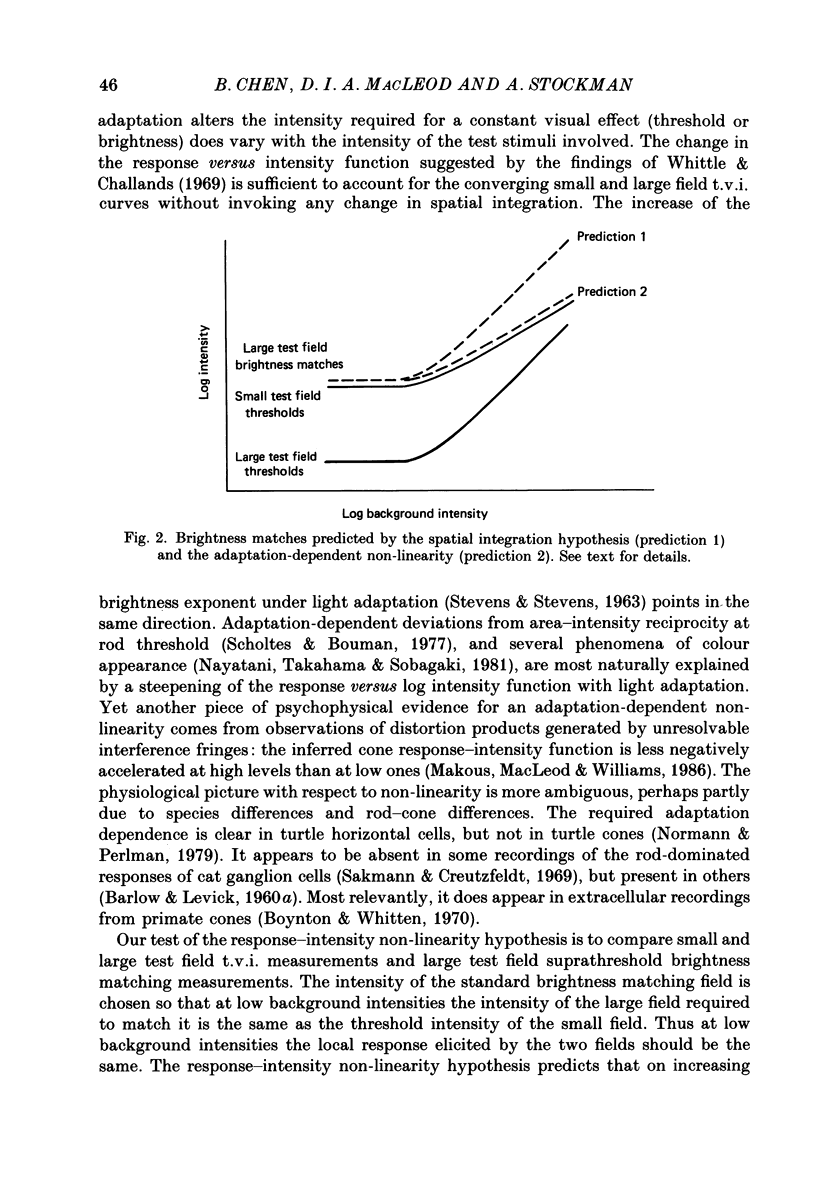
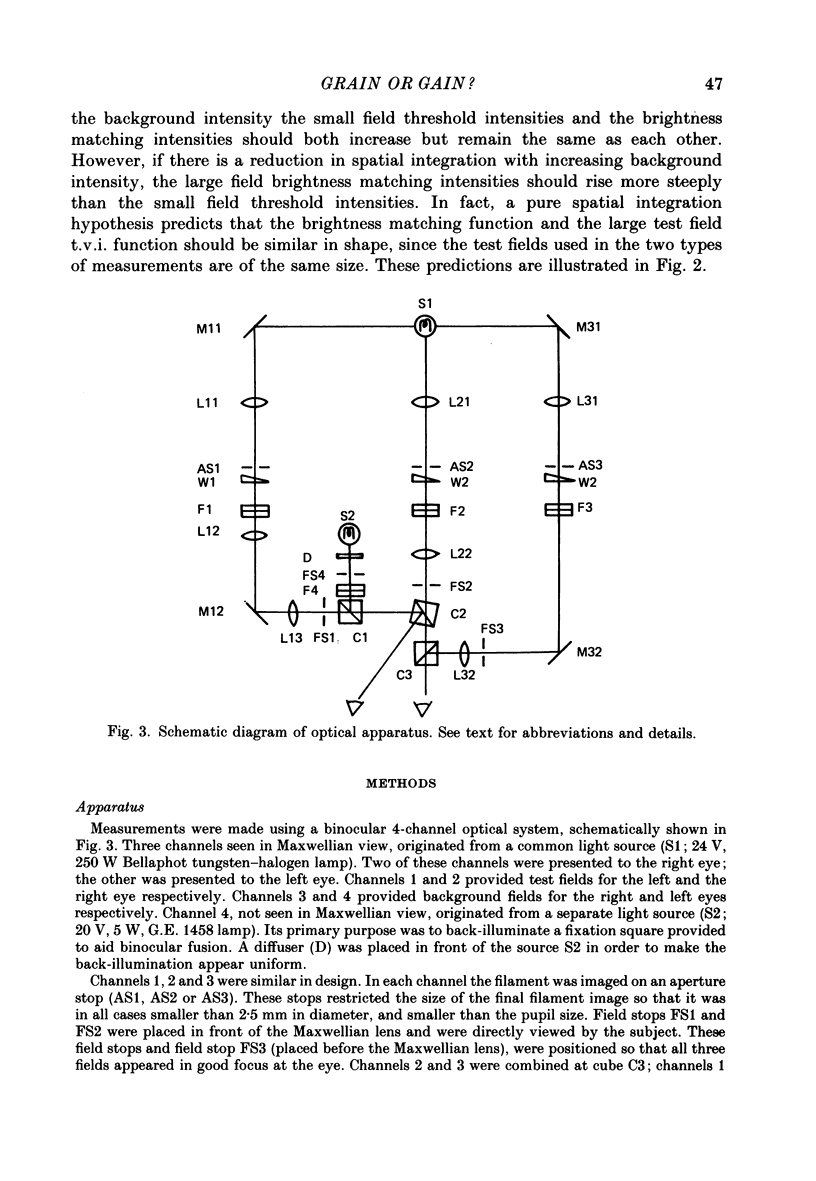
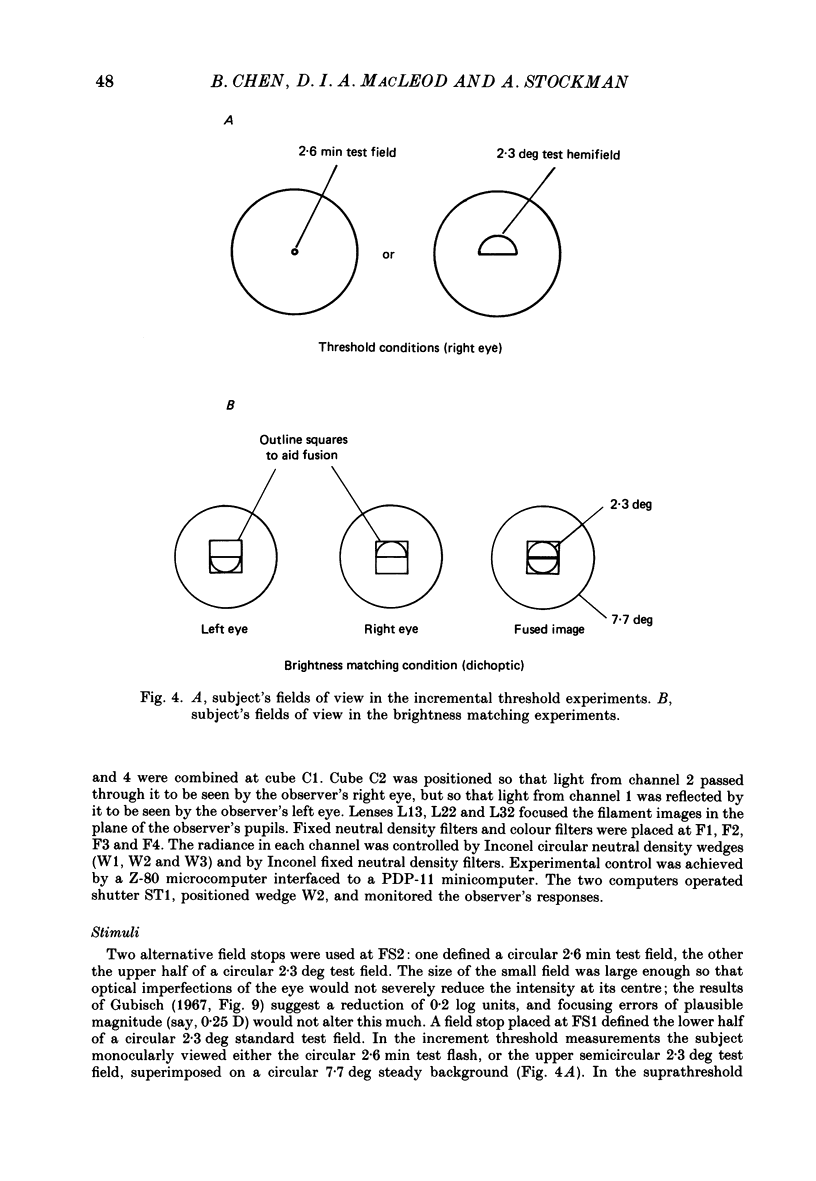
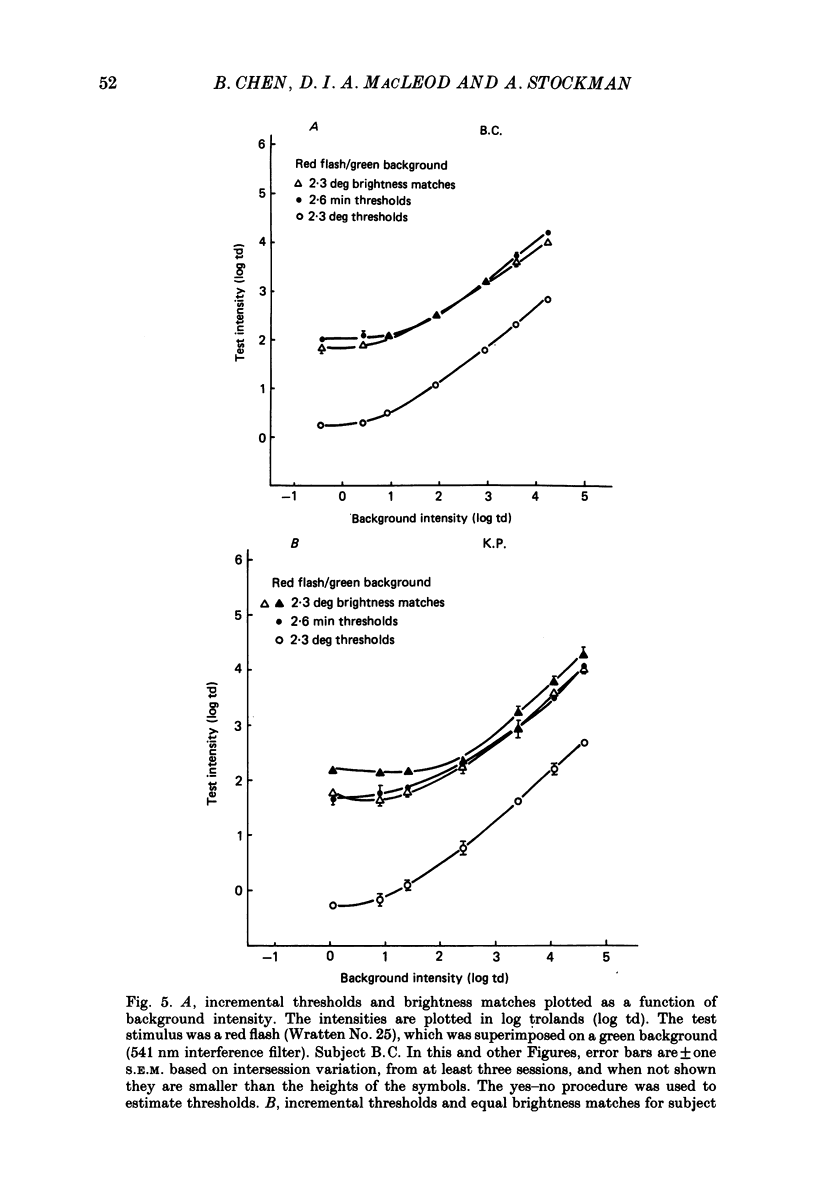
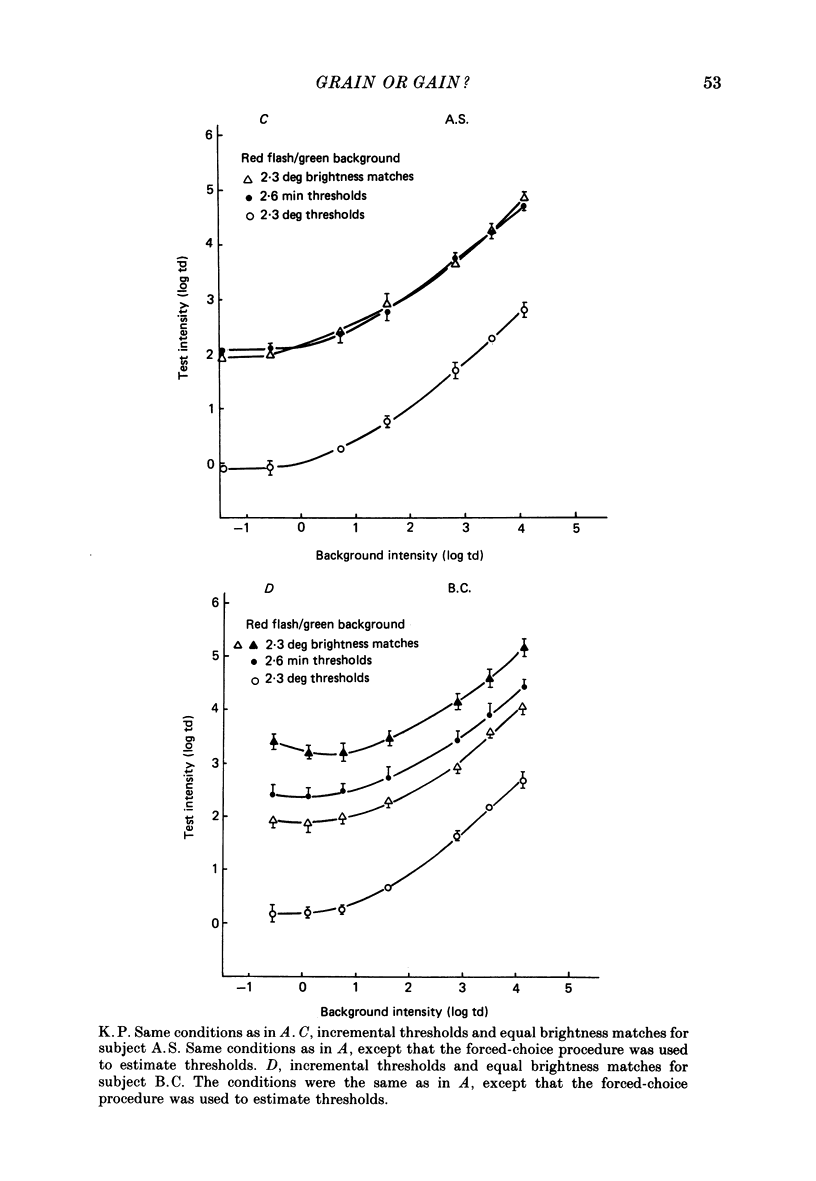
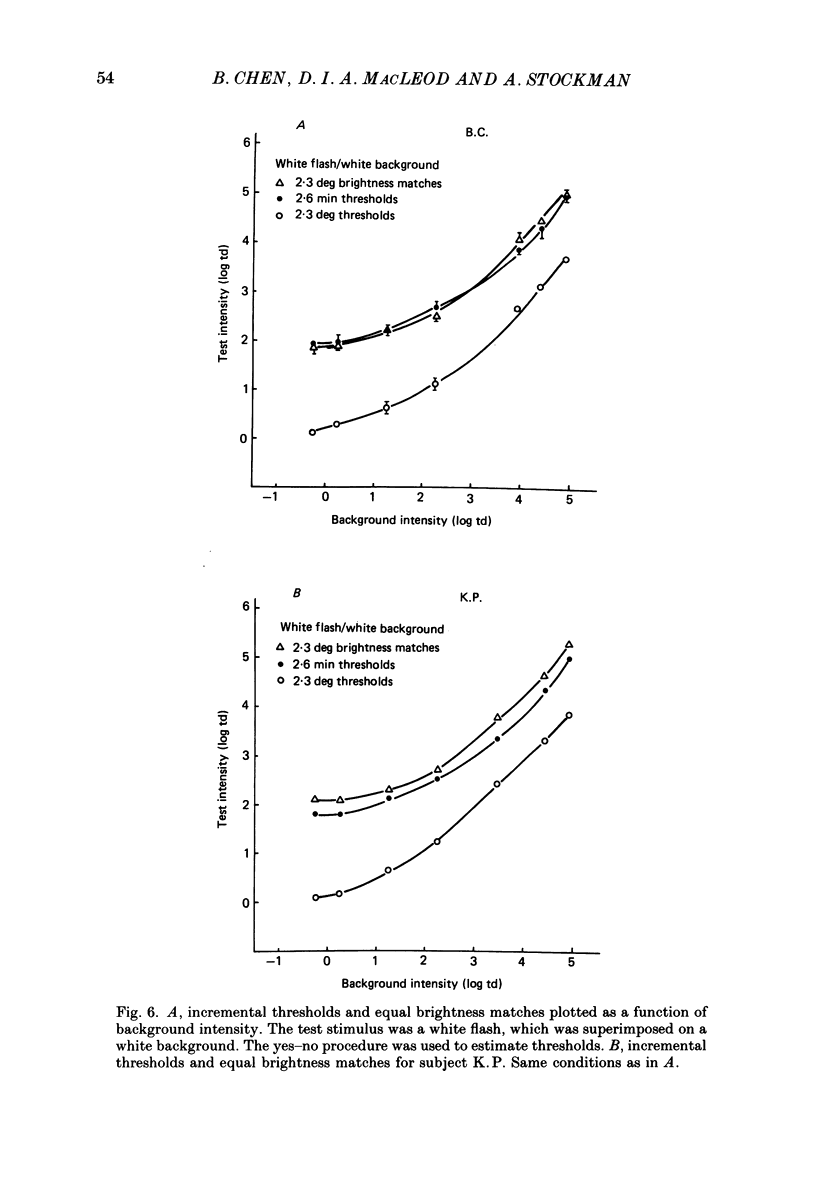
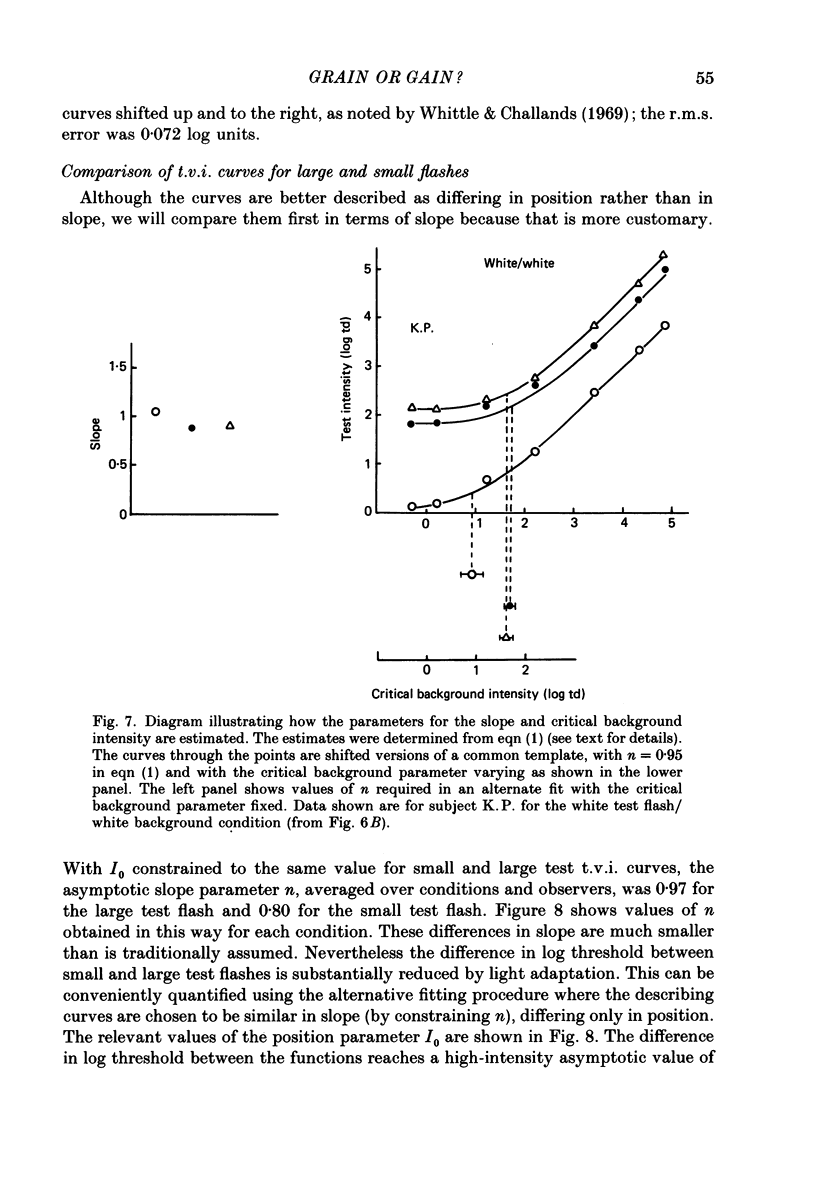
1. The factor by which increment threshold changes with changing background intensity is less if the test flash is small than if it is large. This is commonly attributed to a reduction of the area over which visual signals are integrated as light adaptation increases. 2. We propose and test an alternative hypothesis that the change in slope is the result of purely local processes: if it is assumed that increasing the background intensity increases the exponent of the local response function, but does not alter the extent of spatial integration, then the threshold of the small test flash will rise more slowly than the threshold of the large test flash simply because the small test flash is of a higher intensity than the large and therefore evokes a correspondingly greater local response. 3. We measured small and large test field increment thresholds and dichoptic brightness matches as a function of background intensity. 4. The log-log slopes of the small and large field increment threshold functions differed by not more than about 20%, suggesting that even under the conventional interpretation of such data, the change of spatial integration is less than is usually supposed. 5. The intensity of a large (2.3 deg) suprathreshold test field matched to a standard in the other eye varies with increasing background intensity with the same shallow slope as the small test (2.6 min) threshold versus intensity function; this is in agreement with the predictions of the local non-linearity hypothesis and suggests that there is no substantial change in spatial integration during light adaptation.
Full text
PDF
















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arend L. E., Timberlake G. T. What is psychophysically perfect image stabilization? Do perfectly stabilized images always disappear? J Opt Soc Am A. 1986 Feb;3(2):235–241. doi: 10.1364/josaa.3.000235. [DOI] [PubMed] [Google Scholar]
- BARLOW H. B., FITZHUGH R., KUFFLER S. W. Change of organization in the receptive fields of the cat's retina during dark adaptation. J Physiol. 1957 Aug 6;137(3):338–354. doi: 10.1113/jphysiol.1957.sp005817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARLOW H. B. Temporal and spatial summation in human vision at different background intensities. J Physiol. 1958 Apr 30;141(2):337–350. doi: 10.1113/jphysiol.1958.sp005978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow H. B., Levick W. R. Three factors limiting the reliable detection of light by retinal ganglion cells of the cat. J Physiol. 1969 Jan;200(1):1–24. doi: 10.1113/jphysiol.1969.sp008679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow H. B., Levick W. R. Threshold setting by the surround of cat retinal ganglion cells. J Physiol. 1976 Aug;259(3):737–757. doi: 10.1113/jphysiol.1976.sp011492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Hodgkin A. L. Changes in time scale and sensitivity in turtle photoreceptors. J Physiol. 1974 Nov;242(3):729–758. doi: 10.1113/jphysiol.1974.sp010732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore C. B., Rushton W. A. Dark adaptation and increment threshold in a rod monochromat. J Physiol. 1965 Dec;181(3):612–628. doi: 10.1113/jphysiol.1965.sp007786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boynton R. M., Whitten D. N. Visual adaptation in monkey cones: recordings of late receptor potentials. Science. 1970 Dec 25;170(3965):1423–1426. doi: 10.1126/science.170.3965.1423. [DOI] [PubMed] [Google Scholar]
- Cleland B. G., Enroth-cugell C. Quantitative aspects of sensitivity and summation in the cat retina. J Physiol. 1968 Sep;198(1):17–38. doi: 10.1113/jphysiol.1968.sp008591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daitch J. M., Green D. G. Contrast sensitivity of the human peripheral retina. Vision Res. 1969 Aug;9(8):947–952. doi: 10.1016/0042-6989(69)90100-x. [DOI] [PubMed] [Google Scholar]
- Derrington A. M., Lennie P. The influence of temporal frequency and adaptation level on receptive field organization of retinal ganglion cells in cat. J Physiol. 1982 Dec;333:343–366. doi: 10.1113/jphysiol.1982.sp014457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodge F. A., Jr, Knight B. W., Toyoda J. Voltage noise in Limulus visual cells. Science. 1968 Apr 5;160(3823):88–90. doi: 10.1126/science.160.3823.88. [DOI] [PubMed] [Google Scholar]
- Drum B. Cone threshold vs. retinal eccentricity: changes with dark adaptation. Invest Ophthalmol Vis Sci. 1980 Apr;19(4):432–435. [PubMed] [Google Scholar]
- Enroth-Cugell C., Hertz B. G., Lennie P. Convergence of rod and cone signals in the cat's retina. J Physiol. 1977 Jul;269(2):297–318. doi: 10.1113/jphysiol.1977.sp011903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enroth-Cugell C., Hertz G., Lennie P. Cone signals in the cat's retina. J Physiol. 1977 Jul;269(2):273–296. doi: 10.1113/jphysiol.1977.sp011902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enroth-Cugell C., Robson J. G. The contrast sensitivity of retinal ganglion cells of the cat. J Physiol. 1966 Dec;187(3):517–552. doi: 10.1113/jphysiol.1966.sp008107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enroth-Cugell C., Shapley R. M. Adaptation and dynamics of cat retinal ganglion cells. J Physiol. 1973 Sep;233(2):271–309. doi: 10.1113/jphysiol.1973.sp010308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fain G. L., Dowling J. E. Intracellular recordings from single rods and cones in the mudpuppy retina. Science. 1973 Jun 15;180(4091):1178–1181. doi: 10.1126/science.180.4091.1178. [DOI] [PubMed] [Google Scholar]
- Geisler W. S. Evidence for the equivalent-background hypothesis in cones. Vision Res. 1979;19(7):799–805. doi: 10.1016/0042-6989(79)90156-1. [DOI] [PubMed] [Google Scholar]
- Hayhoe M. M. After-effects of small adapting fields. J Physiol. 1979 Nov;296:141–158. doi: 10.1113/jphysiol.1979.sp012996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecht S. THE RELATION BETWEEN VISUAL ACUITY AND ILLUMINATION. J Gen Physiol. 1928 Jan 20;11(3):255–281. doi: 10.1085/jgp.11.3.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs G. H., Yolton R. L. Center-surround balance in receptive fields of cells in the lateral geniculate nucleus. Vision Res. 1970 Nov;10(11):1127–1144. doi: 10.1016/0042-6989(70)90030-1. [DOI] [PubMed] [Google Scholar]
- KELLY D. H. Visual response to time-dependent stimuli. I. Amplitude sensitivity measurements. J Opt Soc Am. 1961 Apr;51:422–429. doi: 10.1364/josa.51.000422. [DOI] [PubMed] [Google Scholar]
- Kishto B. N., Saunders R. Variation of the visual threshold with retinal location. II. The fovea. Vision Res. 1970 Aug;10(8):762–767. [PubMed] [Google Scholar]
- Kleinschmidt J., Dowling J. E. Intracellular recordings from gecko photoreceptors during light and dark adaptation. J Gen Physiol. 1975 Nov;66(5):617–648. doi: 10.1085/jgp.66.5.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulikowski J. J. Effective contrast constancy and linearity of contrast sensation. Vision Res. 1976;16(12):1419–1431. doi: 10.1016/0042-6989(76)90161-9. [DOI] [PubMed] [Google Scholar]
- MacLeod D. I. Visual sensitivity. Annu Rev Psychol. 1978;29:613–645. doi: 10.1146/annurev.ps.29.020178.003145. [DOI] [PubMed] [Google Scholar]
- Normann R. A., Perlman I. Signal transmission from red cones to horizontal cells in the turtle retina. J Physiol. 1979 Jan;286:509–524. doi: 10.1113/jphysiol.1979.sp012634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STEVENS J. C., STEVENS S. S. Brightness function: effects of adaptation. J Opt Soc Am. 1963 Mar;53:375–385. doi: 10.1364/josa.53.000375. [DOI] [PubMed] [Google Scholar]
- Sakmann B., Creutzfeldt O. D. Scotopic and mesopic light adaptation in the cat's retina. Pflugers Arch. 1969;313(2):168–185. doi: 10.1007/BF00586245. [DOI] [PubMed] [Google Scholar]
- Scholtes A. M., Bouman M. A. Psychophysical experiments on spatial summation at threshold level of the human peripheral retina. Vision Res. 1977;17(7):867–873. doi: 10.1016/0042-6989(77)90131-6. [DOI] [PubMed] [Google Scholar]
- Spillmann L., Ransom-Hogg A., Oehler R. A comparison of perceptive and receptive fields in man and monkey. Hum Neurobiol. 1987;6(1):51–62. [PubMed] [Google Scholar]
- VAN DEN BRINK G., BOUMAN M. A. Variation of integrative actions in the retinal system; an adaptational phenomenon. J Opt Soc Am. 1954 Aug;44(8):616–620. doi: 10.1364/josa.44.000616. [DOI] [PubMed] [Google Scholar]
- Whittle P., Challands P. D. The effect of background luminance on the brightness of flashes. Vision Res. 1969 Sep;9(9):1095–1110. doi: 10.1016/0042-6989(69)90050-9. [DOI] [PubMed] [Google Scholar]


