Abstract
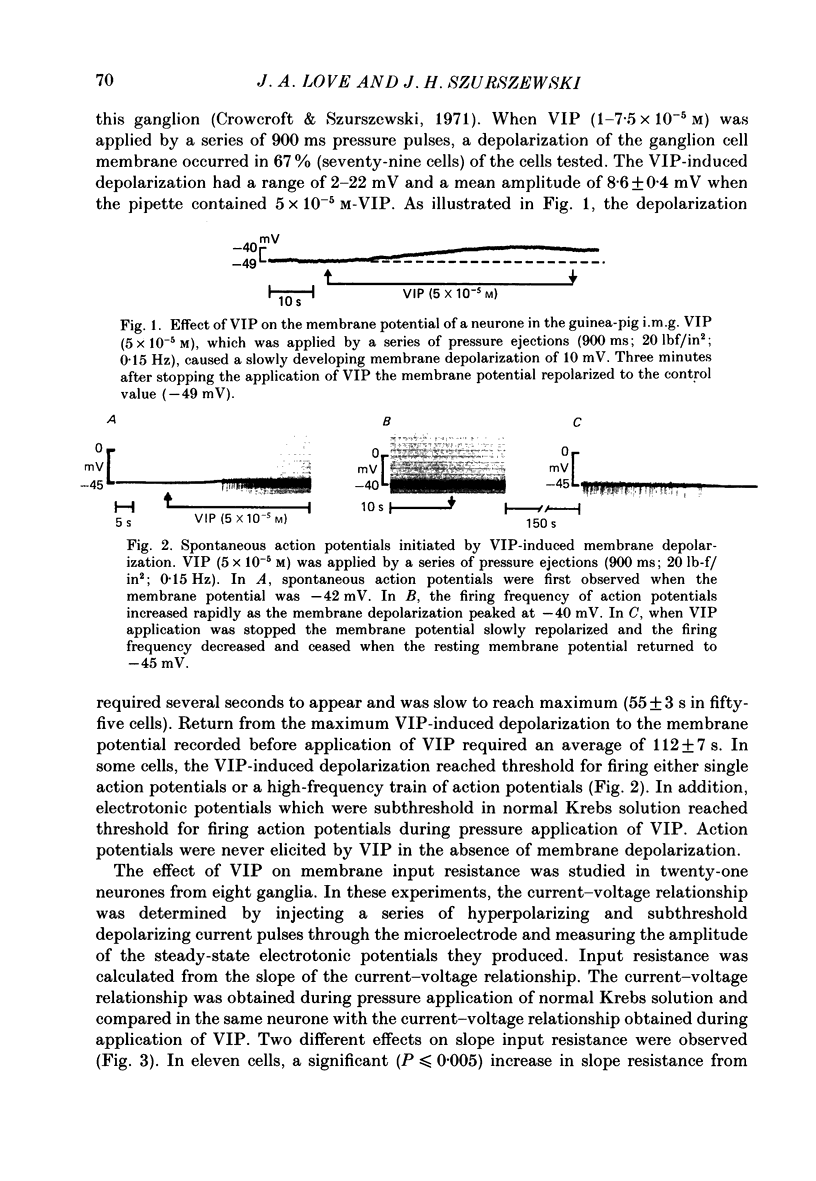
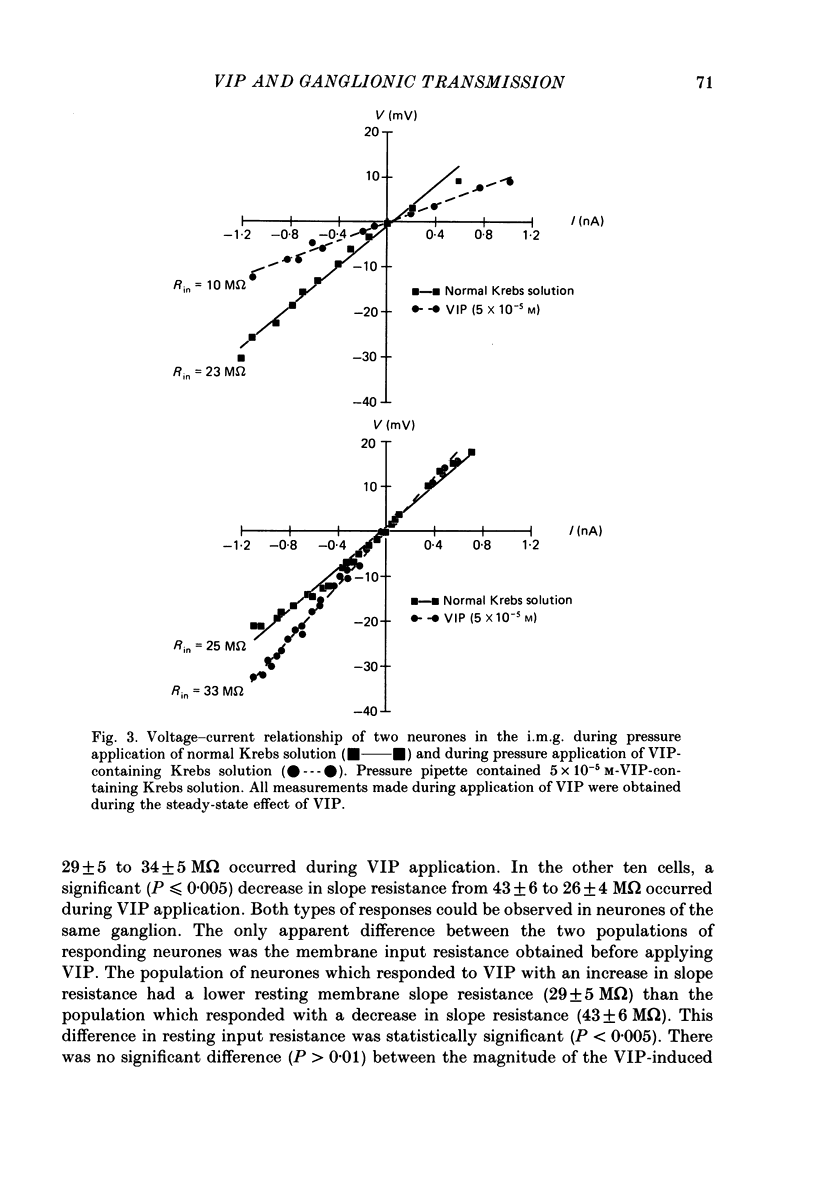
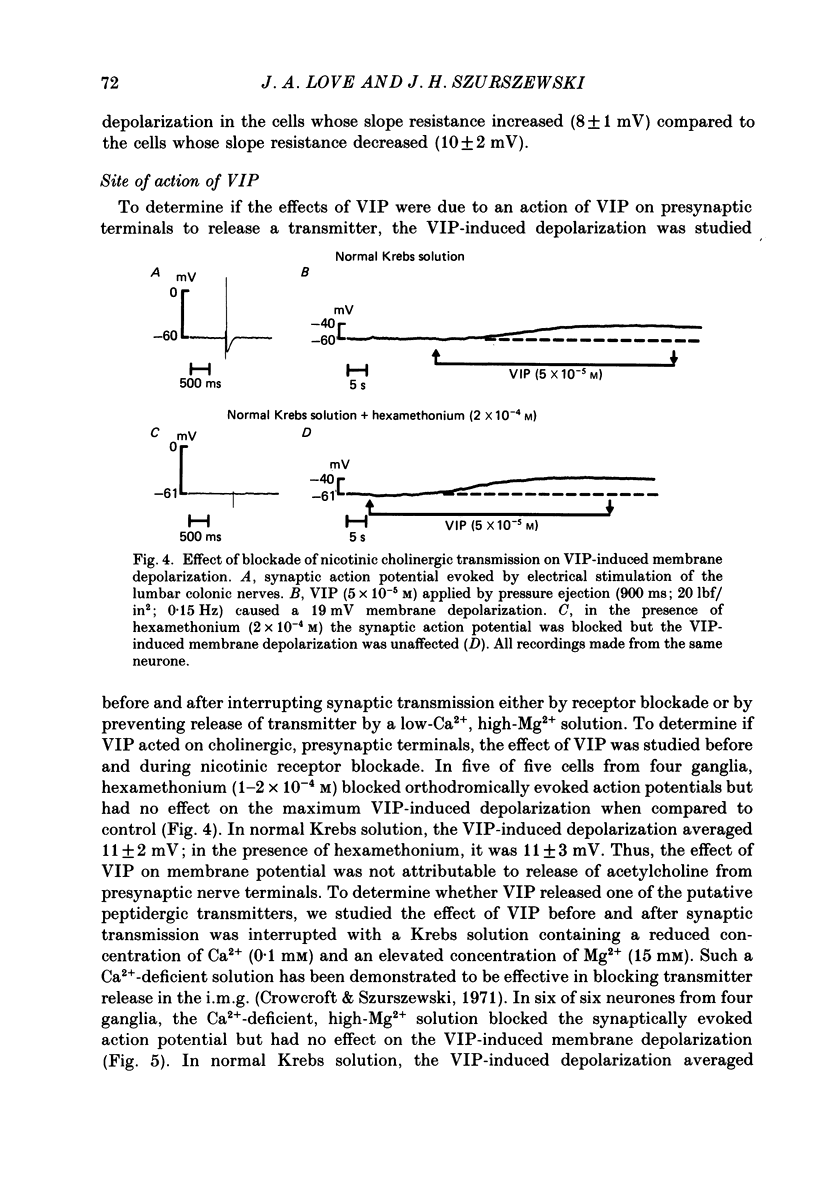
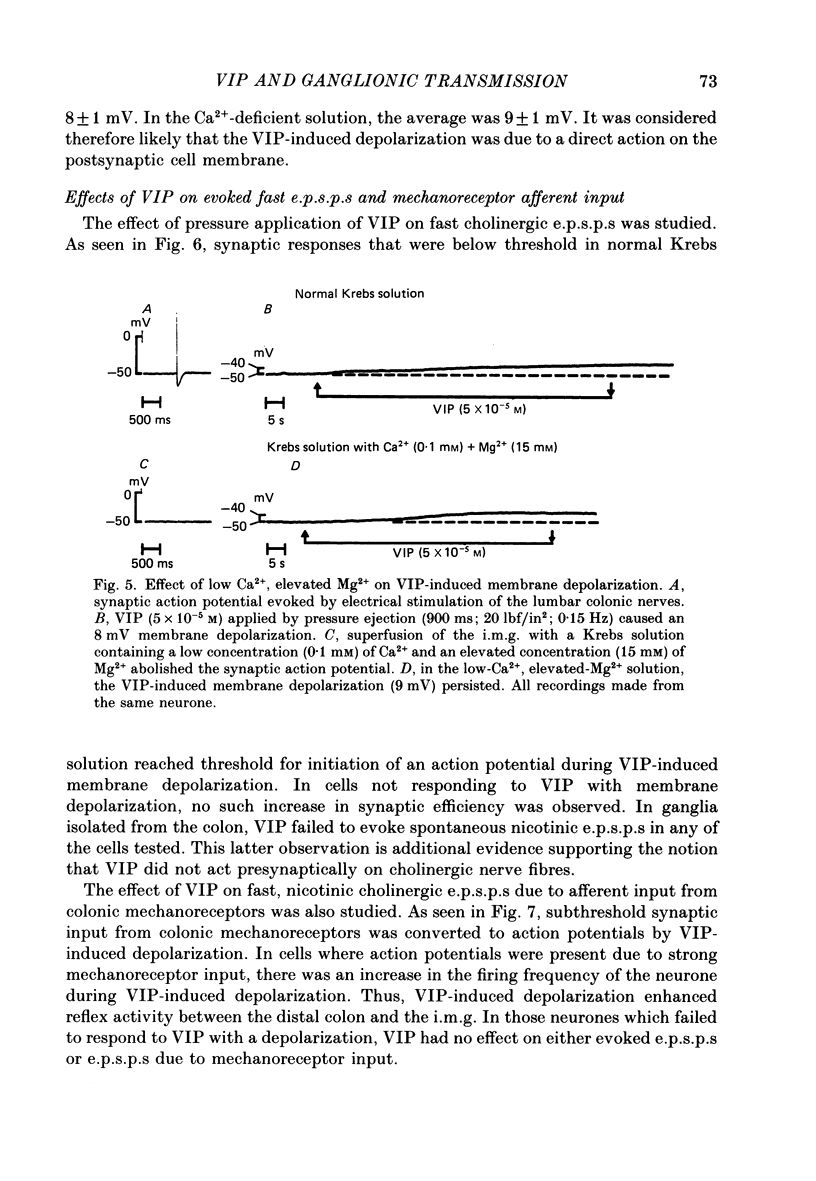
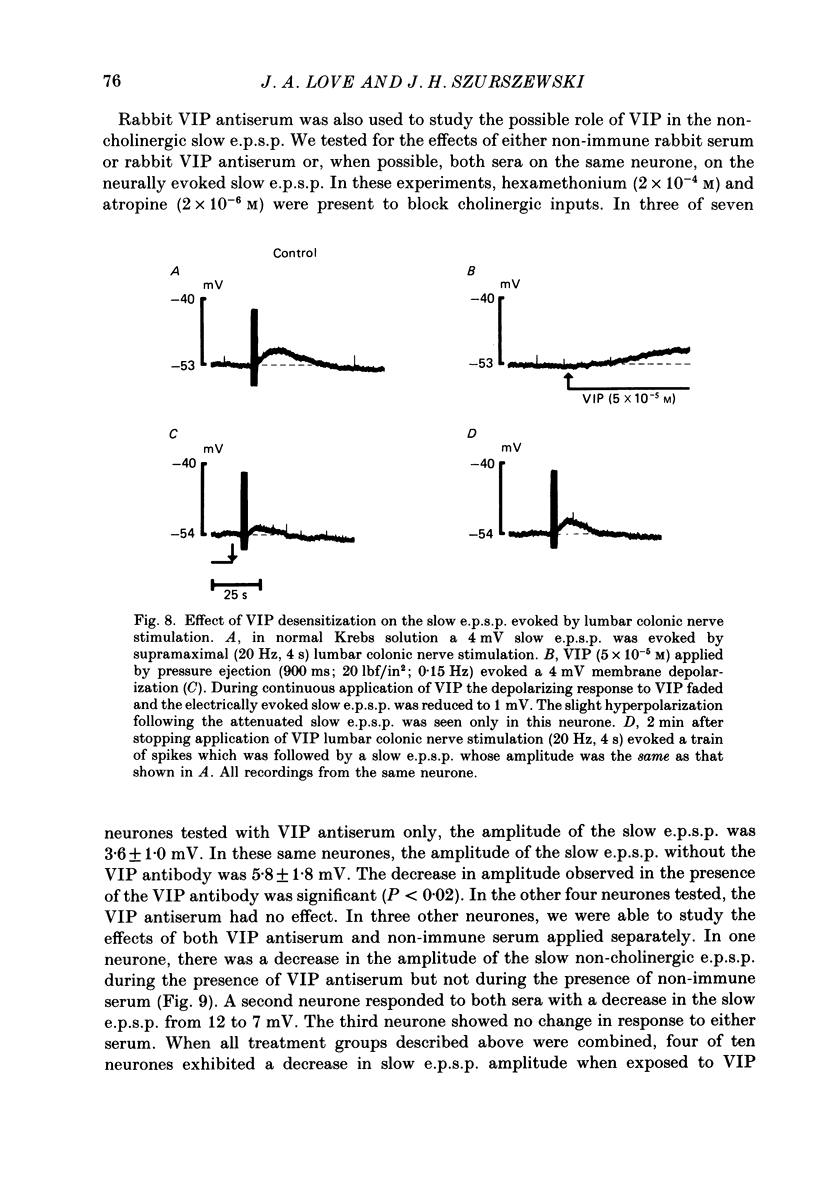
1. The effects of vasoactive intestinal polypeptide (VIP) on the inferior mesenteric ganglion of the guinea-pig were studied in vitro. 2. In 67% of the neurones tested, application of VIP (1-7.5 X 10(-5) M) by pressure ejection caused a depolarization of the membrane potential which averaged 8.6 +/- 0.4 mV. 3. In 52% of the cells that were responsive to VIP, the membrane depolarization was accompanied by a decrease in membrane input resistance. In another 48% of the cells tested, there was an increase in membrane input resistance. 4. Membrane depolarization caused by VIP enhanced the excitability of post-ganglionic neurones and converted subthreshold electrotonic and subthreshold synaptic potentials to action potentials. 5. The effects of VIP persisted during nicotinic and muscarinic synaptic blockade. The effects of VIP also persisted in a low-Ca2+, high-Mg2+ solution. Thus, the site of action of VIP was on the postsynaptic membrane. 6. Electrical stimulation of the lumbar colonic nerves evoked a slow noncholinergic depolarization of the membrane potential. 7. VIP appeared to be one of the transmitters involved in the electrically evoked e.p.s.p. because both prior desensitization with exogenous VIP and VIP antiserum reduced the amplitude of the slow, non-cholinergic e.p.s.p. 8. Radial distension of a segment of colon attached to the inferior mesenteric ganglion (i.m.g.) evoked a non-cholinergic depolarization of the membrane potential in neurones in the i.m.g. 9. The distension-induced non-cholinergic depolarization was reduced by VIP antiserum. 10. The data support the hypothesis that a population of the mechanosensory afferent nerves running between the colon and the i.m.g. utilize VIP or a VIP-like peptide as a transmitter to modulate reflex activity between the colon and the i.m.g.
Full text
PDF


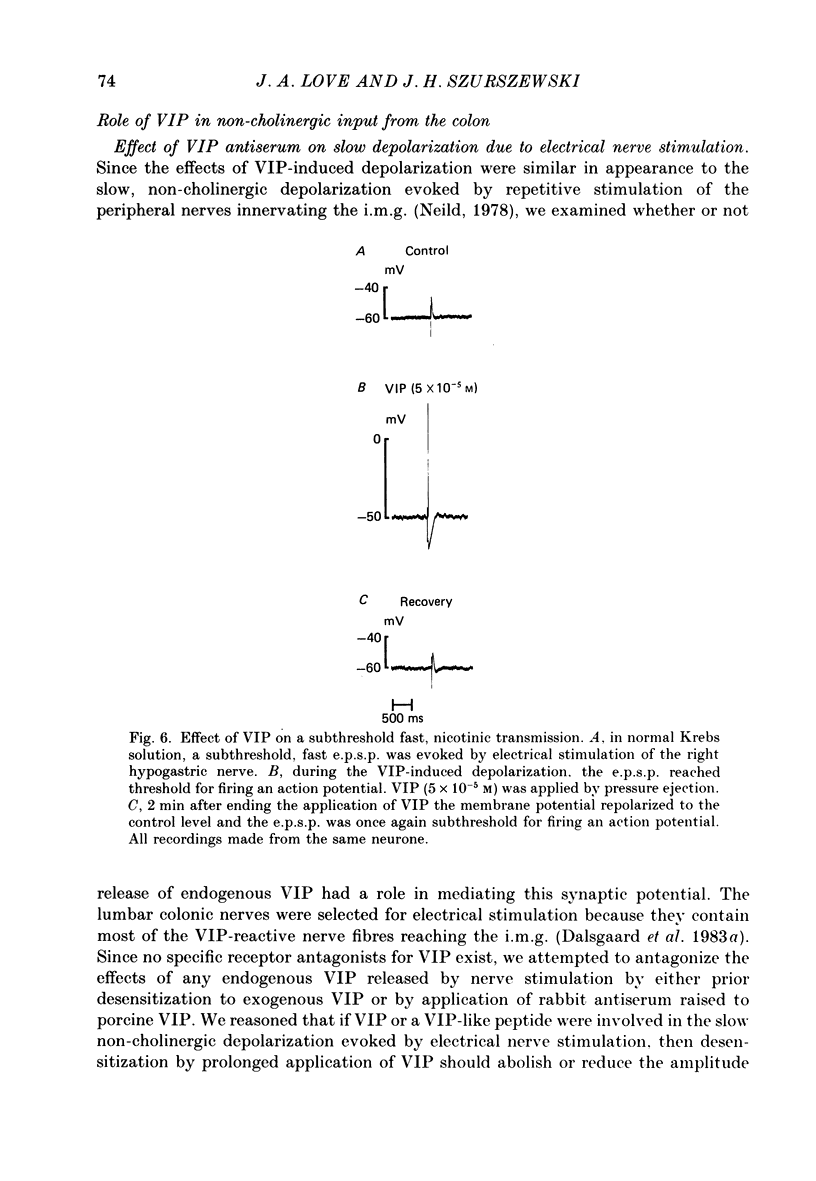
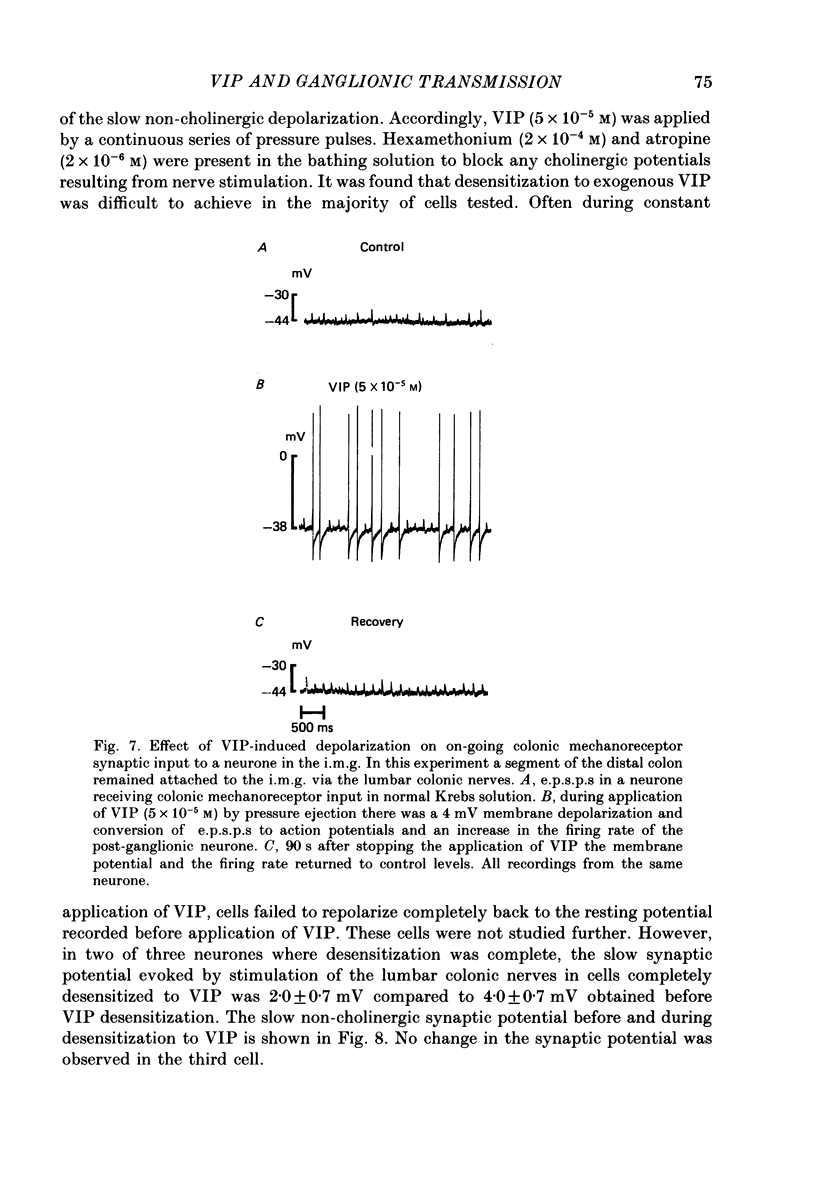
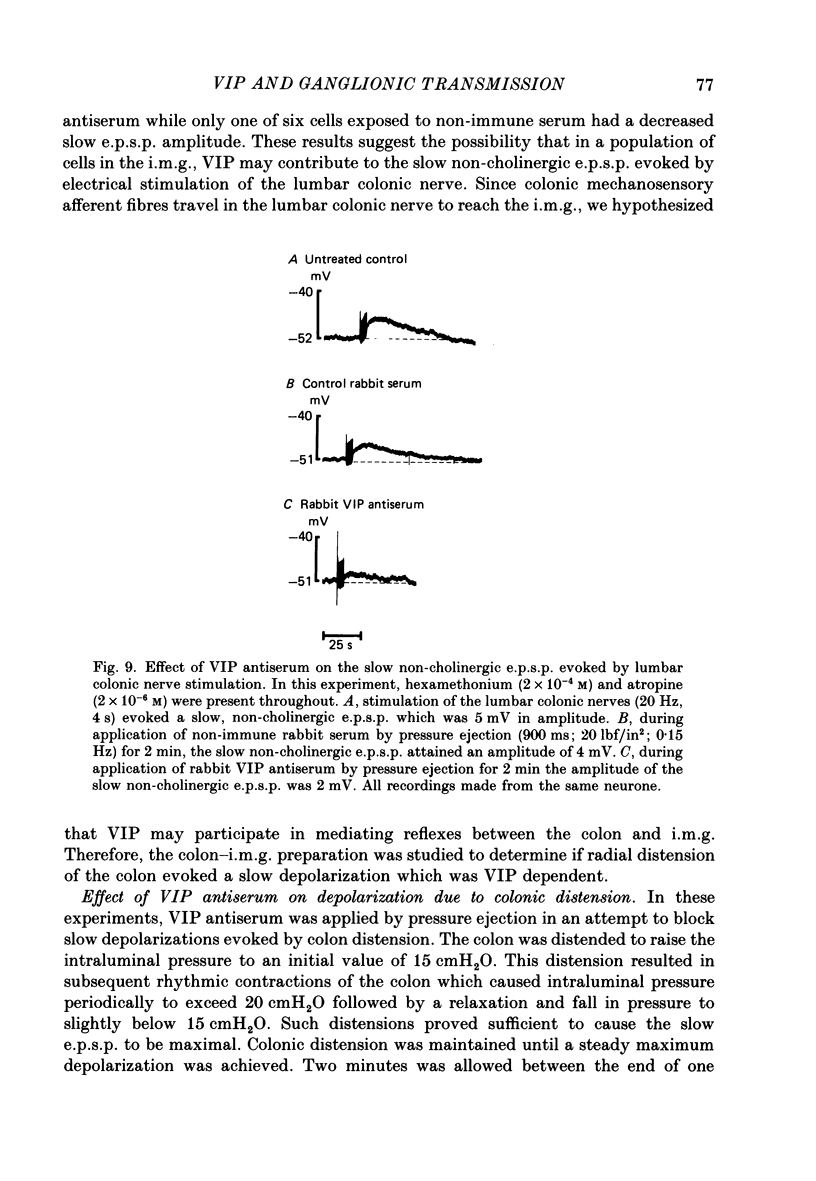
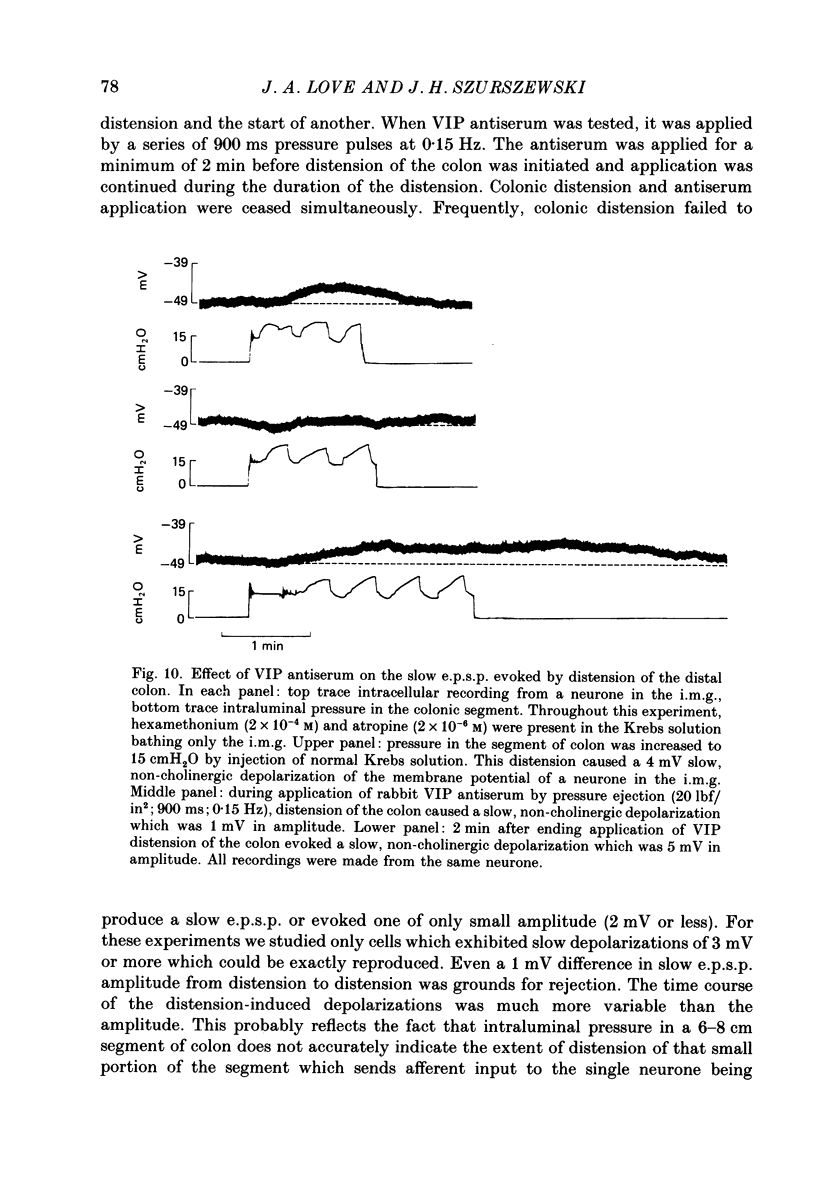






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angel F., Go V. L., Schmalz P. F., Szurszewski J. H. Vasoactive intestinal polypeptide: a putative transmitter in the canine gastric muscularis mucosa. J Physiol. 1983 Aug;341:641–654. doi: 10.1113/jphysiol.1983.sp014830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angel F., Go V. L., Szurszewski J. H. Innervation of the muscularis mucosae of canine proximal colon. J Physiol. 1984 Dec;357:93–108. doi: 10.1113/jphysiol.1984.sp015491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen M. L., Landry A. S. Vasoactive intestinal polypeptide: increased tone, enhancement of acetylcholine release, and stimulation of adenylate cyclase in intestinal smooth muscle. Life Sci. 1980 Mar 10;26(10):811–822. doi: 10.1016/0024-3205(80)90288-x. [DOI] [PubMed] [Google Scholar]
- Costa M., Furness J. B. The origins, pathways and terminations of neurons with VIP-like immunoreactivity in the guinea-pig small intestine. Neuroscience. 1983 Apr;8(4):665–676. doi: 10.1016/0306-4522(83)90002-7. [DOI] [PubMed] [Google Scholar]
- Crowcroft P. J., Szurszewski J. H. A study of the inferior mesenteric and pelvic ganglia of guinea-pigs with intracellular electrodes. J Physiol. 1971 Dec;219(2):421–441. doi: 10.1113/jphysiol.1971.sp009670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalsgaard C. J., Hökfelt T., Schultzberg M., Lundberg J. M., Terenius L., Dockray G. J., Goldstein M. Origin of peptide-containing fibers in the inferior mesenteric ganglion of the guinea-pig: immunohistochemical studies with antisera to substance P, enkephalin, vasoactive intestinal polypeptide, cholecystokinin and bombesin. Neuroscience. 1983 May;9(1):191–211. doi: 10.1016/0306-4522(83)90056-8. [DOI] [PubMed] [Google Scholar]
- Dalsgaard C. J., Vincent S. R., Schultzberg M., Hökfelt T., Elfvin L. G., Terenius L., Dockray G. J. Capsaicin-induced depletion of substance P-like immunoreactivity in guinea pig sympathetic ganglia. J Auton Nerv Syst. 1983 Dec;9(4):595–606. doi: 10.1016/0165-1838(83)90116-9. [DOI] [PubMed] [Google Scholar]
- Dun N. J., Minota S. Effects of substance P on neurones of the inferior mesenteric ganglia of the guinea-pig. J Physiol. 1981 Dec;321:259–271. doi: 10.1113/jphysiol.1981.sp013982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold M. R. The effects of vasoactive intestinal peptide on neuromuscular transmission in the frog. J Physiol. 1982 Jun;327:325–335. doi: 10.1113/jphysiol.1982.sp014234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haskins J. T., Samson W. K., Moss R. L. Evidence for vasoactive intestinal polypeptide (VIP) altering the firing rate of preoptic, septal and midbrain central gray neurons. Regul Pept. 1982 Feb;3(2):113–123. doi: 10.1016/0167-0115(82)90088-x. [DOI] [PubMed] [Google Scholar]
- Jiang Z. G., Dun N. J. Multiple conductance change associated with the slow excitatory potential in mammalian sympathetic neurons. Brain Res. 1981 Dec 14;229(1):203–208. doi: 10.1016/0006-8993(81)90758-7. [DOI] [PubMed] [Google Scholar]
- Kawatani M., Rutigliano M., De Groat W. C. Selective facilitatory effect of vasoactive intestinal polypeptide (VIP) on muscarinic firing in vesical ganglia of the cat. Brain Res. 1985 Jun 17;336(2):223–234. doi: 10.1016/0006-8993(85)90649-3. [DOI] [PubMed] [Google Scholar]
- Kawatani M., Rutigliano M., de Groat W. C. Depolarization and muscarinic excitation induced in a sympathetic ganglion by vasoactive intestinal polypeptide. Science. 1985 Aug 30;229(4716):879–881. doi: 10.1126/science.3895438. [DOI] [PubMed] [Google Scholar]
- Konishi S., Tsunoo A., Otsuka M. Enkephalins presynaptically inhibit cholinergic transmission in sympathetic ganglia. Nature. 1979 Nov 29;282(5738):515–516. doi: 10.1038/282515a0. [DOI] [PubMed] [Google Scholar]
- Kreulen D. L., Peters S. Non-cholinergic transmission in a sympathetic ganglion of the guinea-pig elicited by colon distension. J Physiol. 1986 May;374:315–334. doi: 10.1113/jphysiol.1986.sp016081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuba K., Koketsu K. Synaptic events in sympathetic ganglia. Prog Neurobiol. 1978;11(2):77–169. doi: 10.1016/0301-0082(78)90010-2. [DOI] [PubMed] [Google Scholar]
- Lasater E. M., Watling K. J., Dowling J. E. Vasoactive intestinal peptide alters membrane potential and cyclic nucleotide levels in retinal horizontal cells. Science. 1983 Sep 9;221(4615):1070–1072. doi: 10.1126/science.6308770. [DOI] [PubMed] [Google Scholar]
- Lundberg J. M. Evidence for coexistence of vasoactive intestinal polypeptide (VIP) and acetylcholine in neurons of cat exocrine glands. Morphological, biochemical and functional studies. Acta Physiol Scand Suppl. 1981;496:1–57. [PubMed] [Google Scholar]
- Matthews M. R., Cuello A. C. The origin and possible significance of substance P immunoreactive networks in the prevertebral ganglia and related structures in the guinea-pig. Philos Trans R Soc Lond B Biol Sci. 1984 Aug 14;306(1128):247–276. doi: 10.1098/rstb.1984.0087. [DOI] [PubMed] [Google Scholar]
- Minota S., Dun N. J., Karczmar A. G. Substance P-induced depolarization in sympathetic neurons: not simple K-inactivation. Brain Res. 1981 Jul 6;216(1):224–228. doi: 10.1016/0006-8993(81)91294-4. [DOI] [PubMed] [Google Scholar]
- Mo N., Dun N. J. Vasoactive intestinal polypeptide facilitates muscarinic transmission in mammalian sympathetic ganglia. Neurosci Lett. 1984 Nov 23;52(1-2):19–23. doi: 10.1016/0304-3940(84)90344-6. [DOI] [PubMed] [Google Scholar]
- Neild T. O. Slowly-developing depolarization of neurones in the guinea-pig inferior mesenteric ganglion following repetitive stimulation of the preganglionic nerves. Brain Res. 1978 Jan 27;140(2):231–239. doi: 10.1016/0006-8993(78)90457-2. [DOI] [PubMed] [Google Scholar]
- Peters S., Kreulen D. L. A slow EPSP in mammalian inferior mesenteric ganglion persists after in vivo capsaicin. Brain Res. 1984 Jun 11;303(1):186–189. doi: 10.1016/0006-8993(84)90227-0. [DOI] [PubMed] [Google Scholar]
- Peters S., Kreulen D. L. Fast and slow synaptic potentials produced in a mammalian sympathetic ganglion by colon distension. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1941–1944. doi: 10.1073/pnas.83.6.1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillis J. W., Kirkpatrick J. R., Said S. I. Vasoactive intestinal polypeptide excitation of central neurons. Can J Physiol Pharmacol. 1978 Apr;56(2):337–340. doi: 10.1139/y78-052. [DOI] [PubMed] [Google Scholar]
- Phillis J. W., Kirkpatrick J. R. The actions of motilin, luteinizing hormone releasing hormone, cholecystokinin, somatostatin, vasoactive intestinal peptide, and other peptides on rat cerebral cortical neurons. Can J Physiol Pharmacol. 1980 Jun;58(6):612–623. doi: 10.1139/y80-102. [DOI] [PubMed] [Google Scholar]
- Reinecke M., Forssmann W. G., Thiekötter G., Triepel J. Localization of neurotensin-immunoreactivity in the spinal cord and peripheral nervous system of the guinea pig. Neurosci Lett. 1983 May 27;37(1):37–42. doi: 10.1016/0304-3940(83)90501-3. [DOI] [PubMed] [Google Scholar]
- Shu H. D., Love J. A., Szurszewski J. H. Effect of enkephalins on colonic mechanoreceptor synaptic input to inferior mesenteric ganglion. Am J Physiol. 1987 Jan;252(1 Pt 1):G128–G135. doi: 10.1152/ajpgi.1987.252.1.G128. [DOI] [PubMed] [Google Scholar]
- Szurszewski J. H. Physiology of mammalian prevertebral ganglia. Annu Rev Physiol. 1981;43:53–68. doi: 10.1146/annurev.ph.43.030181.000413. [DOI] [PubMed] [Google Scholar]
- Szurszewski J. H., Weems W. A. A study of peripheral input to and its control by post-ganglionic neurones of the inferior mesenteric ganglion. J Physiol. 1976 Apr;256(3):541–556. doi: 10.1113/jphysiol.1976.sp011338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent S. R., Dalsgaard C. J., Schultzberg M., Hökfelt T., Christensson I., Terenius L. Dynorphin-immunoreactive neurons in the autonomic nervous system. Neuroscience. 1984 Apr;11(4):973–987. doi: 10.1016/0306-4522(84)90208-2. [DOI] [PubMed] [Google Scholar]
- Yasui S., Young L. R. On the predictive control of foveal eye tracking and slow phases of optokinetic and vestibular nystagmus. J Physiol. 1984 Feb;347:17–33. doi: 10.1113/jphysiol.1984.sp015050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yau W. M., Youther M. L., Verdun P. R. A presynaptic site of action of substance P and vasoactive intestinal polypeptide on myenteric neurons. Brain Res. 1985 Mar 25;330(2):382–385. doi: 10.1016/0006-8993(85)90703-6. [DOI] [PubMed] [Google Scholar]
- Zafirov D. H., Palmer J. M., Nemeth P. R., Wood J. D. Bombesin, gastrin releasing peptide and vasoactive intestinal peptide excite myenteric neurons. Eur J Pharmacol. 1985 Sep 10;115(1):103–107. doi: 10.1016/0014-2999(85)90590-4. [DOI] [PubMed] [Google Scholar]