Abstract
1. Intracellular recordings of the voltage responses of rods and both functional classes of bipolar cell were made in the isolated, perfused retina of the tiger salamander, Ambystoma tigrinum. 2. Brief, dim flashes of 519 nm light delivered to the receptive-field centres were used to measure the flash sensitivities of twenty-one on-centre bipolar cells and thirty-six off-centre cells. In each experiment the flash sensitivity of a rod was also measured using diffuse illumination of the same duration and wave-length. 3. The mean flash sensitivity of the rods (fifty-nine cells) was 4.47 mV photon-1 micron 2 flash. The mean flash sensitivity of the off-centre bipolar cells was 35.4 mV photon-1 micron 2 flash (thirty-six cells). The mean flash sensitivity of the on-centre bipolar cells was 12.5 mV photon-1 micron 2 flash. 4. The ratio of the flash sensitivity of the bipolar cell to that of a rod recorded in the same retina defined the gain of voltage transfer from rod to bipolar cell. For signal transfer to on-centre bipolar cells the mean value of the voltage gain was 5.05 +/- 1.34 (S.E. of mean). For signal transfer to the off-centre bipolar cells, the mean value of the gain was 10.4 +/- 1.29. 5. The on-centre cell gain in the salamander was smaller by a factor of 27 than that of the on-centre cells in the dogfish retina (Ashmore & Falk, 1980 a), while the off-centre cell gain was comparable in the two species. Possible reasons for the large difference between the voltage gains of on-centre cells in the dogfish and salamander are considered.
Full text
PDF

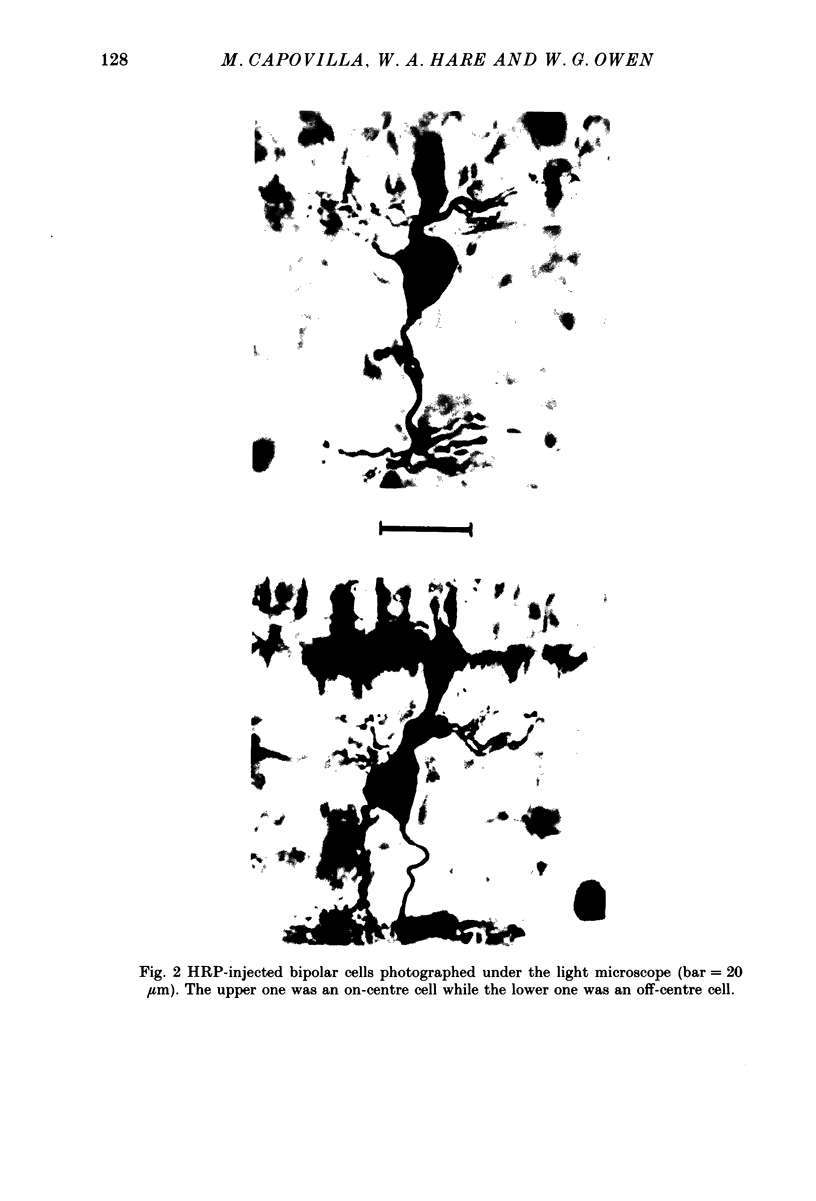


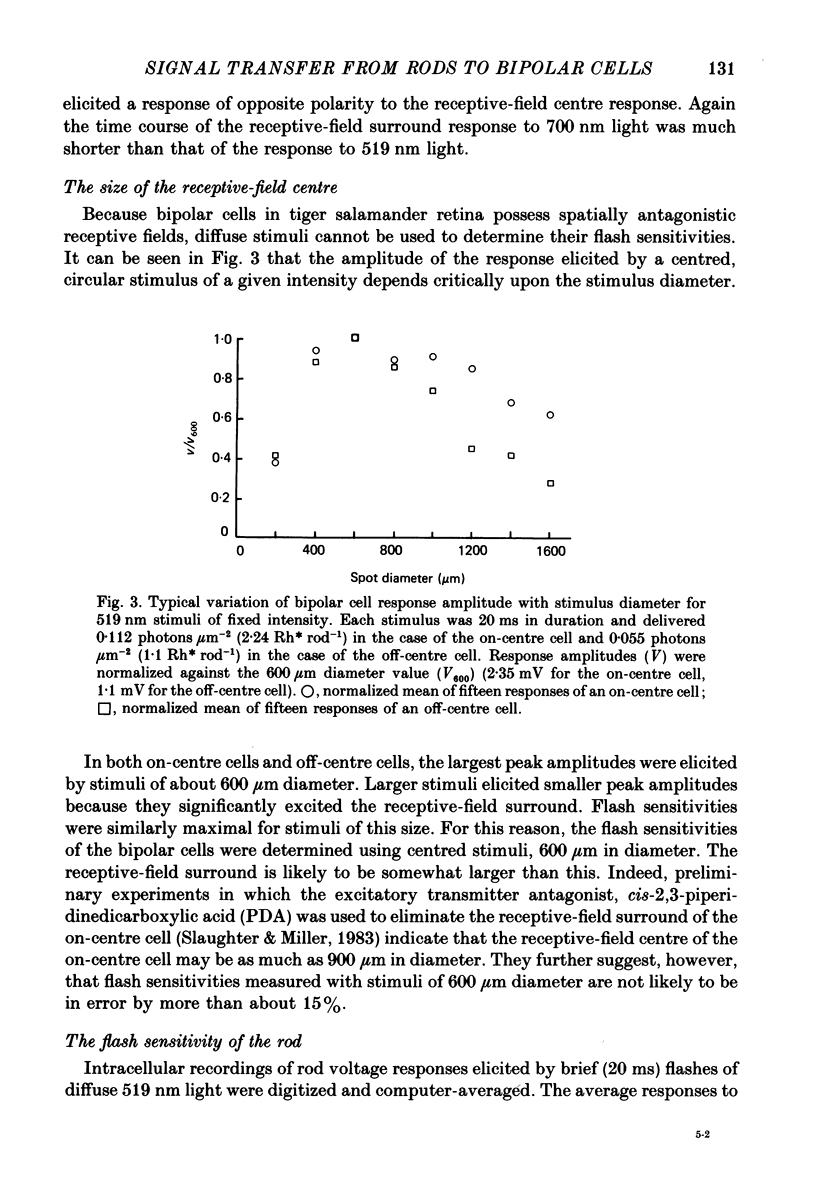
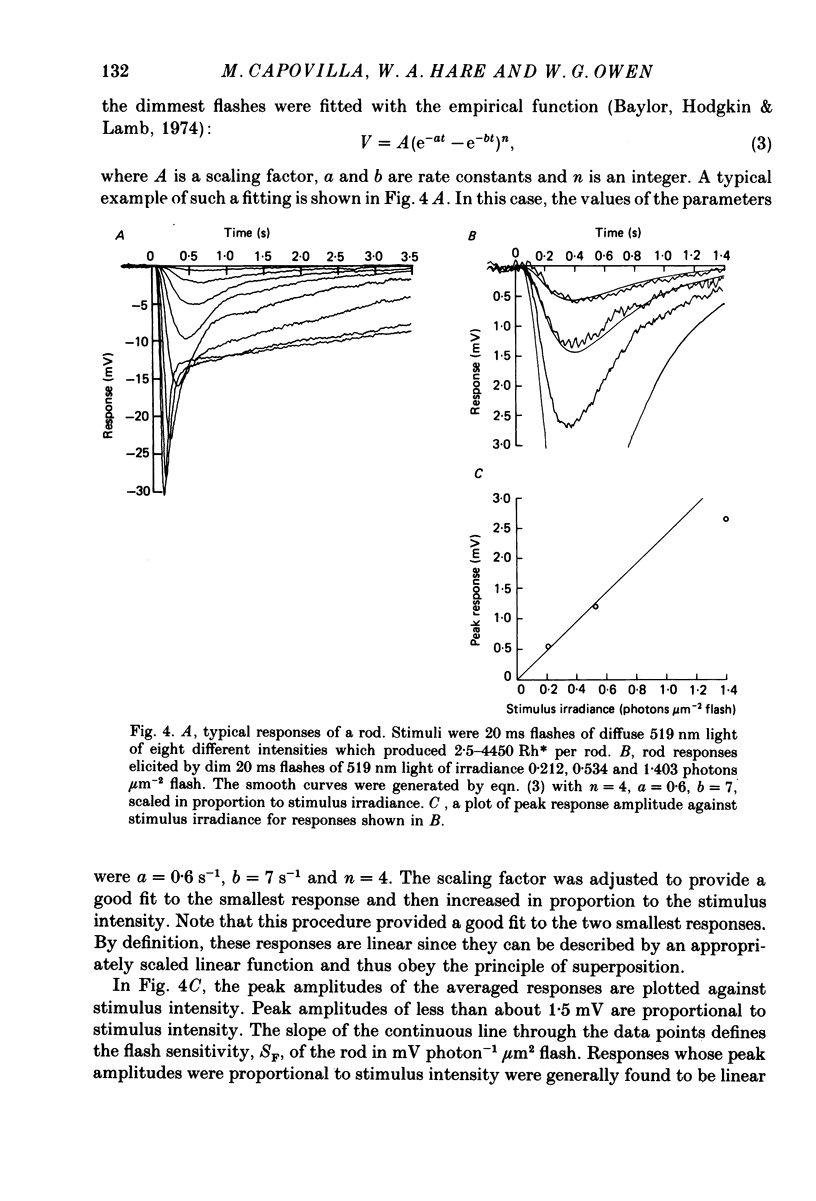
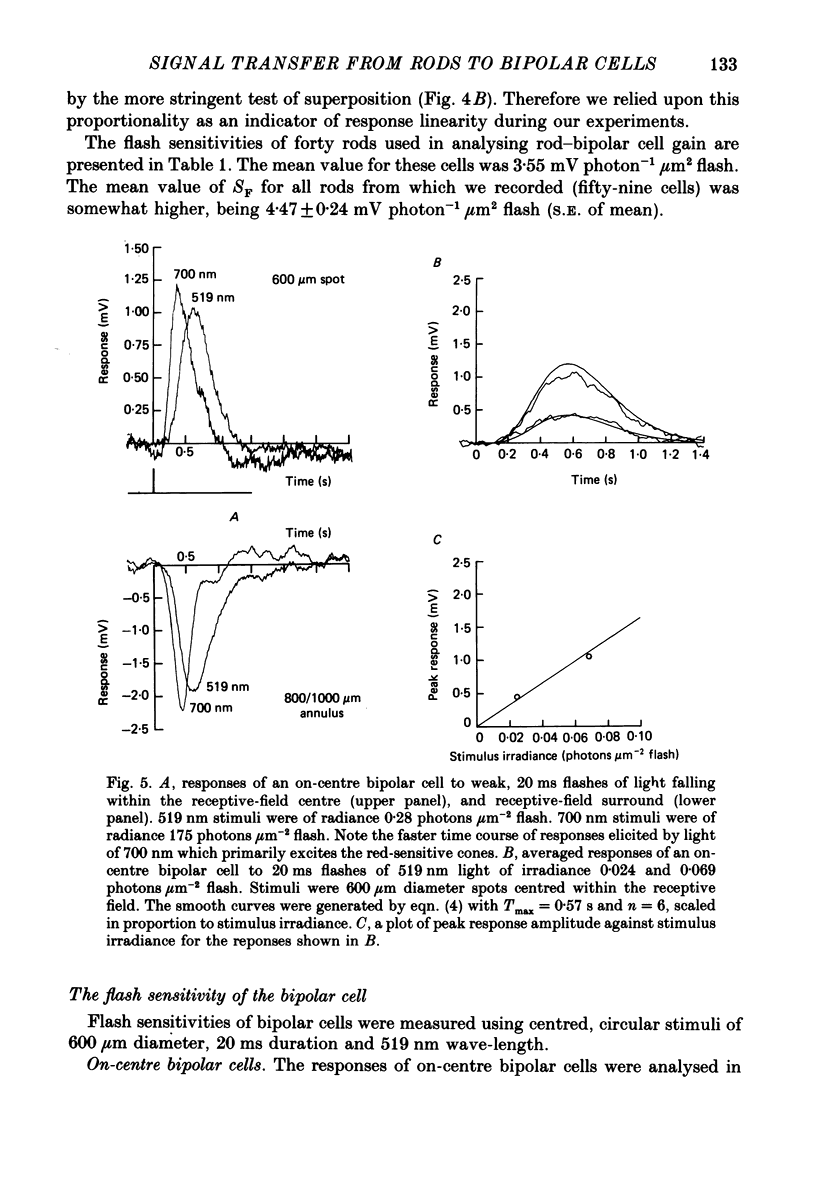
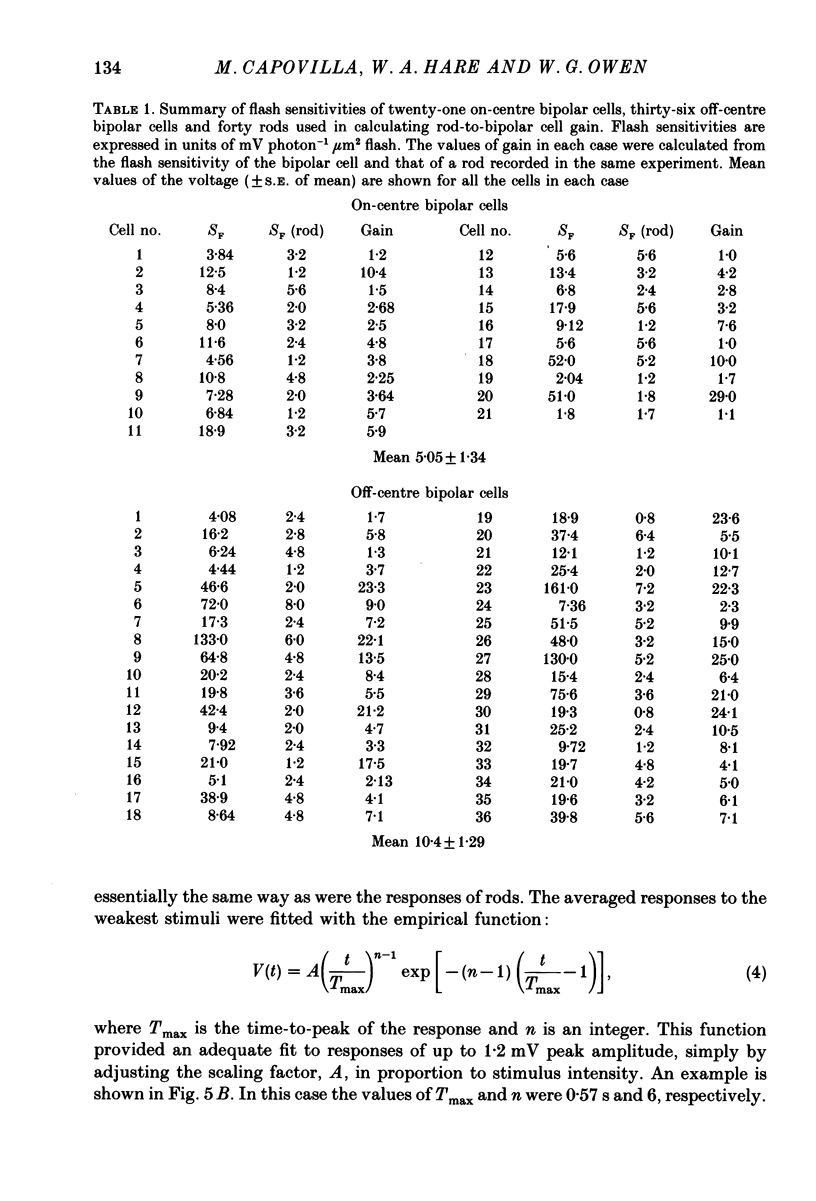
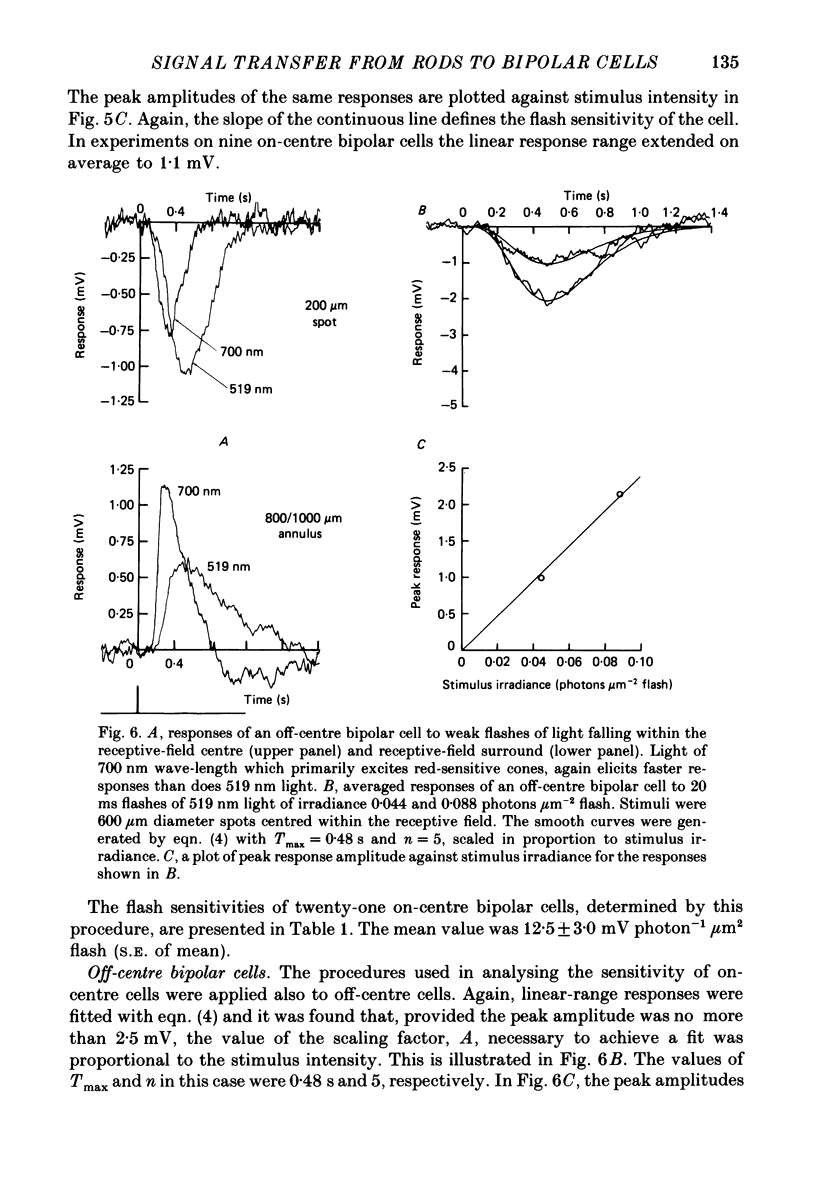




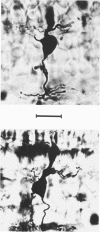
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashmore J. F., Falk G. An analysis of voltage noise in rod bipolar cells of the dogfish retina. J Physiol. 1982 Nov;332:273–297. doi: 10.1113/jphysiol.1982.sp014413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashmore J. F., Falk G. Dark noise in retinal bipolar cells and stability of rhodopsin in rods. Nature. 1977 Nov 3;270(5632):69–71. doi: 10.1038/270069a0. [DOI] [PubMed] [Google Scholar]
- Ashmore J. F., Falk G. Responses of rod bipolar cells in the dark-adapted retina of the dogfish, Scyliorhinus canicula. J Physiol. 1980 Mar;300:115–150. doi: 10.1113/jphysiol.1980.sp013155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashmore J. F., Falk G. Transmission of visual signals to bipolar cells near absolute threshold. Vision Res. 1979;19(4):419–423. doi: 10.1016/0042-6989(79)90107-x. [DOI] [PubMed] [Google Scholar]
- Attwell D., Wilson M. Behaviour of the rod network in the tiger salamander retina mediated by membrane properties of individual rods. J Physiol. 1980 Dec;309:287–315. doi: 10.1113/jphysiol.1980.sp013509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Hodgkin A. L. Detection and resolution of visual stimuli by turtle photoreceptors. J Physiol. 1973 Oct;234(1):163–198. doi: 10.1113/jphysiol.1973.sp010340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Lamb T. D., Yau K. W. Responses of retinal rods to single photons. J Physiol. 1979 Mar;288:613–634. [PMC free article] [PubMed] [Google Scholar]
- Copenhagen D. R., Owen W. G. Functional characteristics of lateral interactions between rods in the retina of the snapping turtle. J Physiol. 1976 Jul;259(2):251–282. doi: 10.1113/jphysiol.1976.sp011465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornwall M. C., MacNichol E. F., Jr, Fein A. Absorptance and spectral sensitivity measurements of rod photoreceptors of the tiger salamander, Ambystoma tigrinum. Vision Res. 1984;24(11):1651–1659. doi: 10.1016/0042-6989(84)90323-7. [DOI] [PubMed] [Google Scholar]
- Falk G., Fatt P. Letter: The dynamic voltage-transfer function for rod-bipolar cell transmission. Vision Res. 1974 Aug;14(8):739–741. doi: 10.1016/0042-6989(74)90073-x. [DOI] [PubMed] [Google Scholar]
- Hare W. A., Lowe J. S., Owen G. Morphology of physiologically identified bipolar cells in the retina of the tiger salamander, Ambystoma tigrinum. J Comp Neurol. 1986 Oct 1;252(1):130–138. doi: 10.1002/cne.902520108. [DOI] [PubMed] [Google Scholar]
- Hecht S., Shlaer S., Pirenne M. H. ENERGY, QUANTA, AND VISION. J Gen Physiol. 1942 Jul 20;25(6):819–840. doi: 10.1085/jgp.25.6.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hárosi F. I. Absorption spectra and linear dichroism of some amphibian photoreceptors. J Gen Physiol. 1975 Sep;66(3):357–382. doi: 10.1085/jgp.66.3.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko A., Saito T. Ionic mechanisms underlying the responses of off-center bipolar cells in the carp retina. II. Studies on responses evoked by transretinal current stimulation. J Gen Physiol. 1983 Apr;81(4):603–612. doi: 10.1085/jgp.81.4.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasansky A. Organization of the outer synaptic layer in the retina of the larval tiger salamander. Philos Trans R Soc Lond B Biol Sci. 1973;265(872):471–489. doi: 10.1098/rstb.1973.0033. [DOI] [PubMed] [Google Scholar]
- Saito T., Kaneko A. Ionic mechanisms underlying the responses of off-center bipolar cells in the carp retina. I. Studies on responses evoked by light. J Gen Physiol. 1983 Apr;81(4):589–601. doi: 10.1085/jgp.81.4.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito T., Kondo H., Toyoda J. I. Ionic mechanisms of two types of on-center bipolar cells in the carp retina. I. The responses to central illumination. J Gen Physiol. 1979 Jan;73(1):73–90. doi: 10.1085/jgp.73.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito T., Kondo H., Toyoda J. Ionic mechanisms of two types of on-center bipolar cells in the carp retina. II. The responses to annular illumination. J Gen Physiol. 1981 Nov;78(5):569–589. doi: 10.1085/jgp.78.5.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slaughter M. M., Miller R. F. An excitatory amino acid antagonist blocks cone input to sign-conserving second-order retinal neurons. Science. 1983 Mar 11;219(4589):1230–1232. doi: 10.1126/science.6131536. [DOI] [PubMed] [Google Scholar]
- Toyoda J., Kujiraoka T. Analyses of bipolar cell responses elicited by polarization of horizontal cells. J Gen Physiol. 1982 Jan;79(1):131–145. doi: 10.1085/jgp.79.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkovsky P., Stell W. K. Retinal structure in the smooth dogfish Mustelus canis: electron microscopy of serially sectioned bipolar cell synaptic terminals. J Comp Neurol. 1973 Jul 15;150(2):147–167. doi: 10.1002/cne.901500204. [DOI] [PubMed] [Google Scholar]
- Wu S. M. Synaptic transmission from rods to bipolar cells in the tiger salamander retina. Proc Natl Acad Sci U S A. 1985 Jun;82(11):3944–3947. doi: 10.1073/pnas.82.11.3944. [DOI] [PMC free article] [PubMed] [Google Scholar]