Abstract
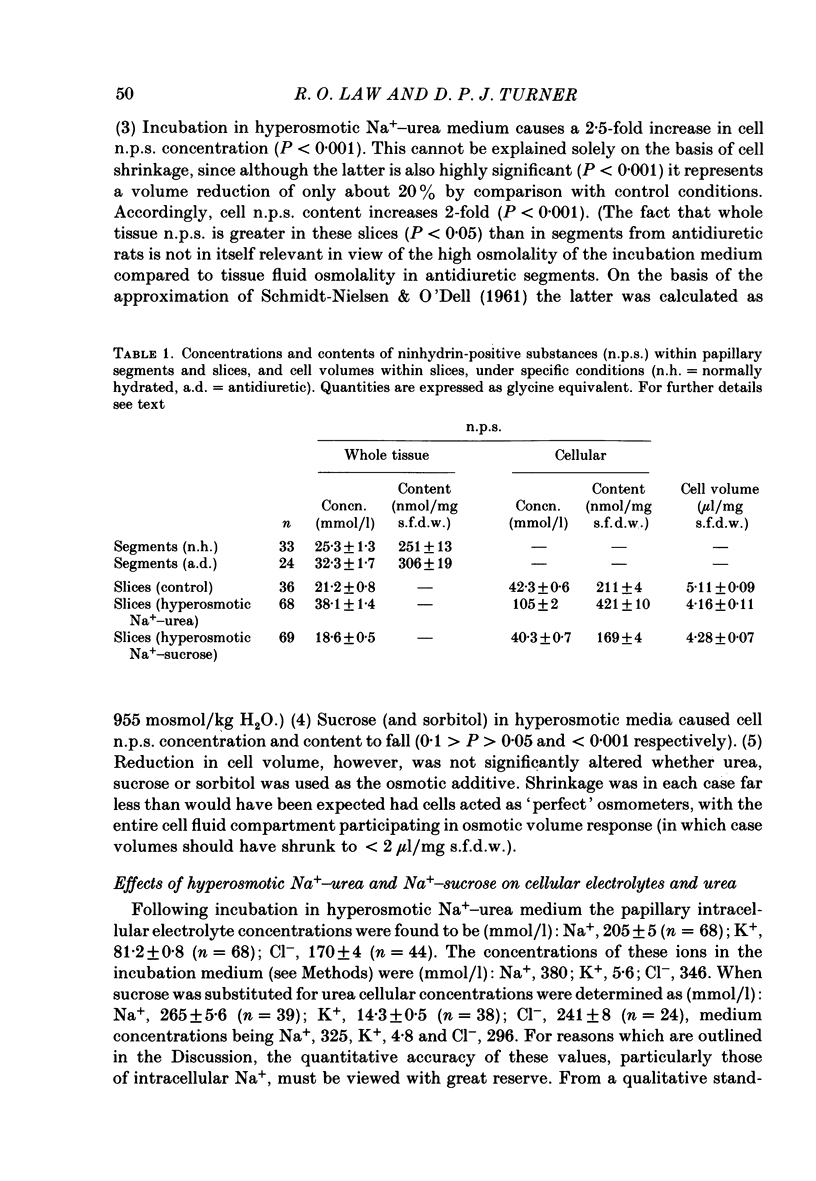
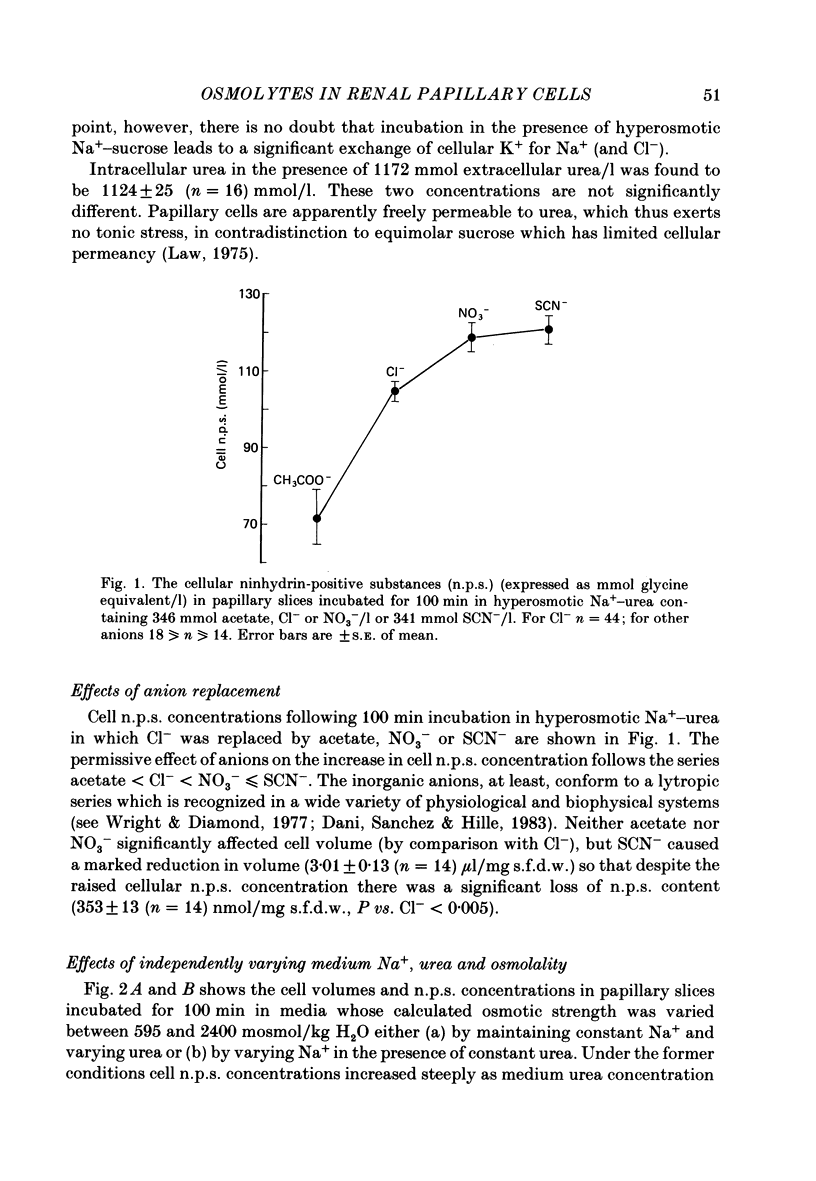
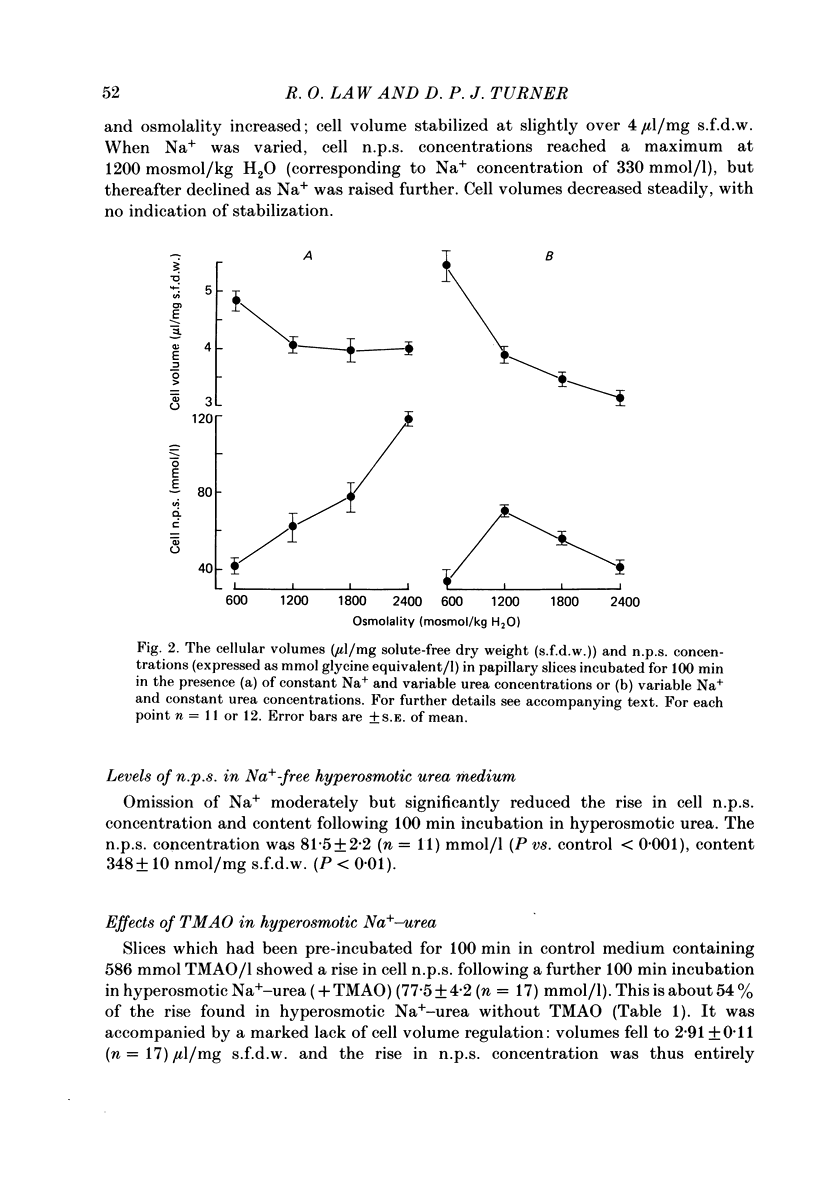
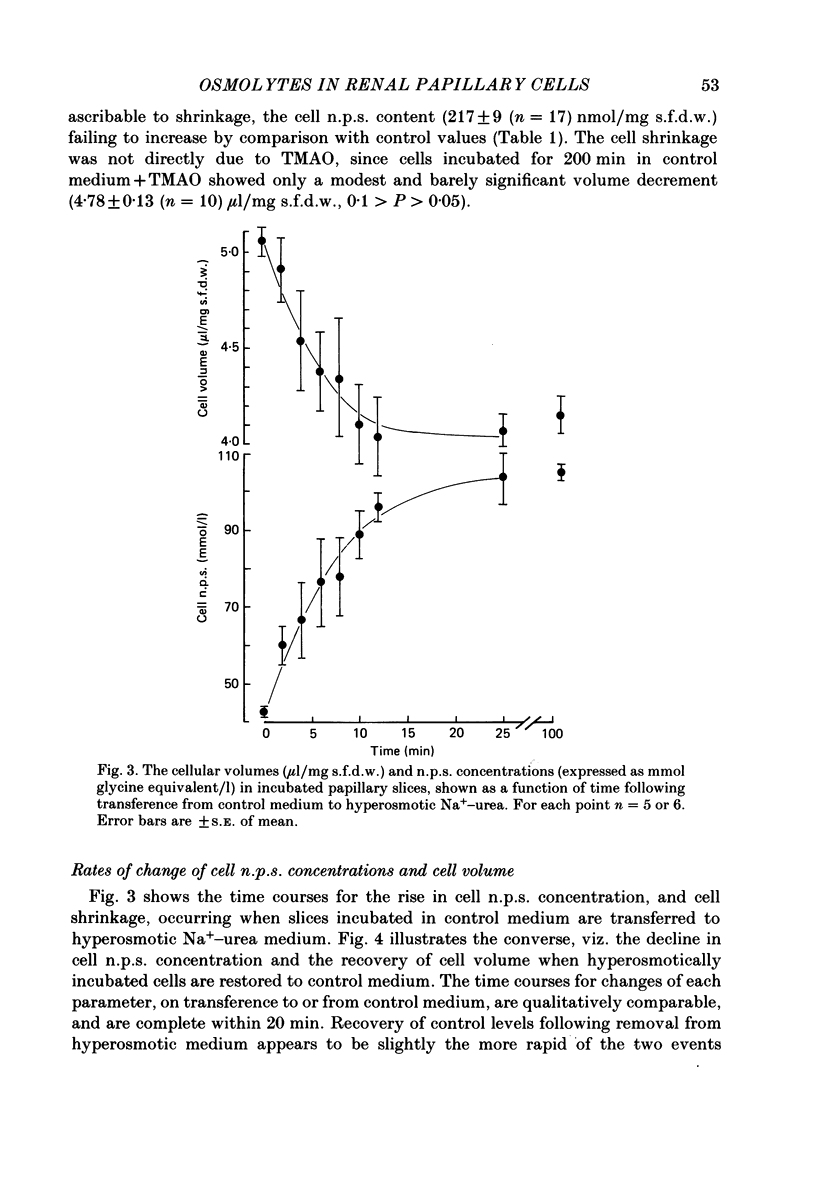
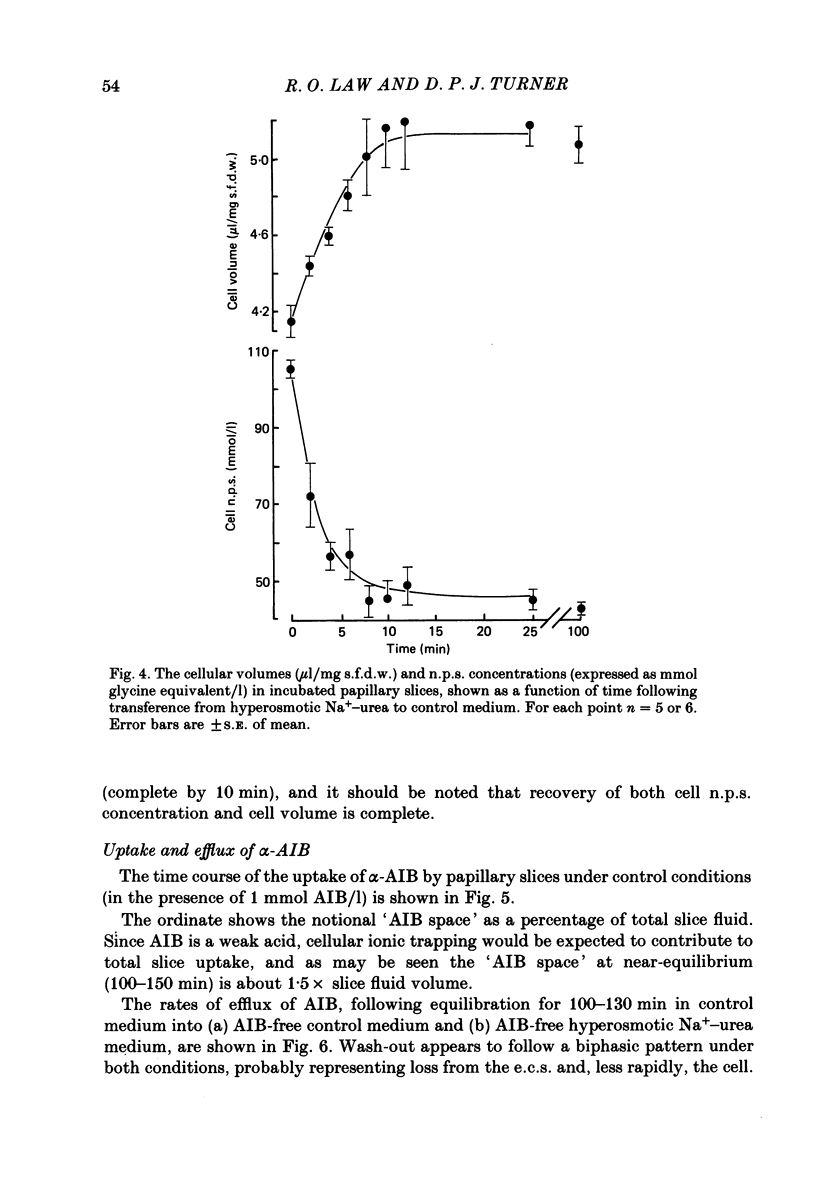
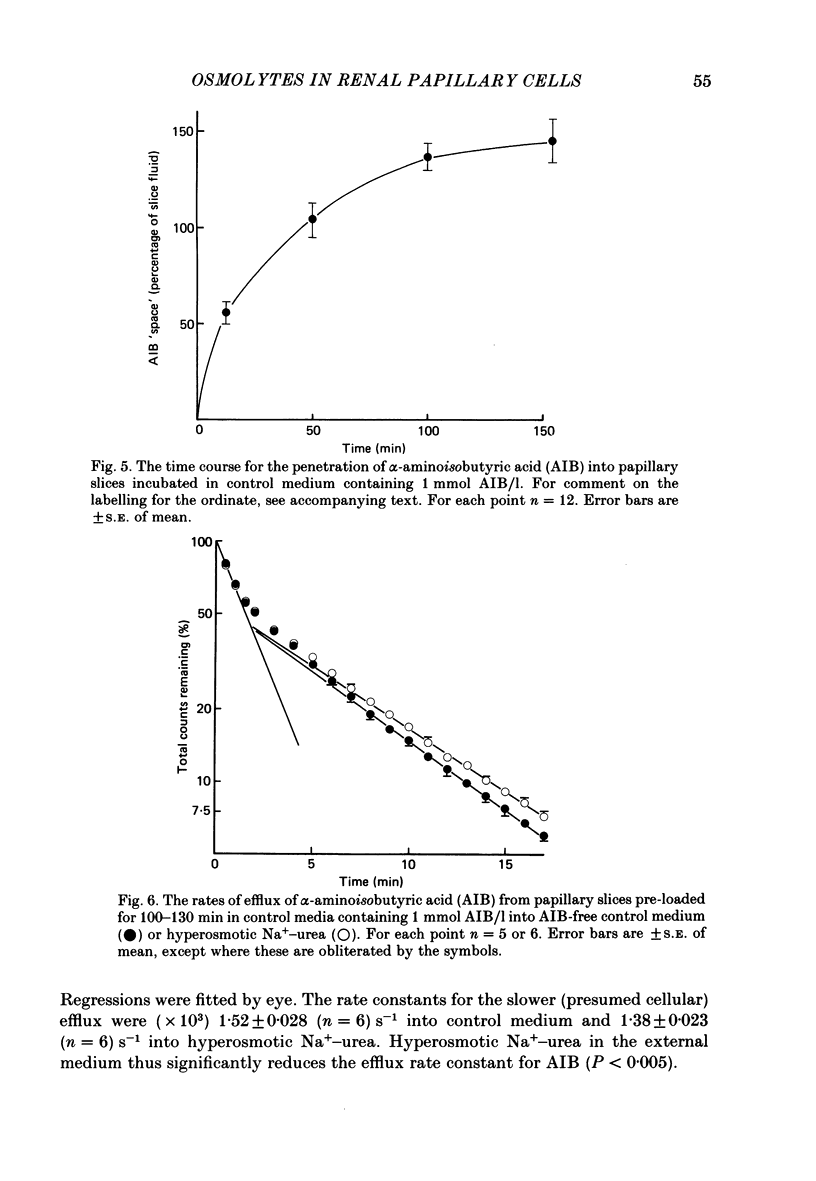
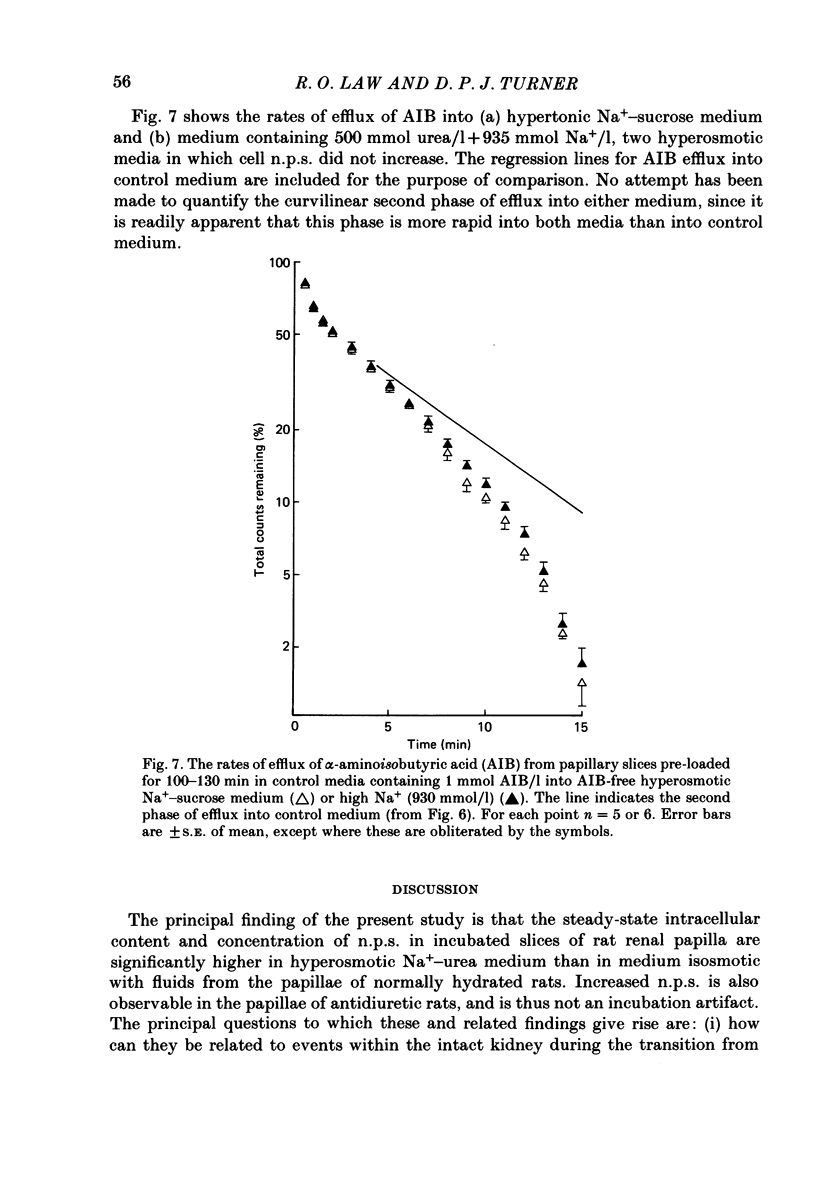
1. A study has been made of the concentrations and contents of ninhydrin-positive substances (n.p.s.), presumed to be predominantly but not exclusively amino acids, in the cells of rat renal papillary slices incubated in variously modified Krebs phosphate-bicarbonate Ringer solution. 2. When the medium osmolality was increased from 710 (control) to 2000 mosmol/kg H2O by additional NaCl and urea, the steady-state cellular n.p.s. concentration rose from 42.3 +/- 0.6 (mean +/- S.E. of mean; n = 36) to 105 +/- 2 (n = 68) mmol/l (glycine equivalent). Cell fluid content fell from 5.11 +/- 0.09 (n = 36) to 4.16 +/- 0.11 (n = 68) microliter/mg solute-free dry weight. Hence cell n.p.s. content increased from 211 +/- 4 (n = 36) to 421 +/- 10 (n = 68) nmol/mg solute-free dry weight. 3. A comparable loss of cell fluid was observed when urea was replaced by sucrose or sorbitol. No increase in cell n.p.s. occurred, and there was a marked cell Na+-for-K+ exchange. 4. The extent of the increase in cell n.p.s. in the presence of 2000 mosmol/kg H2O (NaCl + urea) was sensitive to the presence of external anions in the sequence acetate less than Cl- less than NO3- less than or equal to SCN-. 5. Cell n.p.s. concentration increased progressively as the medium osmolality was increased by the addition of urea, but Na+ at a concentration above 330 mmol/l had an inhibitory effect. The increase in n.p.s. concentration was also significantly reduced in hyperosmotic media in which Na+ was replaced by choline. 6. The increase in cell n.p.s. content due to hyperosmotic NaCl + urea was completely inhibited by pre-incubation in control medium containing trimethylamine N-oxide. 7. On transference of slices from control to hyperosmotic media (NaCl + urea) the steady-state increase in cell n.p.s. concentration was complete within 20 min and followed a time course similar to that for cell fluid loss. The n.p.s. concentration and cell fluid content returned to control levels, with similar time courses, following re-immersion in control medium. 8. Efflux of alpha-amino[1-14C]isobutyric acid (AIB) from slices pre-loaded in control medium containing 1 mmol AIB/l was slightly but significantly slower into AIB-free hyperosmotic NaCl + urea than into AIB-free control medium. The rate of efflux was greatly increased by the presence of hyperosmotic sucrose or very high Na+ (935 mmol/l).(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atherton J. C., Evans J. A., Green R., Thomas S. Influence of variations in hydration and in solute excretion of the effects of lysine-vasopressin infusion on urinary and renal tissue composition in the conscious rat. J Physiol. 1971 Mar;213(2):311–327. doi: 10.1113/jphysiol.1971.sp009384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck F., Dörge A., Rick R., Thurau K. Intra- and extracellular element concentrations of rat renal papilla in antidiuresis. Kidney Int. 1984 Feb;25(2):397–403. doi: 10.1038/ki.1984.30. [DOI] [PubMed] [Google Scholar]
- Beck F., Dörge A., Rick R., Thurau K. Osmoregulation of renal papillary cells. Pflugers Arch. 1985;405 (Suppl 1):S28–S32. doi: 10.1007/BF00581776. [DOI] [PubMed] [Google Scholar]
- Boron W. F., Sackin H. Measurement of intracellular ionic composition and activities in renal tubules. Annu Rev Physiol. 1983;45:483–496. doi: 10.1146/annurev.ph.45.030183.002411. [DOI] [PubMed] [Google Scholar]
- Bulger R. E., Beeuwkes R., 3rd, Saubermann A. J. Application of scanning electron microscopy to x-ray analysis of frozen-hydrated sections. III. Elemental content of cells in the rat renal papillary tip. J Cell Biol. 1981 Feb;88(2):274–280. doi: 10.1083/jcb.88.2.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dani J. A., Sanchez J. A., Hille B. Lyotropic anions. Na channel gating and Ca electrode response. J Gen Physiol. 1983 Feb;81(2):255–281. doi: 10.1085/jgp.81.2.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davson H. The influence of the lyotropic series of anions on cation permeability. Biochem J. 1940 Jun;34(6):917–925. doi: 10.1042/bj0340917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fugelli K., Thoroed S. M. Taurine transport associated with cell volume regulation in flounder erythrocytes under anisosmotic conditions. J Physiol. 1986 May;374:245–261. doi: 10.1113/jphysiol.1986.sp016077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hai M. A., Thomas S. The time-course of changes in renal tissue composition during lysine vasopressin infusion in the rat. Pflugers Arch. 1969;310(4):297–317. doi: 10.1007/BF00587241. [DOI] [PubMed] [Google Scholar]
- Hoffmann E. K., Lambert I. H. Amino acid transport and cell volume regulation in Ehrlich ascites tumour cells. J Physiol. 1983 May;338:613–625. doi: 10.1113/jphysiol.1983.sp014692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JARAUSCH K. H., ULLRICH K. J. Untersuchungen zum Problem der Harnkonzentrierung und Harnverdünnung; Uber die Verteilung von Elektrolyten (Na, K, Ca, Mg, Cl, anorganischem Phosphat), Harnstoff, Aminosäuren und exogenem Kreatinin in Rinde und Mark der Hundeniere bei verschiedenen Diuresezuständen. Pflugers Arch. 1956;262(6):537–550. doi: 10.1007/BF00362116. [DOI] [PubMed] [Google Scholar]
- KREBS H. A. Body size and tissue respiration. Biochim Biophys Acta. 1950 Jan;4(1-3):249–269. doi: 10.1016/0006-3002(50)90032-1. [DOI] [PubMed] [Google Scholar]
- Larsson L., Aperia A., Lechene C. Ionic transport in individual renal epithelial cells from adult and young rats. Acta Physiol Scand. 1986 Mar;126(3):321–332. doi: 10.1111/j.1748-1716.1986.tb07823.x. [DOI] [PubMed] [Google Scholar]
- Law R. O., Rowen D. The influence of hyaluronidase on urinary and renal medullary composition following antidiuretic stimulus in the rat. J Physiol. 1981 Feb;311:341–354. doi: 10.1113/jphysiol.1981.sp013588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law R. O. Volume adjustment by renal medullary cells in hypo- and hyperosmolal solutions containing permeant and impermeant solutes. J Physiol. 1975 May;247(1):55–70. doi: 10.1113/jphysiol.1975.sp010920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macknight A. D., Leaf A. Regulation of cellular volume. Physiol Rev. 1977 Jul;57(3):510–573. doi: 10.1152/physrev.1977.57.3.510. [DOI] [PubMed] [Google Scholar]
- McIver D. J., Macknight A. D. Extracellular space in some isolated tissues. J Physiol. 1974 May;239(1):31–49. doi: 10.1113/jphysiol.1974.sp010554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson R. R., Owen E. E., Schmidt-Nielsen B. Intra-renal distribution of free amino acids in antidiuretic ruminants. Comp Biochem Physiol. 1966 Sep;19(1):187–195. doi: 10.1016/0010-406x(66)90559-7. [DOI] [PubMed] [Google Scholar]
- SAIKIA T. C. COMPOSITION OF THE RENAL CORTEX AND MEDULLA OF RATS DURING WATER DIURESIS AND ANTIDIURESIS. Q J Exp Physiol Cogn Med Sci. 1965 Apr;50:146–157. doi: 10.1113/expphysiol.1965.sp001777. [DOI] [PubMed] [Google Scholar]
- SCHMIDT-NIELSEN B., O'DELL R. Structure and concentrating mechanism in the mammalian kidney. Am J Physiol. 1961 Jun;200:1119–1124. doi: 10.1152/ajplegacy.1961.200.6.1119. [DOI] [PubMed] [Google Scholar]
- Saubermann A. J., Dobyan D. C., Scheid V. L., Bulger R. E. Rat renal papilla: comparison of two techniques for x-ray analysis. Kidney Int. 1986 Mar;29(3):675–681. doi: 10.1038/ki.1986.51. [DOI] [PubMed] [Google Scholar]
- Schultz S. G., Curran P. F. Coupled transport of sodium and organic solutes. Physiol Rev. 1970 Oct;50(4):637–718. doi: 10.1152/physrev.1970.50.4.637. [DOI] [PubMed] [Google Scholar]
- Wright E. M., Diamond J. M. Anion selectivity in biological systems. Physiol Rev. 1977 Jan;57(1):109–156. doi: 10.1152/physrev.1977.57.1.109. [DOI] [PubMed] [Google Scholar]
- Yancey P. H., Clark M. E., Hand S. C., Bowlus R. D., Somero G. N. Living with water stress: evolution of osmolyte systems. Science. 1982 Sep 24;217(4566):1214–1222. doi: 10.1126/science.7112124. [DOI] [PubMed] [Google Scholar]
- Yancey P. H., Somero G. N. Counteraction of urea destabilization of protein structure by methylamine osmoregulatory compounds of elasmobranch fishes. Biochem J. 1979 Nov 1;183(2):317–323. doi: 10.1042/bj1830317. [DOI] [PMC free article] [PubMed] [Google Scholar]


