Abstract
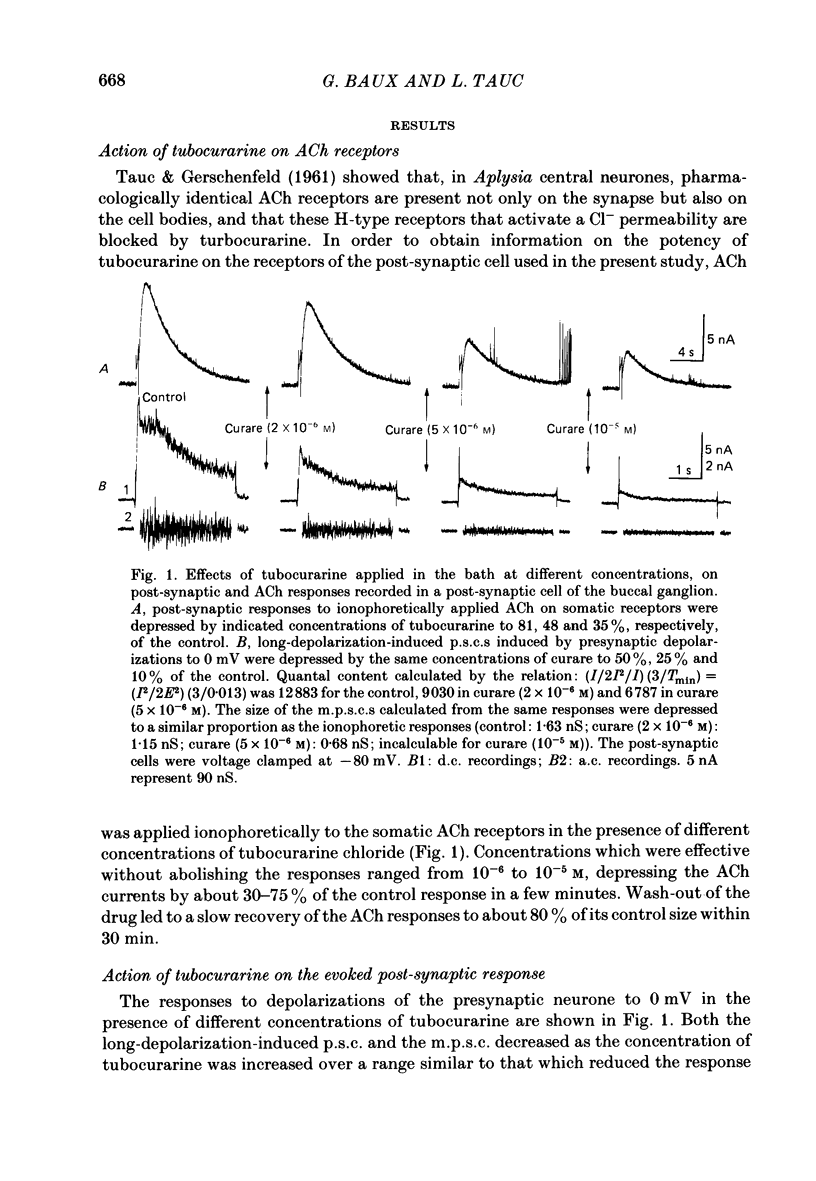
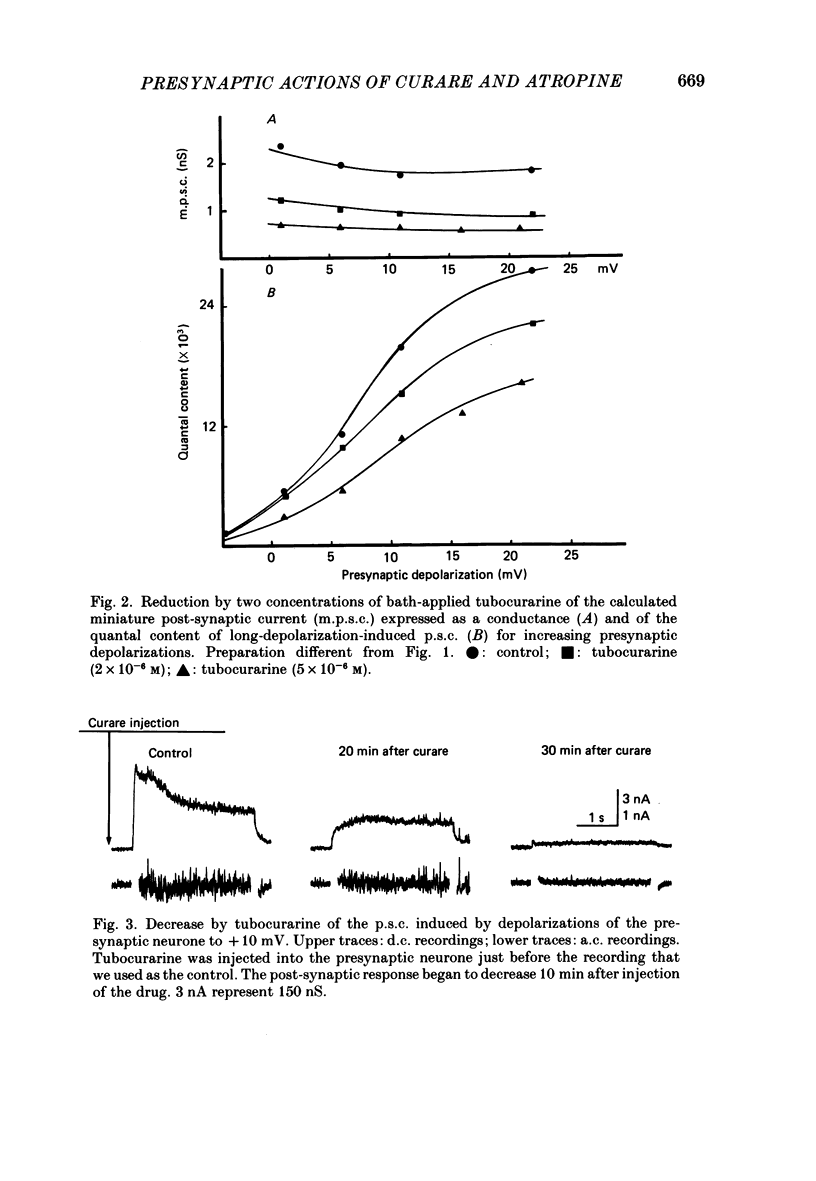
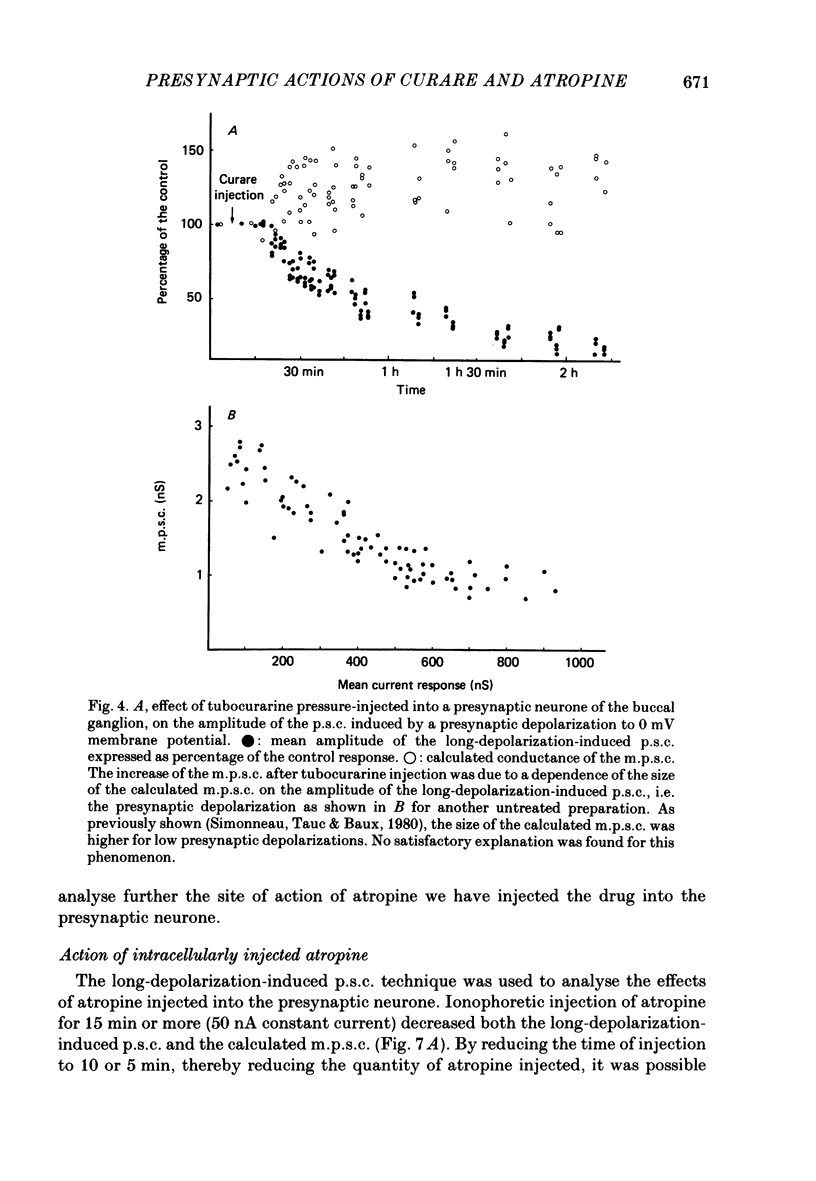
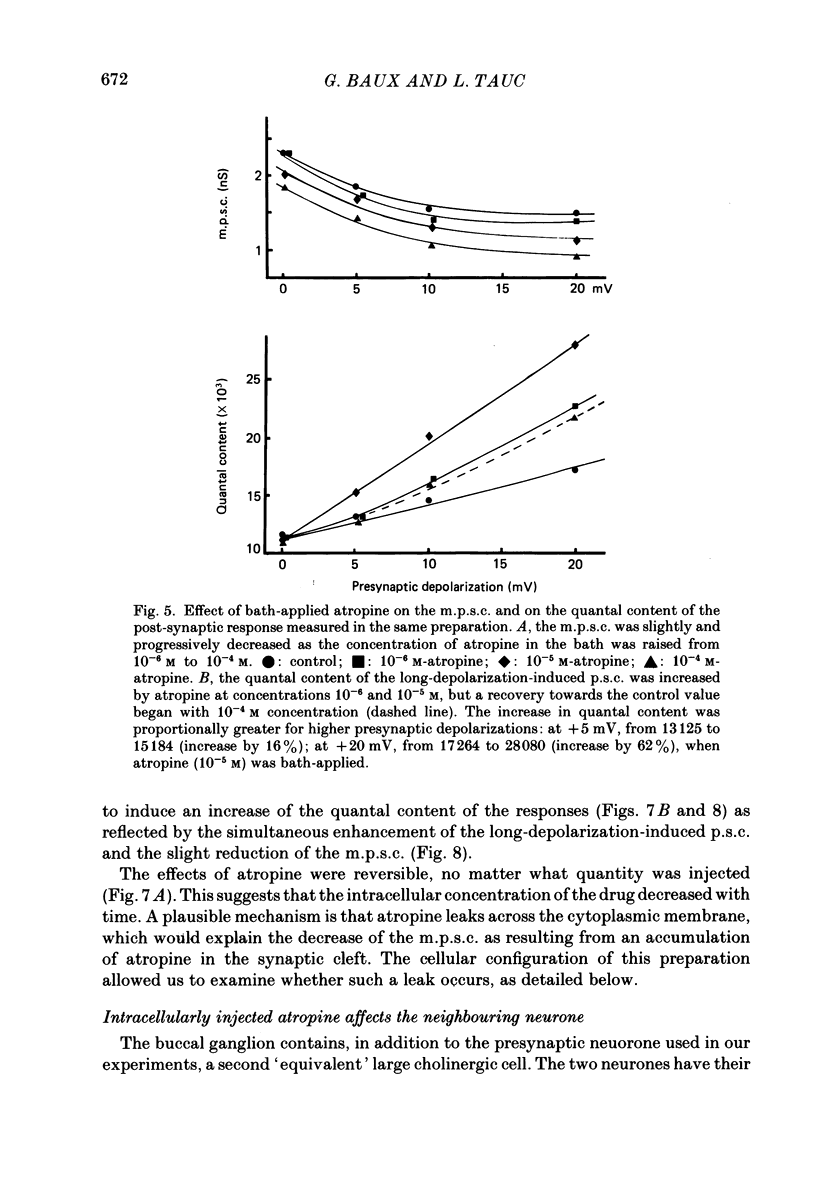
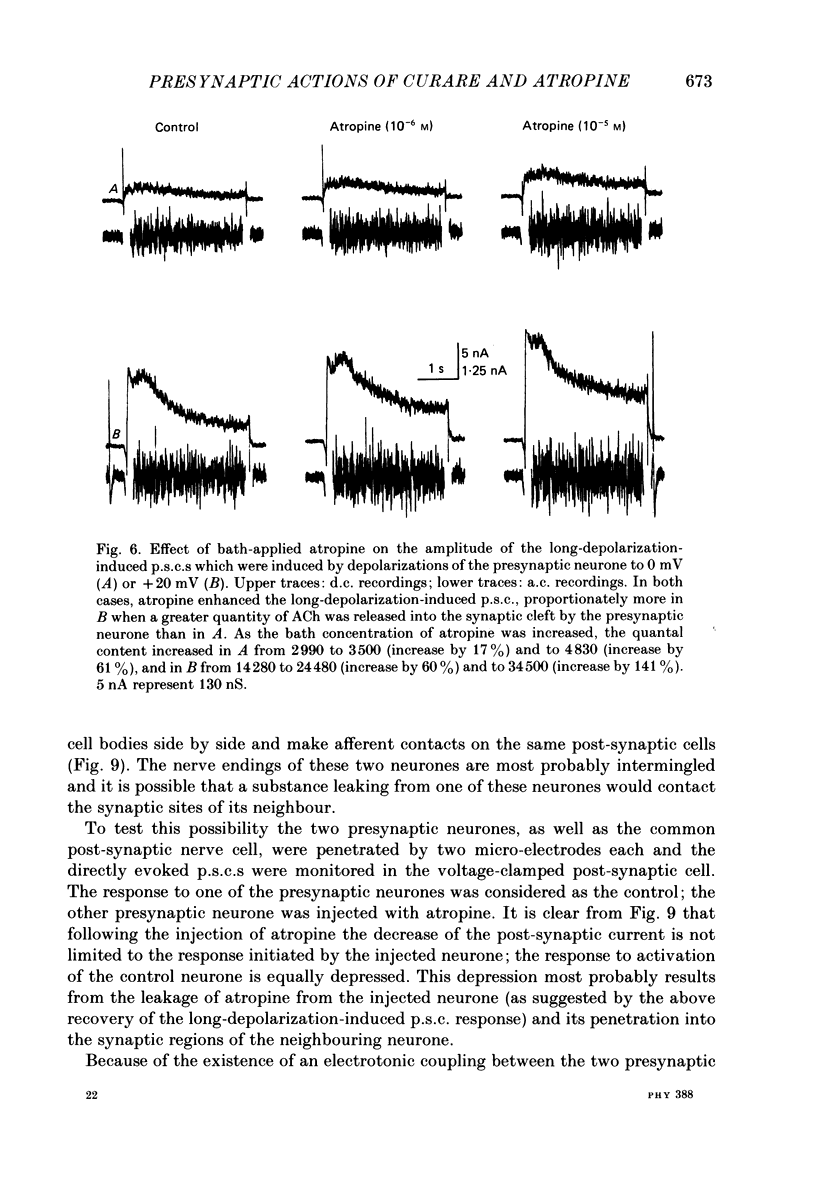
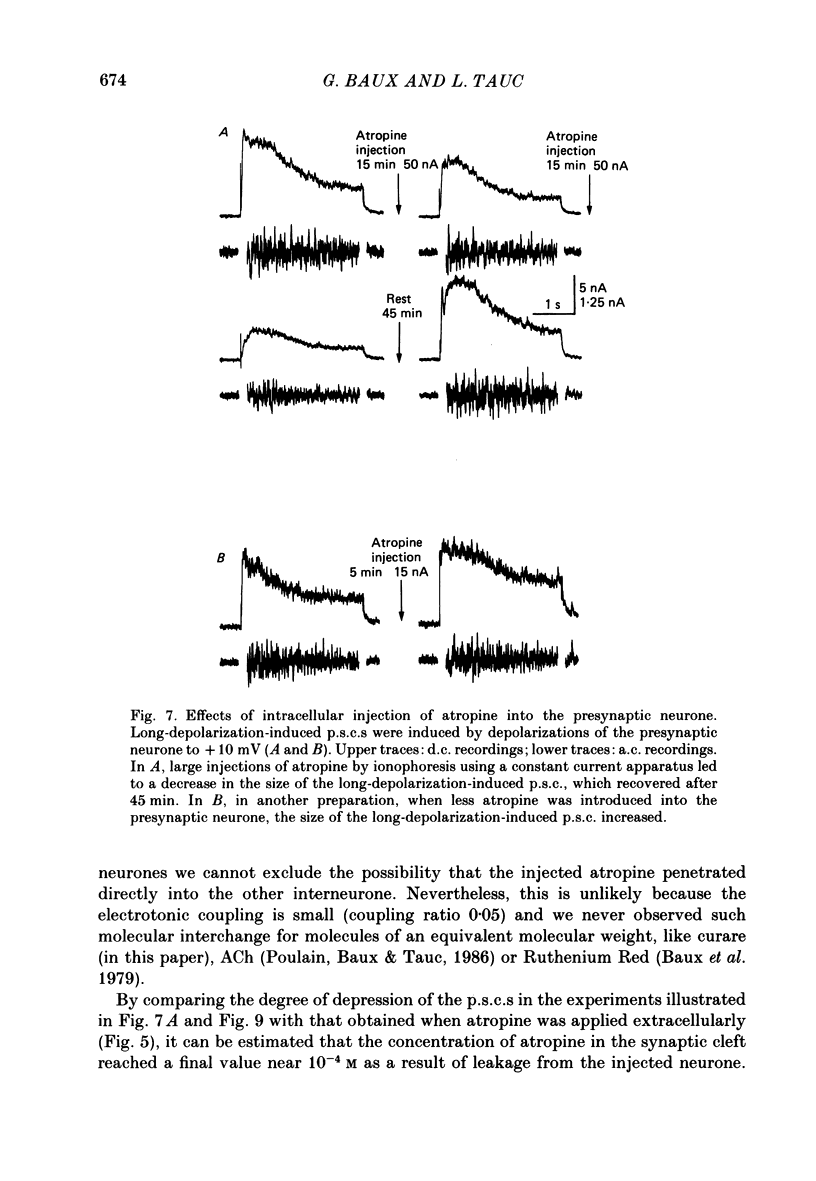
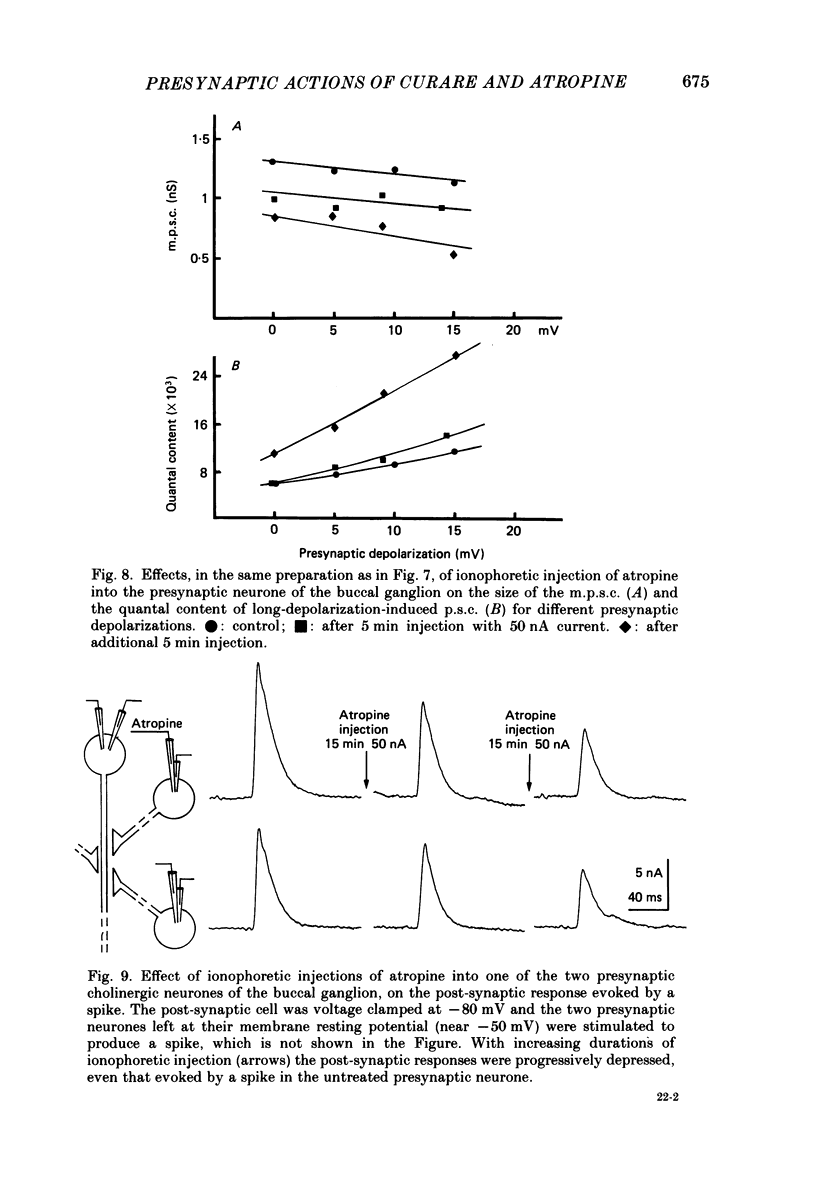
1. In a cholinergic synaptic couple in the buccal ganglion of Aplysia california, where the synaptic areas are situated close to the somata (500 micron), we were able to control transmitter release by stimulating the cell body of the presynaptic neurone with long depolarizing pulses in the presence of tetrodotoxin (TTX). 2. Statistical analysis of noise occurring at the peak of the long-depolarization-induced post-synaptic current (p.s.c.) responses allowed us to calculate the amplitude and the decay time of the miniature post-synaptic currents (m.p.s.c.s). These data were used to calculate the quantal content of the responses. 3. Bath-applied tubocurarine reduced the amplitude of the long-depolarization-induced p.s.c. more than that of the m.p.s.c.s, indicating that tubocurarine exerts a depressive presynaptic action on the quantal content of the post-synaptic responses. 4. Tubocurarine injected into the presynaptic neurone blocked synaptic transmission without decreasing the size of the m.p.s.c.s probably by acting on the mechanism of transmitter release. 5. Bath-applied atropine (10(-6) and 10(-5) M) caused a slight decrease of the m.p.s.c.s but the long-depolarization-induced p.s.c.s increased, as did the quantal content. Higher concentrations of atropine depressed strongly both the m.p.s.c. and the quantal content. 6. Injection of atropine into the presynaptic neurone had the same effect as its bath application, probably due to the leakage of the drug into the synaptic cleft; the effect depended on the concentration reached in the cleft, i.e. on the quantity of injected drug. The synapses of the neighbouring cholinergic neurone were also affected by this leak of atropine. 7. The presence of nicotinic presynaptic receptors blocked by tubocurarine, and muscarinic presynaptic receptors blocked by atropine, which regulate synaptic transmission by facilitating and depressing the ACh release respectively, is discussed.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ascher P., Marty A., Neild T. O. The mode of action of antagonists of the excitatory response to acetylcholine in Aplysia neurones. J Physiol. 1978 May;278:207–235. doi: 10.1113/jphysiol.1978.sp012300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baux G., Simonneau M., Tauc L. Transmitter release: ruthenium red used to demonstrate a possible role of sialic acid containing substrates. J Physiol. 1979 Jun;291:161–178. doi: 10.1113/jphysiol.1979.sp012805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baux G., Tauc L. Carbachol can be released at a cholinergic ganglionic synapse as a false transmitter. Proc Natl Acad Sci U S A. 1983 Aug;80(16):5126–5128. doi: 10.1073/pnas.80.16.5126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beránek R., Vyskocil F. The action of tubocurarine and atropine on the normal and denervated rat diaphragm. J Physiol. 1967 Jan;188(1):53–66. doi: 10.1113/jphysiol.1967.sp008123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatnagar S. P., MacIntosh F. C. Effects of quaternary bases and inorganic cations on acetylcholine synthesis in nervous tissue. Can J Physiol Pharmacol. 1967 Mar;45(2):249–268. doi: 10.1139/y67-028. [DOI] [PubMed] [Google Scholar]
- Bourdois P. S., Mitchell J. F., Somogyi G. T., Szerb J. C. The output per stimulus of acetylcholine from cerebral cortical slices in the presence or absence of cholinesterase inhibition. Br J Pharmacol. 1974 Dec;52(4):509–517. doi: 10.1111/j.1476-5381.1974.tb09718.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs C. A., Cooper J. R. Cholinergic modulation of the release of [3H]acetylcholine from synaptosomes of the myenteric plexus. J Neurochem. 1982 Feb;38(2):501–508. doi: 10.1111/j.1471-4159.1982.tb08656.x. [DOI] [PubMed] [Google Scholar]
- Chesselet M. F. Presynaptic regulation of neurotransmitter release in the brain: facts and hypothesis. Neuroscience. 1984 Jun;12(2):347–375. doi: 10.1016/0306-4522(84)90058-7. [DOI] [PubMed] [Google Scholar]
- Colquhoun D., Dreyer F., Sheridan R. E. The actions of tubocurarine at the frog neuromuscular junction. J Physiol. 1979 Aug;293:247–284. doi: 10.1113/jphysiol.1979.sp012888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale H. H., Feldberg W., Vogt M. Release of acetylcholine at voluntary motor nerve endings. J Physiol. 1936 May 4;86(4):353–380. doi: 10.1113/jphysiol.1936.sp003371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies M. A., Hinzen D. H. Is there an intracellular presynaptic effect of curare on neurotransmission? Brain Res. 1979 Jun 15;169(1):199–207. doi: 10.1016/0006-8993(79)90389-5. [DOI] [PubMed] [Google Scholar]
- Dunant Y., Walker A. I. Cholinergic inhibition of acetylcholine release in the electric organ of Torpedo. Eur J Pharmacol. 1982 Feb 26;78(2):201–212. doi: 10.1016/0014-2999(82)90237-0. [DOI] [PubMed] [Google Scholar]
- Feltz A., Large W. A., Trautmann A. Analysis of atropine action at the frog neutromuscular junction. J Physiol. 1977 Jul;269(1):109–130. doi: 10.1113/jphysiol.1977.sp011895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galindo A. Prejunctional effect of curare: its relative importance. J Neurophysiol. 1971 Mar;34(2):289–301. doi: 10.1152/jn.1971.34.2.289. [DOI] [PubMed] [Google Scholar]
- Gardner D. Bilateral symmetry and interneuronal organization in the buccal ganglia of Aplysia. Science. 1971 Aug 6;173(3996):550–553. doi: 10.1126/science.173.3996.550. [DOI] [PubMed] [Google Scholar]
- Glavinović M. I. Presynaptic action of curare. J Physiol. 1979 May;290(2):499–506. doi: 10.1113/jphysiol.1979.sp012786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadházy P., Szerb J. C. The effect of cholinergic drugs on [3H]acetylcholine release from slices of rat hippocampus, striatum and cortex. Brain Res. 1977 Mar 11;123(2):311–322. doi: 10.1016/0006-8993(77)90482-6. [DOI] [PubMed] [Google Scholar]
- Hubbard J. I., Schmidt R. F., Yokota T. The effect of acetylcholine upon mammalian motor nerve terminals. J Physiol. 1965 Dec;181(4):810–829. doi: 10.1113/jphysiol.1965.sp007799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard J. I., Wilson D. F., Miyamoto M. Reduction of transmitter release by D-tubocurarine. Nature. 1969 Aug 2;223(5205):531–533. doi: 10.1038/223531a0. [DOI] [PubMed] [Google Scholar]
- Jones B. E., Guyenet P., Chéramy A., Gauchy C., Glowinski J. The in vivo release of acetylcholine from cat caudate nucleus after pharmacological and surgical manipulations of dopaminergic nigrostriatal neurons. Brain Res. 1973 Dec 21;64:355–369. doi: 10.1016/0006-8993(73)90189-3. [DOI] [PubMed] [Google Scholar]
- Katz B., Miledi R. A re-examination of curare action at the motor endplate. Proc R Soc Lond B Biol Sci. 1978 Dec 4;203(1151):119–133. doi: 10.1098/rspb.1978.0096. [DOI] [PubMed] [Google Scholar]
- Katz B., Miledi R. The effect of atropine on acetylcholine action at the neuromuscular junction. Proc R Soc Lond B Biol Sci. 1973 Nov 27;184(1075):221–226. doi: 10.1098/rspb.1973.0046. [DOI] [PubMed] [Google Scholar]
- Katz B., Miledi R. The statistical nature of the acetycholine potential and its molecular components. J Physiol. 1972 Aug;224(3):665–699. doi: 10.1113/jphysiol.1972.sp009918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilbinger H., Wessler I. Inhibition by acetylcholine of the stimulation-evoked release of [3H]acetylcholine from the guinea-pig myenteric plexus. Neuroscience. 1980;5(7):1331–1340. doi: 10.1016/0306-4522(80)90205-5. [DOI] [PubMed] [Google Scholar]
- Kuriyama K., Roberts E., Vos J. Some characteristics of binding of gamma-aminobutyric acid and acetylcholine to a synaptic vesicle fraction form mouse brain. Brain Res. 1968 Jul;9(2):231–252. doi: 10.1016/0006-8993(68)90232-1. [DOI] [PubMed] [Google Scholar]
- LILLEHEIL G., NAESS K. A presynaptic effect of d-tubocurarine in the neuromuscular junction. Acta Physiol Scand. 1961 Jun;52:120–136. doi: 10.1111/j.1748-1716.1961.tb02208.x. [DOI] [PubMed] [Google Scholar]
- Lambert J. J., Volle R. L., Henderson E. G. An attempt to distinguish between the actions of neuromuscular blocking drugs on the acetylcholine receptor and on its associated ionic channel. Proc Natl Acad Sci U S A. 1980 Aug;77(8):5003–5007. doi: 10.1073/pnas.77.8.5003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magleby K. L., Pallotta B. S., Terrar D. A. The effect of (+)-tubocurarine on neuromuscular transmission during repetitive stimulation in the rat, mouse, and frog. J Physiol. 1981 Mar;312:97–113. doi: 10.1113/jphysiol.1981.sp013618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer E. M., Otero D. H. Pharmacological and ionic characterizations of the muscarinic receptors modulating [3H]acetylcholine release from rat cortical synaptosomes. J Neurosci. 1985 May;5(5):1202–1207. doi: 10.1523/JNEUROSCI.05-05-01202.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaelson D. M., Avissar S., Kloog Y., Sokolovsky M. Mechanism of acetylcholine release: possible involvement of presynaptic muscarinic receptors in regulation of acetylcholine release and protein phosphorylation. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6336–6340. doi: 10.1073/pnas.76.12.6336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell J. F. The spontaneous and evoked release of acetylcholine from the cerebral cortex. J Physiol. 1963 Jan;165(1):98–116. doi: 10.1113/jphysiol.1963.sp007045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molenaar P. C., Polak R. L. Stimulation by atropine of acetylcholine release and synthesis in cortical slices from rat brain. Br J Pharmacol. 1970 Nov;40(3):406–417. doi: 10.1111/j.1476-5381.1970.tb10622.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray T. F., Mpitsos G. J., Siebenaller J. F., Barker D. L. Stereoselective L-[3H]quinuclidinyl benzilate-binding sites in nervous tissue of Aplysia californica: evidence for muscarinic receptors. J Neurosci. 1985 Dec;5(12):3184–3188. doi: 10.1523/JNEUROSCI.05-12-03184.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordström O., Bartfai T. Muscarinic autoreceptor regulates acetylcholine release in rat hippocampus: in vitro evidence. Acta Physiol Scand. 1980 Apr;108(4):347–353. doi: 10.1111/j.1748-1716.1980.tb06543.x. [DOI] [PubMed] [Google Scholar]
- Pinchasi I., Burstein M., Michaelson D. M. Metabolism of arachidonic acid and prostaglandins in the Torpedo electric organ: modulation by the presynaptic muscarinic acetylcholine receptor. Neuroscience. 1984 Dec;13(4):1359–1364. doi: 10.1016/0306-4522(84)90304-x. [DOI] [PubMed] [Google Scholar]
- Poulain B., Baux G., Tauc L. Presynaptic transmitter content controls the number of quanta released at a neuro-neuronal cholinergic synapse. Proc Natl Acad Sci U S A. 1986 Jan;83(1):170–173. doi: 10.1073/pnas.83.1.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raiteri M., Leardi R., Marchi M. Heterogeneity of presynaptic muscarinic receptors regulating neurotransmitter release in the rat brain. J Pharmacol Exp Ther. 1984 Jan;228(1):209–214. [PubMed] [Google Scholar]
- Riker W. F., Jr, Okamoto M. Pharmacology of motor nerve terminals. Annu Rev Pharmacol. 1969;9:173–208. doi: 10.1146/annurev.pa.09.040169.001133. [DOI] [PubMed] [Google Scholar]
- Rospars J. P., Lefresne P., Beaujouan J. C., Glowinski J. Effect of external ACh and of atropine on 14C-ACh synthesis and release in rat cortical slices. Naunyn Schmiedebergs Arch Pharmacol. 1977 Nov;300(2):153–161. doi: 10.1007/BF00505046. [DOI] [PubMed] [Google Scholar]
- Rowell P. P., Winkler D. L. Nicotinic stimulation of [3H]acetylcholine release from mouse cerebral cortical synaptosomes. J Neurochem. 1984 Dec;43(6):1593–1598. doi: 10.1111/j.1471-4159.1984.tb06083.x. [DOI] [PubMed] [Google Scholar]
- Simonneau M., Tauc L., Baux G. Quantal release of acetylcholine examined by current fluctuation analysis at an identified neuro-neuronal synapse of Aplysia. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1661–1665. doi: 10.1073/pnas.77.3.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinnakre J., Tauc L. Calcium influx in active Aplysia neurones detected by injected aequorin. Nat New Biol. 1973 Mar 28;242(117):113–115. doi: 10.1038/newbio242113b0. [DOI] [PubMed] [Google Scholar]
- TAUC L., GERSCHENFELD H. M. A cholinergic mechanism of inhibitory synaptic transmission in a molluscan nervous system. J Neurophysiol. 1962 Mar;25:236–262. doi: 10.1152/jn.1962.25.2.236. [DOI] [PubMed] [Google Scholar]
- TAUC L., GERSCHENFELD H. M. Cholinergic transmission mechanisms for both excitation and inhibition in molluscan central synapses. Nature. 1961 Oct 28;192:366–367. doi: 10.1038/192366a0. [DOI] [PubMed] [Google Scholar]
- Tauc L., Baux G. Are there intracellular acetylcholine receptors in the cholinergic synaptic nerve terminals? J Physiol (Paris) 1982;78(4):366–372. [PubMed] [Google Scholar]
- Tauc L., Hoffmann A., Tsuji S., Hinzen D. H., Faille L. Transmission abolished on a cholinergic synapse after injection of acetylcholinesterase into the presynaptic neurone. Nature. 1974 Aug 9;250(5466):496–498. doi: 10.1038/250496a0. [DOI] [PubMed] [Google Scholar]
- Tauc L. Non vesicular release of neurotransmitter. Physiol Rev. 1982 Jul;62(3):857–893. doi: 10.1152/physrev.1982.62.3.857. [DOI] [PubMed] [Google Scholar]
- Trautmann A. Curare can open and block ionic channels associated with cholinergic receptors. Nature. 1982 Jul 15;298(5871):272–275. doi: 10.1038/298272a0. [DOI] [PubMed] [Google Scholar]
- Weiler M. H., Misgeld U., Cheong D. K. Presynaptic muscarinic modulation of nicotinic excitation in the rat neostriatum. Brain Res. 1984 Mar 26;296(1):111–120. doi: 10.1016/0006-8993(84)90516-x. [DOI] [PubMed] [Google Scholar]


