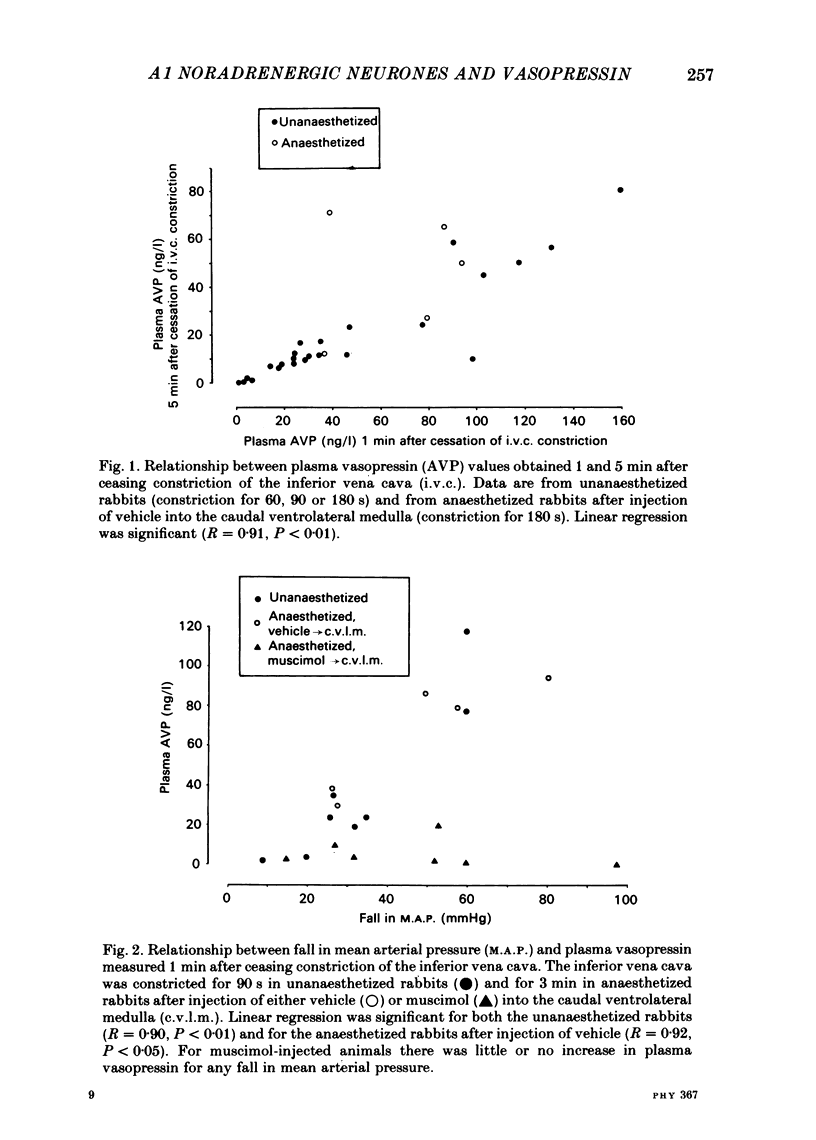
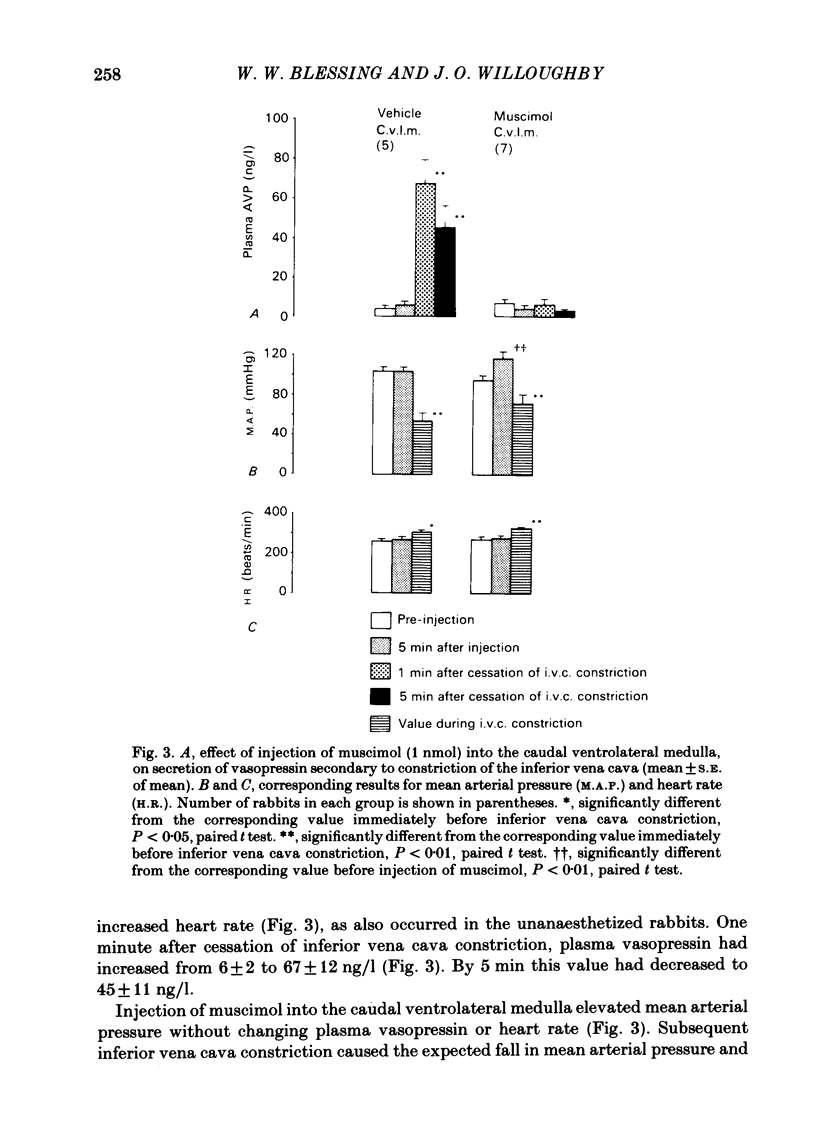
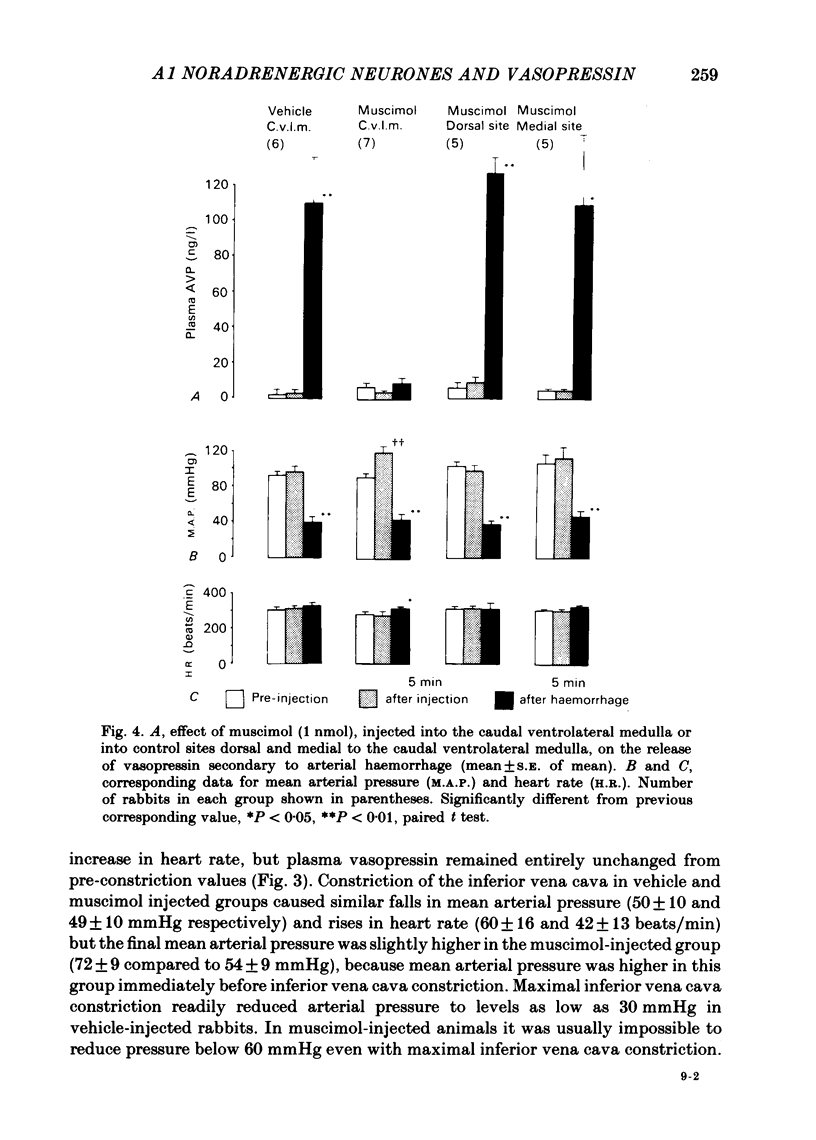
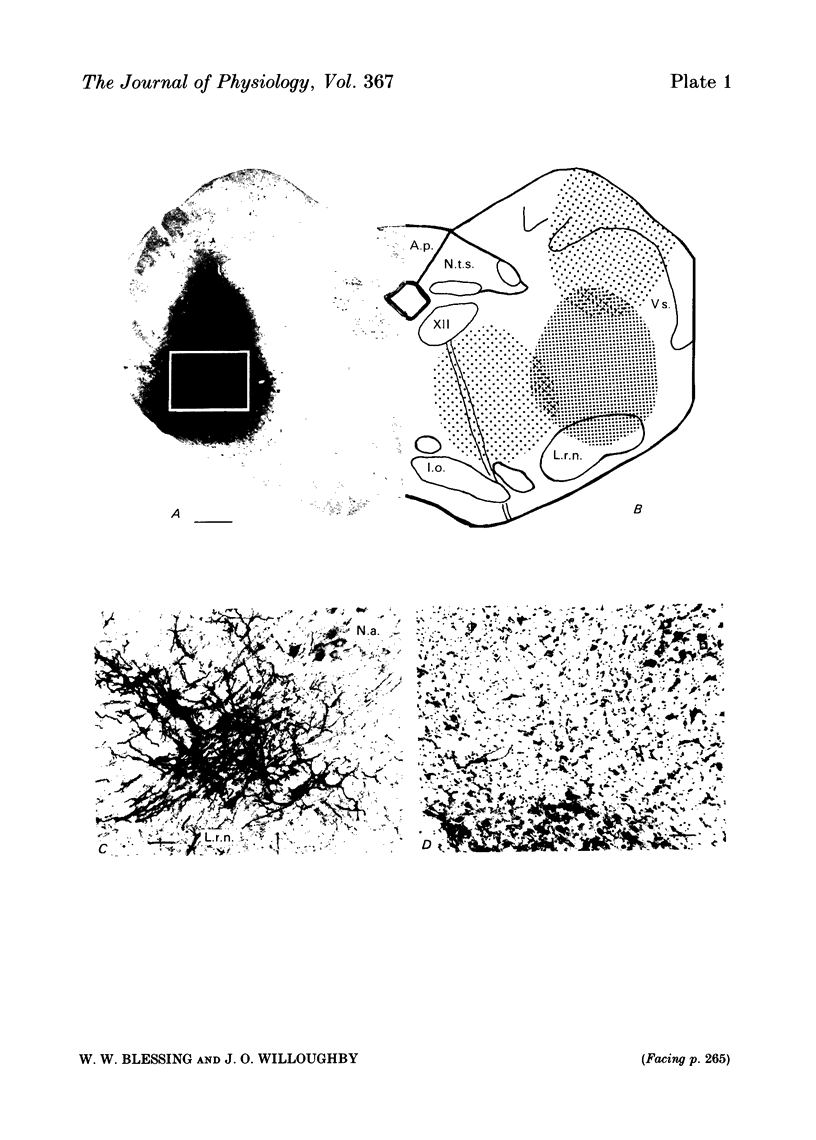
Abstract
The A 1 noradrenergic neurones are known to project from the caudal ventrolateral medulla to the vasopressin-secreting neuroendocrine cells in the hypothalamus. They therefore represent a possible central pathway from the medulla to the hypothalamus for baroreceptor-initiated secretion of vasopressin. We tested this hypothesis in the anaesthetized rabbit. Muscimol, a gamma-aminobutyric-acid-receptor agonist, was injected into the caudal ventrolateral medulla to inhibit the A 1 noradrenergic neurones. Secretion of vasopressin, measured by radioimmunoassay, was initiated either by arterial haemorrhage or by constriction of the inferior vena cava. After injection of vehicle into the caudal ventrolateral medulla, or after injection of muscimol into nearby control areas, both haemorrhage and constriction of the inferior vena cava produced the expected elevation in plasma vasopressin. After injection of muscimol into the caudal ventrolateral medulla, secretion of vasopressin in response to haemorrhage and to constriction of the inferior vena cava, was completely abolished. The A 1 noradrenergic neurones may be the sole pathway transmitting the reflex for baroreceptor-initiated secretion of vasopressin from the medulla to the hypothalamus.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong D. M., Saper C. B., Levey A. I., Wainer B. H., Terry R. D. Distribution of cholinergic neurons in rat brain: demonstrated by the immunocytochemical localization of choline acetyltransferase. J Comp Neurol. 1983 May 1;216(1):53–68. doi: 10.1002/cne.902160106. [DOI] [PubMed] [Google Scholar]
- Arnauld E., Cirino M., Layton B. S., Renaud L. P. Contrasting actions of amino acids, acetylcholine, noradrenaline and leucine enkephalin on the excitability of supraoptic vasopressin-secreting neurons. A microiontophoretic study in the rat. Neuroendocrinology. 1983;36(3):187–196. doi: 10.1159/000123455. [DOI] [PubMed] [Google Scholar]
- Barker J. L., Crayton J. W., Nicoll R. A. Supraoptic neurosecretory cells: adrenergic and cholinergic sensitivity. Science. 1971 Jan 15;171(3967):208–210. doi: 10.1126/science.171.3967.208. [DOI] [PubMed] [Google Scholar]
- Bisset G. W., Chowdrey H. S. A cholinergic link in the reflex release of vasopressin by hypotension in the rat. J Physiol. 1984 Sep;354:523–545. doi: 10.1113/jphysiol.1984.sp015391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blessing W. W., Chalmers J. P., Howe P. R. Distribution of catecholamine-containing cell bodies in the rabbit central nervous system. J Comp Neurol. 1978 May 15;179(2):407–423. doi: 10.1002/cne.901790210. [DOI] [PubMed] [Google Scholar]
- Blessing W. W., Goodchild A. K., Dampney R. A., Chalmers J. P. Cell groups in the lower brain stem of the rabbit projecting to the spinal cord, with special reference to catecholamine-containing neurons. Brain Res. 1981 Sep 21;221(1):35–55. doi: 10.1016/0006-8993(81)91062-3. [DOI] [PubMed] [Google Scholar]
- Blessing W. W., Jaeger C. B., Ruggiero D. A., Reis D. J. Hypothalamic projections of medullary catecholamine neurons in the rabbit: a combined catecholamine fluorescence and HRP transport study. Brain Res Bull. 1982 Jul-Dec;9(1-6):279–286. doi: 10.1016/0361-9230(82)90141-1. [DOI] [PubMed] [Google Scholar]
- Blessing W. W., Oertel W. H., Willoughby J. O. Glutamic acid decarboxylase immunoreactivity is present in perikarya of neurons in nucleus tractus solitarius of rat. Brain Res. 1984 Nov 26;322(2):346–350. doi: 10.1016/0006-8993(84)90131-8. [DOI] [PubMed] [Google Scholar]
- Blessing W. W., Reis D. J. Evidence that GABA and glycine-like inputs inhibit vasodepressor neurons in the caudal ventrolateral medulla of the rabbit. Neurosci Lett. 1983 May 27;37(1):57–62. doi: 10.1016/0304-3940(83)90504-9. [DOI] [PubMed] [Google Scholar]
- Blessing W. W., Reis D. J. Inhibitory cardiovascular function of neurons in the caudal ventrolateral medulla of the rabbit: relationship to the area containing A1 noradrenergic cells. Brain Res. 1982 Dec 16;253(1-2):161–171. doi: 10.1016/0006-8993(82)90683-7. [DOI] [PubMed] [Google Scholar]
- Blessing W. W., Willoughby J. O. Tetrodotoxin elevates arterial pressure but not plasma vasopressin when injected into the caudal ventrolateral medulla of the rabbit. Neurosci Lett. 1985 Feb 4;53(3):259–262. doi: 10.1016/0304-3940(85)90547-6. [DOI] [PubMed] [Google Scholar]
- Ciriello J., Caverson M. M. Direct pathway from neurons in the ventrolateral medulla relaying cardiovascular afferent information to the supraoptic nucleus in the cat. Brain Res. 1984 Feb 6;292(2):221–228. doi: 10.1016/0006-8993(84)90758-3. [DOI] [PubMed] [Google Scholar]
- Ciriello J., Caverson M. M. Ventrolateral medullary neurons relay cardiovascular inputs to the paraventricular nucleus. Am J Physiol. 1984 Jun;246(6 Pt 2):R968–R978. doi: 10.1152/ajpregu.1984.246.6.R968. [DOI] [PubMed] [Google Scholar]
- Clark B. J., Silva MR Jr E. An afferent pathway for the selective release of vasopressin in response to carotid occlusion and haemorrhage in the cat. J Physiol. 1967 Aug;191(3):529–542. doi: 10.1113/jphysiol.1967.sp008266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day T. A., Ferguson A. V., Renaud L. P. Facilitatory influence of noradrenergic afferents on the excitability of rat paraventricular nucleus neurosecretory cells. J Physiol. 1984 Oct;355:237–249. doi: 10.1113/jphysiol.1984.sp015416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day T. A., Renaud L. P. Electrophysiological evidence that noradrenergic afferents selectively facilitate the activity of supraoptic vasopressin neurons. Brain Res. 1984 Jun 15;303(2):233–240. doi: 10.1016/0006-8993(84)91209-5. [DOI] [PubMed] [Google Scholar]
- DeFeudis F. V. Binsing studies with muscimol; relation ty synaptic gamma-aminobutyrate receptors. Neuroscience. 1980;5(4):675–688. doi: 10.1016/0306-4522(80)90165-7. [DOI] [PubMed] [Google Scholar]
- Feldberg W., Guertzenstein P. G., Rocha e Silva M., Jr Vasopressin release by nicotine: the site of action. Br J Pharmacol. 1975 Aug;54(4):463–474. doi: 10.1111/j.1476-5381.1975.tb07592.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldberg W., Guertzenstein P. G. Vasodepressor effects obtained by drugs acting on the ventral surface of the brain stem. J Physiol. 1976 Jun;258(2):337–355. doi: 10.1113/jphysiol.1976.sp011423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldberg W., Rocha e Silva M., Jr Inhibition of vasopressin release to carotid occlusion by gamma-aminobutyric acid and glycine. Br J Pharmacol. 1981 Jan;72(1):17–24. doi: 10.1111/j.1476-5381.1981.tb09099.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldberg W., Rocha e Silva M., Jr Vasopressin release produced in anaesthetized cats by antagonists of gamma-aminobutyric acid and glycine. Br J Pharmacol. 1978 Jan;62(1):99–106. doi: 10.1111/j.1476-5381.1978.tb07011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feuerstein G., Johnson A. K., Zerbe R. L., Davis-Kramer R., Faden A. I. Anteroventral hypothalamus and hemorrhagic shock: cardiovascular and neuroendocrine responses. Am J Physiol. 1984 Apr;246(4 Pt 2):R551–R557. doi: 10.1152/ajpregu.1984.246.4.R551. [DOI] [PubMed] [Google Scholar]
- Guertzenstein P. G., Lopes O. U. Cardiovascular responses evoked from the nicotine-sensitive area on the ventral surface of the medulla oblongata in the cat. J Physiol. 1984 Feb;347:345–360. doi: 10.1113/jphysiol.1984.sp015069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston G. A., Curtis D. R., De Groat W. C., Duggan A. W. Central actions of ibotenic acid and muscimol. Biochem Pharmacol. 1968 Dec;17(12):2488–2489. doi: 10.1016/0006-2952(68)90141-x. [DOI] [PubMed] [Google Scholar]
- Kalia M., Mesulam M. M. Brain stem projections of sensory and motor components of the vagus complex in the cat: I. The cervical vagus and nodose ganglion. J Comp Neurol. 1980 Sep 15;193(2):435–465. doi: 10.1002/cne.901930210. [DOI] [PubMed] [Google Scholar]
- Kannan H., Yamashita H., Osaka T. Paraventricular neurosecretory neurons: synaptic inputs from the ventrolateral medulla in rats. Neurosci Lett. 1984 Oct 12;51(2):183–188. doi: 10.1016/0304-3940(84)90548-2. [DOI] [PubMed] [Google Scholar]
- Kimura H., McGeer P. L., Peng J. H., McGeer E. G. The central cholinergic system studied by choline acetyltransferase immunohistochemistry in the cat. J Comp Neurol. 1981 Aug 1;200(2):151–201. doi: 10.1002/cne.902000202. [DOI] [PubMed] [Google Scholar]
- Korner P. I., Shaw J., West M. J., Oliver J. R. Central nervous system control of baroreceptor reflexes in the rabbit. Circ Res. 1972 Nov;31(5):637–652. doi: 10.1161/01.res.31.5.637. [DOI] [PubMed] [Google Scholar]
- Lightman S. L., Todd K., Everitt B. J. Ascending noradrenergic projections from the brainstem: evidence for a major role in the regulation of blood pressure and vasopressin secretion. Exp Brain Res. 1984;55(1):145–151. doi: 10.1007/BF00240508. [DOI] [PubMed] [Google Scholar]
- Loewy A. D., Burton H. Nuclei of the solitary tract: efferent projections to the lower brain stem and spinal cord of the cat. J Comp Neurol. 1978 Sep 15;181(2):421–449. doi: 10.1002/cne.901810211. [DOI] [PubMed] [Google Scholar]
- McKellar S., Loewy A. D. Organization of some brain stem afferents to the paraventricular nucleus of the hypothalamus in the rat. Brain Res. 1981 Aug 3;217(2):351–357. doi: 10.1016/0006-8993(81)90010-x. [DOI] [PubMed] [Google Scholar]
- Milton A. S., Paterson A. T. A microinjection study of the control of antidiuretic hormone release by the supraoptic nucleus of the hypothalamus in the cat. J Physiol. 1974 Sep;241(3):607–628. doi: 10.1113/jphysiol.1974.sp010674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miselis R. R., Shapiro R. E., Hand P. J. Subfornical organ efferents to neural systems for control of body water. Science. 1979 Sep 7;205(4410):1022–1025. doi: 10.1126/science.472723. [DOI] [PubMed] [Google Scholar]
- Moss R. L., Urban I., Cross B. A. Microelectrophoresis of cholinergic and aminergic drugs on paraventricular neurons. Am J Physiol. 1972 Aug;223(2):310–318. doi: 10.1152/ajplegacy.1972.223.2.310. [DOI] [PubMed] [Google Scholar]
- Ricardo J. A., Koh E. T. Anatomical evidence of direct projections from the nucleus of the solitary tract to the hypothalamus, amygdala, and other forebrain structures in the rat. Brain Res. 1978 Sep 15;153(1):1–26. doi: 10.1016/0006-8993(78)91125-3. [DOI] [PubMed] [Google Scholar]
- Sawchenko P. E., Swanson L. W. The organization of noradrenergic pathways from the brainstem to the paraventricular and supraoptic nuclei in the rat. Brain Res. 1982 Nov;257(3):275–325. doi: 10.1016/0165-0173(82)90010-8. [DOI] [PubMed] [Google Scholar]
- Spyer K. M. Central nervous integration of cardiovascular control. J Exp Biol. 1982 Oct;100:109–128. doi: 10.1242/jeb.100.1.109. [DOI] [PubMed] [Google Scholar]
- Sun M. Mary Lasker's Latest Pet Project. Science. 1982 Aug 13;217(4560):611–611. doi: 10.1126/science.217.4560.611. [DOI] [PubMed] [Google Scholar]
- Sved A. F., Blessing W. W., Reis D. J. Caudal ventrolateral medulla can alter vasopressin and arterial pressure. Brain Res Bull. 1985 Mar;14(3):227–232. doi: 10.1016/0361-9230(85)90087-5. [DOI] [PubMed] [Google Scholar]
- Tanaka J., Kaba H., Saito H., Seto K. The action of the A1 noradrenergic region on phasically firing neurons in the rat paraventricular nucleus. Brain Res. 1984 Sep 17;310(1):138–141. doi: 10.1016/0006-8993(84)90017-9. [DOI] [PubMed] [Google Scholar]
- Urano A., Kobayashi H. Effects of noradrenaline and dopamine injected into the supraoptic nucleus on urine flow rate in hydrated rats. Exp Neurol. 1978 May 15;60(1):140–150. doi: 10.1016/0014-4886(78)90173-5. [DOI] [PubMed] [Google Scholar]
- Van Itallie C. M., Fernstrom J. D. Osmolal effects on vasopressin secretion in the streptozotocin-diabetic rat. Am J Physiol. 1982 Jun;242(6):E411–E417. doi: 10.1152/ajpendo.1982.242.6.E411. [DOI] [PubMed] [Google Scholar]
- Westlund K. N., Bowker R. M., Ziegler M. G., Coulter J. D. Noradrenergic projections to the spinal cord of the rat. Brain Res. 1983 Mar 14;263(1):15–31. doi: 10.1016/0006-8993(83)91196-4. [DOI] [PubMed] [Google Scholar]
- Willette R. N., Barcas P. P., Krieger A. J., Sapru H. N. Endogenous GABAergic mechanisms in the medulla and the regulation of blood pressure. J Pharmacol Exp Ther. 1984 Jul;230(1):34–39. [PubMed] [Google Scholar]
- Willette R. N., Krieger A. J., Barcas P. P., Sapru H. N. Medullary gamma-aminobutyric acid (GABA) receptors and the regulation of blood pressure in the rat. J Pharmacol Exp Ther. 1983 Sep;226(3):893–899. [PubMed] [Google Scholar]
- Yamashita H., Koizumi K. Influence of carotid and aortic baroreceptors on neurosecretory neurons in supraoptic nuclei. Brain Res. 1979 Jul 13;170(2):259–277. doi: 10.1016/0006-8993(79)90106-9. [DOI] [PubMed] [Google Scholar]



