Abstract
PRODH maps to 22q11 in the region deleted in the velocardiofacial syndrome/DiGeorge syndrome (VCFS/DGS) and encodes proline oxidase (POX), a mitochondrial inner-membrane enzyme that catalyzes the first step in the proline degradation pathway. At least 16 PRODH missense mutations have been identified in studies of type I hyperprolinemia (HPI) and schizophrenia, 10 of which are present at polymorphic frequencies. The functional consequences of these missense mutations have been inferred by evolutionary conservation, but none have been tested directly. Here, we report the effects of these mutations on POX activity. We find that four alleles (R185Q, L289M, A455S, and A472T) result in mild (<30%), six (Q19P, A167V, R185W, D426N, V427M, and R431H) in moderate (30%–70%), and five (P406L, L441P, R453C, T466M, and Q521E) in severe (>70%) reduction in POX activity, whereas one (Q521R) increases POX activity. The POX encoded by one severe allele (T466M) shows in vitro responsiveness to high cofactor (flavin adenine dinucleotide) concentrations. Although there is limited information on plasma proline levels in individuals of known PRODH genotype, extant data suggest that severe hyperprolinemia (>800 μM) occurs in individuals with large deletions and/or PRODH missense mutations with the most-severe effect on function (L441P and R453C), whereas modest hyperprolinemia (300–500 μM) is associated with PRODH alleles with a moderate reduction in activity. Interestingly, three of the four alleles associated with or found in schizophrenia (V427M, L441P, and R453C) resulted in severe reduction of POX activity and hyperprolinemia. These observations plus the high degree of polymorphism at the PRODH locus are consistent with the hypothesis that reduction in POX function is a risk factor for schizophrenia.
Introduction
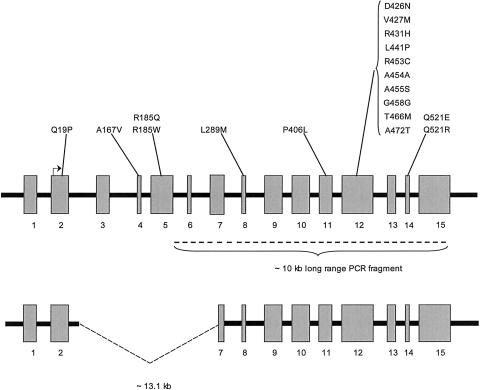
The PRODH gene comprises 15 exons that span 23.77 kb, located at 17.3 Mb (National Center for Biotechnology Information build 34) in 22q11, near the centromeric end of the region typically deleted in the velocardiofacial syndrome/DiGeorge syndrome (VCFS/DGS) (McDermid and Morrow 2002). A PRODH pseudogene (ΨPRODH) located 1.4 Mb telomeric on 22q at 18.7 Mb has >95% sequence identity with PRODH but has an internal deletion that removes a 13.1-kb segment containing exons 2 through the 5′ half of exon 7, along with the intervening introns (Liu et al. 2002a, 2002b; McDermid and Morrow 2002; Williams et al. 2003b) (fig. 1). ΨPRODH has accumulated several missense mutations that, in some instances, have been transferred to PRODH, apparently by gene conversion (Liu et al. 2002b).
Figure 1.
Diagram of PRODH (upper panel) and ΨPRODH (lower panel). The exons, represented by the gray rectangles, are numbered and shown roughly to scale. The intronic sequence is represented by the black heavy line and is not to scale. The exonic location of the missense mutations, by exon, is shown above the rectangles. The translation start site in exon 2 is indicated by the right-angle arrow; the heavy dashed line below the rectangles in the upper panel indicates the ∼10-kb PRODH-specific PCR product. The deletion in ΨPRODH is indicated by the dashed lines in the lower panel.
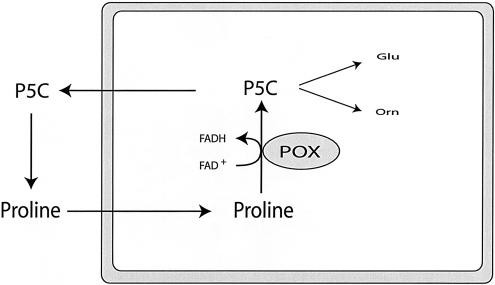
PRODH encodes proline oxidase (POX), a mitochondrial inner-membrane enzyme—expressed in kidney, liver, and brain—that catalyzes the conversion of proline to Δ1-pyrroline-5-carboxylate (P5C) by use of flavin adenine dinucleotide (FAD) as a cofactor (fig. 2). P5C has three possible metabolic fates: oxidation to glutamate in a reaction catalyzed by P5C dehydrogenase, transamination to ornithine in a reaction catalyzed by ornithine aminotransferase, or reduction back to proline in a reaction catalyzed by P5C reductase by use of either nicotinamide adenine dinucleotide phosphate or nicotinamide adenine dinucleotide as cofactor. The latter reaction, coupled with POX, forms a cycle of proline synthesis and degradation that can transfer redox potential between subcellular compartments and between cells (Phang et al. 2001). PRODH was one of 14 genes among 7,202 assayed in a cultured colorectal cancer cell line whose expression was increased ⩾10-fold by p53-induced genes (p53) (Polyak et al. 1997). A similar upregulation of PRODH by p53 was observed in a bladder carcinoma cell line (Maxwell and Davis 2000). Subsequent reports have shown that cells with high PRODH expression demonstrate a proline-dependent increase in reactive-oxygen species (ROS) generation, suggesting that high POX activity with attendant increases in ROS and/or the proline redox cycle may play a role in apoptosis and/or cellular proliferation (Maxwell and Davis 2000; Donald et al. 2001; Maxwell and Rivera 2003).
Figure 2.
Schematic representation of proline metabolic pathways. The rectangle represents a mitochondrion (see text for additional details).
Proline itself has multiple physiological functions. It is a nonessential, protein amino acid with a pyrrolidine ring structure that provides unique characteristics for peptide structure (Berg et al. 2002). Free proline is also utilized as an osmolyte in certain plant and human cells (Kiyosue et al. 1996; Yoshiba et al. 1997; Dall’Asta et al. 1999; Takagi et al. 2000; Bussolati et al. 2001). Additionally, several properties of proline metabolism in the CNS suggest that proline functions as an inhibitory neurotransmitter and/or as a metabolic precursor of glutamate in subpopulations of glutamatergic neurons (reviewed in Phang et al. [2001]). Consistent with this hypothesis, a specific, high-affinity proline transporter (hProt/SLC6A7) has been shown to be expressed in certain glutamatergic neurons, where it localizes to the membranes of small, subsynaptic vesicles or, more rarely, to the plasma membrane of these axon terminals (Fremeau et al. 1992; Shafqat et al. 1995; Velaz-Faircloth et al. 1995; Renick et al. 1999). Proline has also been shown to be a regulator of cortical acetycholinesterase activity in rats (Fremeau et al. 1992; Delwing et al. 2003a, 2003b).
Several defects in the proline catabolic pathway have been associated with increased plasma proline levels (fig. 2). Two monogenic, autosomal recessive inborn errors are known. Hyperprolinemia type I (HPI [MIM 239500]) results from inherited deficiency of POX and is associated with plasma proline levels with a range of 3–10-fold above normal (mean plasma proline in controls after an overnight fast is 161 μM, with a range of 51–271 μM) (Phang et al. 2001). Hyperprolinemia type II (HPII [MIM 239510]) results from inherited deficiency of the second enzyme in the proline catabolic pathway, P5C dehydrogenase, and is characterized by plasma proline levels 10–15-fold above normal and excretion of P5C in urine (Valle et al. 1976; Geraghty et al. 1998). The clinical phenotype of HPII includes an increased frequency of seizures and mild mental retardation (Flynn et al. 1989; Geraghty et al. 1998; Phang et al. 2001). The phenotype of HPI is less well characterized. Although many affected individuals have been described as asymptomatic, a few with severe neurological manifestations, including at least two with schizophrenia, have been reported (Humbertclaude et al. 2001; Jacquet et al. 2002, 2003). It is uncertain whether these associations reflect pathophysiological consequences of the metabolic disturbances in HPI or ascertainment bias. Mild hyperprolinemia to an extent overlapping with that of HPI has also been observed in VCFS/DGS, a spectrum of developmental abnormalities that results from a 1.5–3-Mb deletion in 22q11 (McDermid and Morrow 2002). Prior to the mapping of PRODH, Jaeken et al. (1996) suggested that the hemizygous deletion of PRODH might account for the hyperprolinemia they observed in a patient with VCFS/DGS, and subsequent studies have shown that about half of patients with VCFS/DGS have hyperprolinemia (Goodman et al. 2000). Interestingly, patients with VCFS have a greatly increased risk (∼20–30-fold) of psychiatric disorders, particularly schizophrenia (Pulver et al. 1994; Karayiorgou et al. 1995; Lindsay et al. 1995; Murphy et al. 1999; Ivanov et al. 2003; Williams et al. 2003c). Consistent with this observation, several studies have mapped susceptibility loci for schizophrenia to 22q11. In particular, Liu et al. recently reported association of certain PRODH variants with increased susceptibility to schizophrenia (Liu et al. 2002b). Moreover, the same group described a sensorimotor-gating deficit, an endophenotype also observed in patients with schizophrenia, in the POX-deficient Pro/Re mouse (Gogos et al. 1999).
At least 16 PRODH missense mutations have been identified in patients with HPI (Jacquet et al. 2002), schizophrenia (Jacquet et al. 2002; Liu et al. 2002b; Williams et al. 2003b), unexplained hyperprolinemia (W.-W. Lin, C.-A. Hu, D. Valle, J. Steel, unpublished observations), and control subjects (Jacquet et al. 2002; Liu et al. 2002b; Williams et al. 2003b). The functional consequences of these missense mutations have been inferred by evolutionary conservation of the altered residue, but none have been tested directly. Here, we use transient transfections into cells (CHO-K1-C9) lacking endogenous POX activity, to determine the functional consequences of these 16 PRODH missense mutations singly or as haplotypes with multiple mutations on POX activity.
Material and Methods
Reagents and Chemicals
We purchased restriction enzymes and buffers from New England Biolabs: Pfu-Turbo DNA polymerase from Stratagene and chemicals from Sigma. We obtained 14C-proline from New England Nuclear and purified it prior to use by ion-exchange chromatography on Dowex AG 50w-8x hydrogen form (BioRad) (Phang et al. 2001). To assay green fluorescent protein (GFP) fluorescence, we used a Zeiss LSM 510 Meta confocal laser-scanning microscope.
Cloning and Mutagenesis of Human Proline Oxidase
Utilizing RT-PCR and the PRODH cDNA sequence we previously submitted to GenBank (accession number NM_016335), we cloned a full-length PRODH cDNA and transferred the 2.4-kb EcoRI/KpnI fragment containing the entire 1,800-bp ORF into pBluescript KS (Stratagene). An alternative cDNA (GenBank accession number AF120278) encodes an N-terminal truncated protein lacking 84 N-terminal amino acids and recognizable mitochondrial leader sequence and has not been shown to encode a functionally active enzyme.
We created synonymous mutations that introduced a ClaI (ATC GAC→ATC GAT) and a BstEII (GGC TAC CCC→GGT TAC CCC) site at cDNA positions 966 and 1626, respectively (where +1 is the A of the initiation methionine codon). This step allowed division of the ORF into three fragments by use of restriction enzymes. For expression, we transferred the wild-type PRODH cDNA into pTracer (Invitrogen) by EcoRI/KpnI and verified the integrity of the recombinant plasmid by sequencing. We mutagenized the ClaI/BstEII-modified PRODH cDNA in pBluescript KS by PCR by use of the QuikChange Mutagenesis Kit (Stratagene). All primers are listed in a supplementary tab-delimited ASCII file (online only), which can be imported into a Microsoft Excel spreadsheet. Dependent on the position of the desired mutation, we subcloned either the mutagenized KpnI/ClaI or mutagenized ClaI/BstEII fragment into the pTracer expression construct and resequenced to verify the presence of the mutation and the integrity of the ligations.
Transfection and Assay of POX Activity
For all expression studies, we utilized a subclone (CHO-K1-C9) of CHO-K1 cells that lack endogenous POX activity (Valle et al. 1973). For electroporation, we used 30 μg of the indicated plasmid DNA and 350 volts/400 Ω/960 μF in 300 μl of growth medium containing 1.25% DMSO and 5–7×106 cells. After 48 h, we harvested the cells by washing the monolayer with PBS and scraping them into cold PBS. The cells were collected by centrifugation at 480×g and were resuspended in 0.1 M KPO4 (pH 7.2). The cells and their organelles were disrupted by sonication for 1 min at a setting of 25% (Branson Sonifier 450 [Branson Ultrasonics]). Total protein was determined with the Pierce BCA protein assay (Pierce). POX-specific activity (nmol/prod/mg/hr) was assayed radioisotopically, as described elsewhere (Phang et al. 1975). To account for variation in transfection efficiency, we expressed the data as a percentage of wild type (specific activity of mutant allele/transfection efficiency)/(specific activity of wild-type allele/transfection efficiency). For alleles with severe reduction in POX activity, we repeated the assay with the addition of 1 mM FAD.
Antibodies and Immunoblotting
We generated a rabbit anti-human POX antiserum against a peptide corresponding to the exact C-terminus of POX (-LLRRLRTGNLFHRPA) and used it in a dilution of 1:500. The secondary antibody, goat anti-rabbit horseradish peroxidase, was used in a dilution of 1:5,000. We used a mouse anti-GFP monoclonal antibody (Clontech) to detect GFP. For immunoblotting, we separated the proteins (20 μg/lane) by SDS PAGE and transferred them to Hybond-PVDF membranes (Amersham), according to the protocols of the manufacturer.
Genotyping
For those PRODH alleles whose frequency was not available in the literature (A167V, D426N, Q521E, and Q521R), we genotyped 50 North American controls. Because these mutations are also present in ΨPRODH, we used a long-range PCR strategy to selectively amplify an ∼10-kb fragment present only in PRODH, using primers that are not complementary to any ΨPRODH sequence. This ensures that the genotyping reflects only PRODH (Williams et al. 2003b). From this 10-kb fragment, we amplified the individual exons and genotyped by analysis with restriction enzymes or by hybridization to allele-specific oligonucleotides (ASO), as described elsewhere (Braverman et al. 1997). All primers, restriction sites, and ASO probes are listed in the supplementary data file (online only).
Results
PRODH Alleles
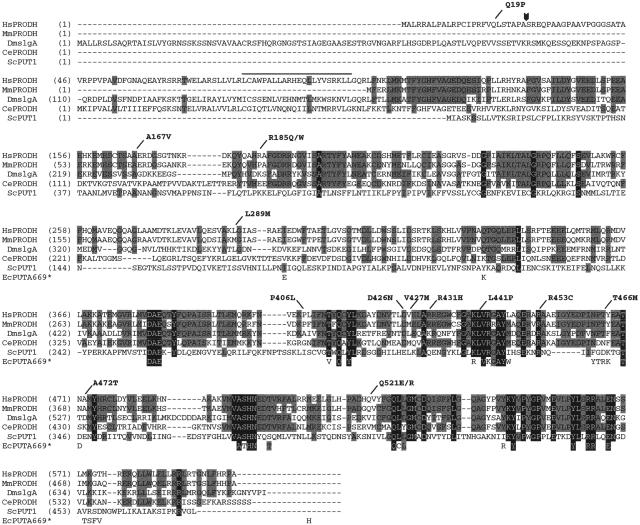
The full-length PRODH cDNA (GenBank accession number NM_016335) encodes a protein of 600 amino acids with a putative N-terminal mitochondrial leader sequence of 25 residues and a possible leucine zipper domain between residues 79 and 100 (fig. 3). The C-terminal 40% of the protein is highly conserved, with 12 blocks of identity of two or more amino acids across representative eukaryotic species, human to yeast. Moreover, in the recently solved structure of the Escherichia coli POX ortholog, PutA669 (Lee et al. 2003), 18 of the 40 residues that are within 5 Å of substrate or cofactor are identical to the corresponding residues in the conserved blocks in eukaryotic POX. Of the 16 missense mutations we studied, 12 alter residues in this region of the protein. The PRODH alleles expressed in this study and their frequency and clinical associations are summarized in table 1. Of the 16 mutations, 10 were polymorphic, with a frequency ⩾0.01 in the general population, in one or more studies.
Figure 3.
Alignment of the predicted PRODH amino acid sequences from various eukaryotic species. Residues conserved across three or four of the sequences are indicated by a gray background, those conserved in all five eukaryotic examples by a black background. Also shown are the 40 residues of E. coli PutA669 that are within 5 Å of substrate and/or cofactor (Lee et al. 2003). These are spaced as they are in PutA669, but, for the sake of clarity, we have not shown the intervening residues. The shading scheme for the E. coli residues follows the convention for the eukaryotic sequences. The locations of the human missense mutations studied in this article are indicated at the top of the figure. The arrowhead indicates the predicted cleavage site for the mitochondrial targeting sequence. The overline indicates a possible leucine zipper motif. Hs=Homo sapiens, Mm=Mus musculus, Dm=Drosophila melanogaster, Ce=Caenorhabditis elegans, Sc=Saccharomyces cerevisiae, and Ec=E. coli.
Table 1.
Analysis of PRODH Alleles
| Allele | Reference SNP (rs) Number | Exon | Allele Frequency in Control Individualsa | Identified inb | Associated with Schizophreniac |
| Q19P | 2008720 | 2 | .29d | C, S | |
| A167V | 4 | .03e | C, S | ||
| R185W | 4819756 | 5 | .37f | C, S | |
| R185Q | 5 | .05f | C | ||
| L289M | 8 | .03f; 0/136g | C, H, S | ||
| P406L | 3970555 | 11 | 0/216f | S | |
| D426N | 12 | 0/94e | H | ||
| V427M | 2238731 | 12 | .02f | C, H, S | +f |
| R431H | 2904552 | 12 | .16d; .10g; .13h | C, H, S | |
| L441P | 2904551 | 12 | 0/74f; 0/136g | H, S | |
| R453C | 3970559 | 12 | .004f; .015g | C, H, S | +f |
| A455S | 1807467 | 12 | 0/136g | H, S | |
| T466M | 2870984 | 12 | 0/156f | S | +f |
| A472T | 2870983 | 12 | .02f; .10g; .07h | C, S | +f |
| Q521R | 450046 | 14 | .05g | C, H, S | |
| Q521E | 14 | 0/94e |
Frequency in controls, as determined by the indicated (footnoted) study. For alleles not detected, the result is presented as 0/number of chromosomes screened.
C=control individuals, H=hyperprolinemics, and S=schizophrenics.
Associated with schizophrenia in at least one study, at P⩾.05.
Williams et al. 2003b.
The present study.
Liu et al. 2002.
Jacquet et al. 2002.
Jacquet et al. 2004.
POX Activity
The specific activity of POX measured in our transient transfection assays could be influenced by transfection efficiency and by level of expression of the introduced recombinant plasmid. To control for the former, we scored the fraction of cells expressing GFP in counts of 500 cells on cover slips included in the cell-culture dish. Transfection efficiencies determined in this way had a range of 12%–15% and were used to normalize the POX activity. To control for the level of expression, we took advantage of the fact that the pTracer vector used in these studies expresses both GFP and the introduced cDNA, which allowed comparison of the relative amounts of GFP and POX in immunoblots on sonicates of the transfected cells (fig. 4). In general, the relative amounts of POX and GFP for each construct were similar, which indicates that PRODH expression and POX stability were the same for each of the mutant alleles. POX-L441P is an exception, with reduced amounts as compared with GFP, which suggests that this mutation reduces the stability of POX, an observation that agrees with a recent report showing that the corresponding mutation in E. coli PutA669 (L432P) results in an unstable enzyme (Zhang et al. 2004). Despite this effect on stability, the amount of POX-L441P present in the sonicates (∼20% of control) was sufficient to assay its enzymatic activity. We also note reduced amounts of GFP in sonicates of cells expressing POX-Q19P. The explanation for this is uncertain, but, relevant to our studies of POX activity, the amount of POX-Q19P is similar to that of the other mutant forms of POX (fig. 4).
Figure 4.
Immunoblot analysis of POX and GFP in sonicates of cells transfected with the indicated recombinant pTracer construct. For each panel, POX is shown above, GFP below. Each lane contains 20 μg of crude cell sonicate. POX migrates as an ∼66-kDa protein.
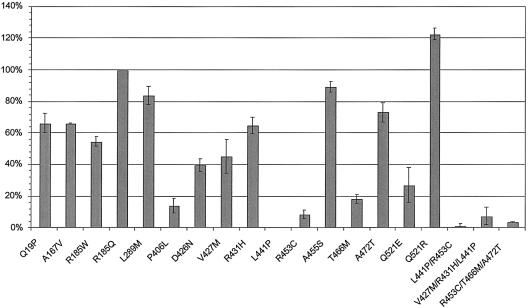
The normalized POX activity encoded by the PRODH alleles in our study varied widely (fig. 5). For purposes of analysis, we grouped the alleles into three categories by activity: group I contains four alleles (R185Q, L289M, A455S, and A472T) with little, if any, effect on POX activity (<30% reduction); group II contains six alleles (Q19P, A167V, R185W, D426N, V427M, and R431H) with moderate (30%–70%) reduction of POX activity; and group III contains five alleles (P406L, L441P, R453C, T466M, and Q521E) with severe (>70%) reduction in POX activity. Interestingly, one allele, O521R, encodes a POX with increased activity (120% of wild type).
Figure 5.
Proline oxidase activity of PRODH alleles. For each transfected allele, the activity was normalized to transfection efficiency and was expressed as a percentage of the wild-type allele (see the “Material and Methods” section). The thin vertical lines indicate the range of mean activity measured in two-to-five independent transient transfection experiments. Bars without these vertical lines indicate the mean of a triplicate assay performed in one transfection experiment. For some alleles (L441P, R453C/T466M, and A472T), the value is from multiple experiments, but the variance is so narrow that it cannot be seen on this scale.
Because some of the PRODH mutations were described on haplotypes with others, we also expressed three PRODH alleles with two or three missense mutations in cis (L441P/R453C, V427M/R431H/L441P, and R453C/T466M/A472T) (Humbertclaude et al. 2001; Jacquet et al. 2002; Liu et al. 2002b). Each of these haplotypes has at least one missense mutation that, in isolation, confers severe reduction of POX activity. We found the POX activity encoded by these haplotype alleles was dictated by the functionally most-severe missense mutation with no evidence of intra-allelic complementation (Turner et al. 1997; Walker et al. 1997).
POX Activity of Severe Mutants Measured under High FAD Conditions
Our standard POX assay depends on FAD already bound to the apoenzyme (Phang et al. 1975). To check for alleles with altered cofactor affinity, we also assayed all five group III alleles in the presence of supplementary FAD (1 mM). Four of these alleles showed little or no response, but one, T466M, showed a 3–4-fold increase in activity, from 5%–10% to 30–40% of control. Structural considerations based on the structure of E. coli PUTA669 suggest that T466 of POX interacts with the adenine moiety of FAD to stabilize the noncovalent binding of the cofactor to the POX apoenzyme. Thus, T466M would be expected to alter the affinity of the POX apoenzyme for FAD. To test this expectation, we assayed POX activity over a range of added FAD concentrations (5–1,000 μM) in partially purified mitochondrial extracts of CHO-K1-C9 cells transfected with either wild-type or T466M PRODH. From these results, we estimated the Km for FAD of wild-type POX to be ∼0.25×10-9 and that of T466M-POX to be ∼3×10-9, or at least 1 order of magnitude higher (data not shown).
Discussion
The extent of polymorphism and its consequences varies considerably from gene to gene across the genome (Cargill et al. 1999; Halushka et al. 1999; Reich and Lander 2001). PRODH is toward the high end of this spectrum, with several recognized variants that occur at polymorphic frequency (>0.01). This high rate of polymorphism appears to result, at least in part, from the existence of a nonprocessed pseudogene 1.4 Mb telomeric on chromosome 22 that serves as a reservoir of variation that can be transferred to PRODH by gene conversion. A similar mechanism is thought to explain the high frequency of polymorphism in CYP21A2, which encodes a key enzyme in steroid hormone biosynthesis and has a nearby pseudogene (CYP21A1P) in 6p21 (Donohoue et al. 2001).
To test the functional consequences of the polymorphic and rare variant PRODH alleles, we expressed all 16 known PRODH missense mutations individually and, in some instances, as haplotypes with multiple mutations in cis. Among these 16 missense mutations, we found 4 that result in a mild (30%) (group I), 6 that result in a moderate (30%–70%) (group II), and 5 that result in severe (>70%) (group III) reduction in POX activity. There was a rough but imperfect correlation between allele frequency and effect on activity: the 10 polymorphic alleles included 4 in group I, 5 in group II, and only 1 (E453C) in group III. By contrast, of the six rare PRODH alleles, four encoded POX with a severely reduced activity (group III), with only one each in groups I and II. This correlation would be consistent with a selective disadvantage against the functionally severe alleles, but additional population-genetics studies would be necessary to confirm this suggestion. Interestingly, one polymorphic mutation (Q521R) with an allele frequency of ∼0.05 encoded an enzyme with an activity ∼20% higher than that of the common Q521 allele (fig. 5).
One caveat regarding our results is that the POX activity we measure reflects the catalytic activity and stability of the holoenzymes that result from expression, targeting, and assembly of POX subunits encoded by the expressed cDNAs. Our experiments circumvent any additional effects these mutations might have on splicing or RNA stability (Cartegni et al. 2002). In particular, a mutation that has only a mild effect on POX activity in our experiments could have a profound effect on POX activity in vivo by alteration of splicing. Testing for this would require RT-PCR analysis in expressing tissues in individuals of known genotypes. As a proxy for this, we analyzed our set of PRODH missense mutations with the online analysis tool ESEfinder (2.0) to search for mutations predicted to alter exon-splice enhancers (Cartegni et al. 2003). Q19P in exon 2 was the only mutation in our mild or moderate categories (groups I and II) predicted to alter a splice enhancer. Interestingly, the 3′ splice site in intron 1 has a weak polypyrimidine tract (ggcgggaccaacag/C… vs. the consensus of (t/c)10 n c/tag/G …) (Shapiro and Senapathy 1987), which suggests the possibility that exon 2 utilizes a splice enhancer (Cartegni et al. 2002). Additional studies of individuals of this genotype will be required to address this possibility.
Ideally, our observations on the functional consequences of these 16 missense mutations on POX activity would be understood in the context of the three-dimensional structure of human POX. Although this is not available, the structure of the POX segment (residues 1–669, designated “PutA669”) of the multifunctional 1,320 residue PutA enzyme of E. coli was recently described (Lee et al. 2003). PutA669 binds FAD, has the POX activity of the full-length protein, and has the overall structure of an interlocking homodimer (Lee et al. 2003; Zhang et al. 2004). Each PutA669 subunit has three domains: domain I (residues 87–139) forms an arm reaching out from one subunit to partially encircle the other, domain II (residues 140–260) does not contact the other subunit or make contributions to the active site and has unknown function, and domain III (residues 261–612) forms a β8α8 barrel that binds FAD and performs the proline oxidase function of PutA669. Although a sequence comparison of PutA669 and human POX over residues 341–570 of the human enzyme shows an amino acid identity of only 15%, this increases to 47% for the 40 human POX residues that correspond to putA669 residues within a 5-Å neighborhood of substrate and/or cofactor. On the basis of the high level of sequence identity of these critical residues and of the common catalytic function, Lee et al. (2003) argue that PutA669 and human POX share a common active site and that the structure of the human protein can be modeled on PutA669.
The model predicts that human POX residues 341–570 form a β8α8 barrel structure. Three of the five mutations with a severe effect on POX activity (group III) are in PRODH exon 12. The segment of POX encoded by this exon (residues 418–477) forms an alpha helix (α4), a beta strand (β5), and a helix-turn-helix structure (α5a/α5). In putA669, β5 stabilizes proline binding, and α5a is important for FAD binding (Dym and Eisenberg 2001; Lee et al. 2003). FAD is composed of an adenosine monophosphate (AMP) connected by a pyrophosphate bond to flavin mononucleotide. The latter has an isoalloxazine-flavin ring structure connected to a ribitol group that, in turn, links to the pyrophosphate. The binding of FAD to its resident apoenzyme typically involves high affinity, noncovalent interactions with the ribityl phosphate and AMP moieties of the cofactor, whereas the catalytic function is concentrated in the isoalloxazine ring (Dym and Eisenberg 2001). Two group III mutations (L441P and Q521E) and the mutation with increased activity (Q521R) alter β5 and would be expected to affect the active site of POX, whereas a third group III mutation, T466M in α5a, would be expected to alter FAD binding (fig. 6). L441P probably disrupts the stabilizing interaction of V442 with the isoalloxazine of FAD, adversely affecting the interaction of substrate and cofactor (fig. 6). In fact, Zhang et al. (2004) recently showed that the equivalent mutation in PutA669 (L432P) results in a protein with significantly lower catalytic activity and stability. They found a 5-fold reduction in Kcat with no significant effect on Km but a markedly reduced thermostability (Zhang et al. 2004)
Figure 6.
Structural models of the active site of human POX, as predicted from the solved structure of E. coli PutA669 (Lee et al. 2003). The location of residues altered in certain of the missense mutations is shown: A, T466M; B, Q521E; C, L441P. The white arrows indicate potential disruptive forces.
The contrasting effects of the Q521R/E substitutions (120% and 20% activity, respectively) can also be understood from the predicted structure of POX. The PutA669 residue corresponding to Q521 (L513) contributes to one wall of the active site and is located only 3.6 Å from proline, near the isoalloxazine end of FAD. Q521R substitutes a basic residue that appears to enhance catalytic activity, whereas Q521E substitutes an acidic residue for a polar neutral one and probably alters the architecture of the active site, with severe reduction of POX activity as the result.
To our knowledge, PRODH-T466M is only the second example of an FAD-responsive mutation in humans. The other is the N324S allele of the methylenetetrahydrofolate reductase gene (MTHFR), which encodes the other β8α8 barrel FAD-binding protein whose structure has been solved (Sibani et al. 2003). The PutA669 residue corresponding to human T466 (R458) is only 3.1 Å from the adenine group of FAD. Since this end of FAD usually participates in the noncovalent binding of the cofactor to the apoenzyme, we speculated that T466M destabilizes this interaction. To test this hypothesis, we assayed T466M and all other mutant POX enzymes with a severe reduction in activity in the presence of high (1 mM) FAD. Of these, only T466M showed a significant response with a 3–4-fold increase in activity to levels that are ∼30% that of control. Using partially purified, mitochondrial preparations from cells expressing either T446M or wild-type POX, we found that T466M increases the Km for FAD at least 10-fold, from ∼0.25 nM to 3 nM. It would be interesting to test for riboflavin responsiveness in individuals carrying the T466M-PRODH allele (Liu et al. 2002b).
How does the POX function of the various PRODH alleles relate to biochemical and clinical phenotypes? HPI, an autosomal recessive inborn error, was shown to be due to inherited deficiency of POX, in classic biochemical studies by Efron, Scriver, Schafer, and their colleagues in the 1960s (Scriver et al. 1961; Schafer et al. 1962; Efron 1965). Because PRODH expression is limited to tissues that are not readily accessible (liver, kidney, and brain), there have been almost no additional studies of POX activity in this disorder. Consequently, HPI has been a diagnosis of exclusion (chronic hyperprolinemia without excretion of P5C and with normal P5C dehydrogenase activity in lymphoblasts or fibroblasts). Although the data are limited, it is possible to combine our results on the POX activity encoded by the various PRODH alleles and plasma proline levels published by others to begin to relate genotype to metabolic phenotype. One patient with HPI, homozygous for a 350-kb deletion that removed the entire PRODH gene, had a proline level of 2,246 μM (Jacquet et al. 2003). Two patients who were homozygous for the most-severe missense mutation (L441P), with essentially undetectable activity in our assay, had proline levels of 1,255 μM and 800 μM (Jacquet et al. 2002). A compound-heterozygote patient with a PRODH deletion and missense allele with low but detectable residual activity (R453C; 9% activity) had a level of 538 μM (Jacquet et al. 2002). At the low end of the spectrum of elevated plasma proline (300–500 μM), the situation is much less clear, and several individuals appear to be heterozygous for alleles with a range of residual activity. Interestingly, Scriver, on the basis of family studies, pointed out many years ago that some individuals predicted to be heterozygous for an HPI allele had mild but significant hyperprolinemia (Scriver 1978). Although many variables—including dietary intake, metabolic state, and drugs—influence plasma proline levels, the emerging picture suggests that there is a broad “dynamic range” in the relationship between POX activity and plasma proline levels. In their classic studies, Kacser and Burns (1981) predicted that this would be the case for enzymes catalyzing the initial step in a pathway, as compared with those catalyzing intermediate steps in a pathway. The latter have more opportunity for metabolic buffering provided by the multiple steps in the pathway, each with substrate pools. Much work needs to be done to relate PRODH genotype and residual POX activity to plasma proline levels and, more importantly, to proline accumulation in key local environments in the CNS.
The clinical phenotype of HPI is not as well understood as the metabolic phenotype. Some individuals with HPI have been described as “normal,” others have had neurological abnormalities, and at least two received a diagnosis of schizophrenia (Jacquet et al. 2002). It would be important to restudy these individuals, to determine if their phenotypes were typical of schizophrenia or if, in retrospect, there were any unusual features (age at onset, response to therapy, etc.).
PRODH variants have also been implicated as susceptibility factors for schizophrenia and schizoaffective disorder in some (Gogos et al. 1999; Chakravarti 2002; Jacquet et al. 2002, 2004; Liu et al. 2002b; Hoogendoorn et al. 2004; Li et al. 2004) but not all studies (Williams et al. 2003a, 2003b; Ohtsuki et al. 2004). To account for increased susceptibility for a common phenotype, a locus must have a substantial reservoir of variation (Reich and Lander 2001; Pritchard and Cox 2002), and this is the case for PRODH (see table 1). Moreover, a role for PRODH variants is consistent with the well-documented 20–30-fold increase in risk of schizophrenia in patients with VCFS/DGS due to 22q11.2 deletions that include the PRODH locus (Shprintzen et al. 1992; Pulver et al. 1994; Karayiorgou et al. 1995; Murphy et al. 1999). The psychiatric phenotype of these individuals has been described either as indistinguishable from that of schizophrenic patients without 22q11.2 deletions (Bassett et al. 2003) or as having a later onset and fewer negative symptoms (Murphy et al. 1999). Our results indicate that nine functionally significant mutations (>30% reduction of POX activity) have been found in the PRODH genes of patients with schizophrenia. Moreover, we find that three of four alleles shown elsewhere to be significantly associated with schizophrenia encode POX with <30% of normal activity. The fourth allele, A472T, encodes an enzyme with 70% activity. True positive associations of a phenotype with a particular variant may occur because the variant is the causative mutation or because the variant is in linkage disequilibrium with the causative mutation (Page et al. 2003). Thus, the association with A472T could reflect an increase in risk conferred by a modest reduction in POX activity or could reflect linkage disequilibrium of A472T with a more severe, as-yet-undetected variant. A role for reduced POX function as a risk factor for schizophrenia is consistent with the results of Jacquet et al., who identified two schizophrenic patients with HPI (Jacquet et al. 2002) and found that hyperprolinemia is a significant risk factor (P=.02) for the related phenotype, schizoaffective disorder (Jacquet et al. 2004). Additionally, the observation of sensorimotor-gating abnormalities in the POX-deficient Pro/Re mouse support a role for reduced POX activity as a risk factor for schizophrenia (Gogos et al. 1999).
Unfortunately, we know little about the relationship of proline concentrations in plasma and CNS, particularly in the subpopulations of glutamatergic neurons for which proline has been proposed to function as a neurotransmitter or as a modulator of neurotransmission (Renick et al. 1999). Variation in proline metabolism is, however, an attractive candidate risk factor for schizophrenia. Because POX is expressed in the CNS and catalyzes the first step in the catabolic pathway that converts proline to glutamate, variation in POX activity has the potential to influence both proline and glutamate levels in CNS. Perturbation of the proline/P5C redox shuttle also could influence the metabolic activity and/or apoptosis in selected neurons. Taken together, our results and these considerations indicate that further studies of proline metabolism and function in the CNS are warranted.
Supplementary Material
Acknowledgments
We thank M. Amsel and J. Phang, for helpful discussions; J. Tanner, for assistance with the PutA669 sequence; and S. Muscelli, for manuscript preparation. Dr. Valle is an Investigator at the Howard Hughes Medical Institute.
Electronic-Database Information
Accession numbers and URLs for data presented herein are as follows:
- ESEfinder (2.0), http://rulai.cshl.edu/tools/ESE/
- GenBank, http://www.ncbi.nih.gov/Genbank/ (for PRODH cDNA [accession number NM_016335] and Homo sapiens proline dehydrogenase mRNA [accession number AF120278])
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim/ (for HPI and HPII)
References
- Bassett AS, Chow EW, AbdelMalik P, Gheorghiu M, Husted J, Weksberg R (2003) The schizophrenia phenotype in 22q11 deletion syndrome. Am J Psychiatry 160:1580–1586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg JM, Tymoczko JL, Stryer L (2002) Biochemistry. W H Freeman, New York [Google Scholar]
- Braverman N, Steel G, Obie C, Moser A, Moser H, Gould SJ, Valle D (1997) Human PEX7 encodes the peroxisomal PTS2 receptor and is responsible for rhizomelic chondrodysplasia punctata. Nat Genet 15:369–376 [DOI] [PubMed] [Google Scholar]
- Bussolati O, Dall’Asta V, Franchi-Gazzola R, Sala R, Rotoli BM, Visigalli R, Casado J, Lopez-Fontanals M, Pastor-Anglada M, Gazzola GC (2001) The role of system A for neutral amino acid transport in the regulation of cell volume. Mol Membr Biol 18:27–38 [DOI] [PubMed] [Google Scholar]
- Cargill M, Altshuler D, Ireland J, Sklar P, Ardlie K, Patil N, Lane CR, Lim EP, Kalyanaraman N, Nemesh J, Ziaugra L, Friedland L, Rolfe A, Warrington J, Lipshutz R, Daley GQ, Lander ES (1999) Characterization of single-nucleotide polymorphisms in coding regions of human genes. Nat Genet 22:231–238 [DOI] [PubMed] [Google Scholar]
- Cartegni L, Chew SL, Krainer AR (2002) Listening to silence and understanding nonsense: exonic mutations that affect splicing. Nat Rev Genet 3:285–298 [DOI] [PubMed] [Google Scholar]
- Cartegni L, Wang J, Zhu Z, Zhang MQ, Krainer AR (2003) ESEfinder: a Web resource to identify exonic splicing enhancers. Nucleic Acids Res 31:3568–3571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakravarti A (2002) A compelling genetic hypothesis for a complex disease: PRODH2/DGCR6 variation leads to schizophrenia susceptibility. Proc Natl Acad Sci USA 99:4755–4756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dall’Asta V, Bussolati O, Sala R, Parolari A, Alamanni F, Biglioli, Gazzola GC (1999) Amino acids are compatible osmolytes for volume recovery after hypertonic shrinkage in vascular endothelial cells. Am J Physiol 276:C865–872 [DOI] [PubMed] [Google Scholar]
- Delwing D, Bavaresco CS, Wannmacher CM, Wajner M, Dutra-Filho CS, Wyse AT (2003a) Proline induces oxidative stress in cerebral cortex of rats. Int J Dev Neurosci 21:105–110 [DOI] [PubMed] [Google Scholar]
- Delwing D, Chiarani F, Bavaresco CS, Wannmacher CM, Wajner M, Wyse AT (2003b) Proline reduces acetylcholinesterase activity in cerebral cortex of rats. Metab Brain Dis 18:79–86 [DOI] [PubMed] [Google Scholar]
- Donald SP, Sun X, Hu C-A, Yu J, Mei JM, Valle D, Phang JM (2001) Proline oxidase, encoded by p53-induced gene-6, catalyzes the generation of proline-dependent reactive oxygen species. Cancer Res 61:1810–1815 [PubMed] [Google Scholar]
- Donohoue PA, Parker KL, Migeon CJ (2001) Congenital adrenal hyperplasia. In: Scriver CR, Beaudet AL, Sly WS, Valle D (eds) The metabolic and molecular bases of inherited disease. McGraw-Hill, New York, pp 4077–4115 [Google Scholar]
- Dym O, Eisenberg D (2001) Sequence-structure analysis of FAD-containing proteins. Protein Sci 10:1712–1728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efron ML (1965) Familial hyperprolinemia. N Engl J Med 272:1243–1254 [DOI] [PubMed] [Google Scholar]
- Flynn MP, Martin MC, Moore PT, Stafford JA, Fleming GA, Phang JM (1989) Type II hyperprolinaemia in a pedigree of Irish travellers (nomads). Arch Dis Child 64:1699–1707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fremeau RTJ, Caron MG, Blakely RD (1992) Molecular cloning and expression of a high affinity L-proline transporter expressed in putative glutamatergic pathways of rat brain. Neuron 8:915–926 [DOI] [PubMed] [Google Scholar]
- Geraghty MT, Vaughn D, Nicholson AJ, Lin W-W, Jimenez-Sanchez G, Obie C, Flynn MP, Valle D, Hu C-A (1998) Mutations in the Δ1-pyrroline 5-carboxylate dehydrogenase gene cause type II hyperprolinemia. Hum Mol Genet 7:1411–1415 [DOI] [PubMed] [Google Scholar]
- Gogos JA, Santha M, Takacs Z, Beck KD, Luine V, Lucas LR, Nadler JV, Karayiorgou M (1999) The gene encoding proline dehydrogenase modulates sensorimotor gating in mice. Nat Genet 21:434–439 [DOI] [PubMed] [Google Scholar]
- Goodman BK, Rutberg J, Lin WW, Pulver AE, Thomas GH, Geraghty MT (2000) Hyperprolinaemia in patients with deletion (22)(q11.2) syndrome. J Inherit Metab Dis 23:847–848 [DOI] [PubMed] [Google Scholar]
- Halushka MK, Fan J-B, Bentley K, Hsie L, Shen N, Weder A, Cooper R, Lipshutz R, Chakravarti A (1999) Patterns of single-nucleotide polymorphisms in candidate genes for blood-pressure homeostasis. Nat Genet 22:239–247 [DOI] [PubMed] [Google Scholar]
- Hoogendoorn B, Coleman SL, Guy CA, Smith K, O’Donovan MC, Buckland PR (2004) Functional analysis of polymorphisms in the promoter regions of genes on 22q11. Hum Mutat 24:35–42 [DOI] [PubMed] [Google Scholar]
- Humbertclaude V, Rivier F, Roubertie A, Echenne B, Bellet H, Vallat C, Morin D (2001) Is hyperprolinemia type I actually a benign trait? report of a case with severe neurologic involvement and vigabatrin intolerance. J Child Neurol 16:622–623 [DOI] [PubMed] [Google Scholar]
- Ivanov D, Kirov G, Norton N, Williams HJ, Williams NM, Nikolov I, Tzwetkova R, Stambolova SM, Murphy KC, Toncheva D, Thapar A, O’Donovan MC, Owen MJ (2003) Chromosome 22q11 deletions, velo-cardio-facial syndrome and early-onset psychosis. molecular genetic study. Br J Psychiatry 183:409–413 [DOI] [PubMed] [Google Scholar]
- Jacquet H, Berthelot J, Bonnemains C, Simard G, Saugier-Veber P, Raux G, Campion D, Bonneau D, Frebourg T (2003) The severe form of type I hyperprolinaemia results from homozygous inactivation of the PRODH gene. J Med Genet 40:E7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacquet H, Demily C, Houy E, Hecketsweiler B, Raux G, Lerond J, Allio G, Haouzir S, Tillaux A, Bellegou C, Fouldrin G, Delamillieure P, Menard JF, Dollfus S, D’Amato T, Petit M, Thibaut F, Frebourg T, Campion D (2004) Hyperprolinemia is a risk factor for schizoaffective disorder. Mol Psychiatry 1–7 [DOI] [PubMed] [Google Scholar]
- Jacquet H, Raux G, Thibaut F, Hecketsweiler B, Houy E, Demilly C, Haouzir S, Allio G, Fouldrin G, Drouin V, Bou J, Petit M, Campion D, Frébourg T (2002) PRODH mutations and hyperprolinemia in a subset of schizophrenic patients. Hum Mol Genet 11:2243–2249 [DOI] [PubMed] [Google Scholar]
- Jaeken J, Goemans N, Fryns JP, Farncois I, DeZegher F (1996) Association of hyperprolinemia type I and heparin cofactor II deficiency with CATCH22 syndrome: evidence for a contiguous gene syndrome locating the proline oxidase gene. J Inher Metab Dis 19:275–277 [DOI] [PubMed] [Google Scholar]
- Kacser H, Burns JA (1981) The molecular basis of dominance. Genetics 97:639–666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karayiorgou M, Morris MA, Morrow B, Shprintzen RJ, Goldberg R, Borrow J, Gos A, Nestadt G, Wolyniec PS, Lasseter VK, Eisen H, Childs B, Kazazian HH, Kucherlapati R, Antonarakis SE, Pulver AE, Housman DE (1995) Schizophrenia susceptibility associated with interstitial deletions of chromosome 22q11. Proc Natl Acad Sci USA 92:7612–7616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyosue T, Yoshiba Y, Yamaguchi-Shinozake K, Shinozaki K (1996) A nuclear gene encoding mitochondrial proline dehydrogenase, an enzyme involved in proline metabolism, is upregulated by proline but downregulated by dehydration in Arabidopsis. Plant Cell 8:1323–1335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YH, Nadaraia S, Gu D, Becker DF, Tanner JJ (2003) Structure of the proline dehydrogenase domain of the multifunctional PutA flavoprotein. Nat Struct Biol 10:109–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T, Ma X, Sham PC, Sun X, Hu X, Wang Q, Meng H, Deng W, Liu X, Murray RM, Collier DA (2004) Evidence for association between novel polymorphisms in the PRODH gene and schizophrenia in a Chinese population. Am J Med Genet 129B:13–15 [DOI] [PubMed] [Google Scholar]
- Lindsay EA, Shaffer LG, Carrozzo R, Greenberg F, Baldini A (1995) De novo tandem duplication of chromosome segment 22q11-q12: clinical, cytogenetic, and molecular characterization. Am J Med Genet 56:296–299 [DOI] [PubMed] [Google Scholar]
- Liu H, Abecasis GR, Heath SC, Knowles A, Demars S, Chen Y-J, Roos JL, Rapoport JL, Gogos JA, Karayiorgou M (2002a) Genetic variation in the 22q11 locus and susceptibility to schizophrenia. Proc Natl Acad Sci USA 99:16859–16864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Heath SC, Sobin C, Roos JL, Galke BL, Blundell ML, Lenane M, Robertson B, Mijsman EM, Rapoport JL, Gogos JA, Karayiorgou M (2002b) Genetic variation at the 22q11 PRODH2/DGCR6 locus presents an unusual pattern and increases susceptibility to schizophrenia. Proc Natl Acad Sci USA 99:3717–3722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell A, Rivera A (2003) Proline oxidase induces apoptosis in tumor cells, and its expression is frequently absent or reduced in renal carcinomas. J Biol Chem 278:9784–9789 [DOI] [PubMed] [Google Scholar]
- Maxwell SA, Davis GE (2000) Differential gene expression in p53-mediated apoptosis-resistant vs apoptosis-sensitive tumor cell lines. Proc Natl Acad Sci USA 97:13009–13014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermid HE, Morrow BE (2002) Genomic disorders on 22q11. Am J Hum Genet 70:1077–1088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy KC, Jones LA, Owen MJ (1999) High rates of schizophrenia in adults with velo-cardio-facial syndrome. Arch Gen Psychiatry 56:940–945 [DOI] [PubMed] [Google Scholar]
- Ohtsuki T, Tanaka S, Ishiguro H, Noguchi E, Arinami T, Tanabe E, Yara K, Okubo T, Matsuura M, Sakai T, Muto M, Kojima T, Matsushima E, Toru M, Inada T (2004) Failure to find association between PRODH deletion and schizophrenia. Schizophr Res 67:111–113 [DOI] [PubMed] [Google Scholar]
- Page GP, George V, Go RC, Page PZ, Allison DB (2003) “Are we there yet?”: deciding when one has demonstrated specific genetic causation in complex diseases and quantitative traits. Am J Hum Genet 73:711–719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phang JM, Downing SJ, Valle D, Kowaloff EM (1975) A radioisotopic assay for proline oxidase activity. J Lab Clin Med 85:312–317 [PubMed] [Google Scholar]
- Phang JM, Hu C-A, Valle D (2001) Disorders of proline and hydroxyproline metabolism. In: Scriver CR, Beaudet AL, Sly WS, Valle D (eds) The metabolic and molecular bases of inherited disease. McGraw Hill, New York, pp 1821–1838 [Google Scholar]
- Polyak K, Xia Y, Zweier JL, Kinzler KW, Vogelstein B (1997) A model for p53-induced apoptosis. Nature 389:300–305 [DOI] [PubMed] [Google Scholar]
- Pritchard JK, Cox NJ (2002) The allelic architecture of human disease genes: common disease–common variant…or not? Hum Mol Genet 11:2417–2423 [DOI] [PubMed] [Google Scholar]
- Pulver AE, Nestadt G, Goldberg R, Shprintzen RJ, Lamacz M, Wolyniec P, Morrow BE, Karayiorgou M, Antonarakis S, Housman D (1994) Psychotic illness in patients diagnosed with velo-cardio-facial syndrome and their relatives. J Nerv Ment Dis 182:476–478 [DOI] [PubMed] [Google Scholar]
- Reich D, Lander ES (2001) On the allelic spectrum of human disease. Trends Genet 17:502–510 [DOI] [PubMed] [Google Scholar]
- Renick SE, Kleven DT, Chan J, Stenius K, Milner TA, Pickel VM, Fremeau RT Jr (1999) The mammalian brain high-affinity L-proline transporter is enriched preferentially in synaptic vesicles in a subpopulation of excitatory nerve terminals in rat forebrain. J Neurosci 19:21–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer IA, Scriver CR, Efron ML (1962) Familial hyperprolinemia, cerebral dysfunction and renal anomalies occurring in a family with hereditary nephritis and deafness. N Engl J Med 267:51–60 [DOI] [PubMed] [Google Scholar]
- Scriver CR (1978) Disorders of proline and hydroxyproline metabolism. In: Stanbury JB, Wyngaarden JB, Fredrickson DS (eds) The metabolic basis of inherited disease. McGraw Hill, New York, pp 336–361 [Google Scholar]
- Scriver CR, Schafer IA, Efron ML (1961) New renal tubular amino acid transport system and a new hereditary disorder of amino acid metabolism. Nature 192:672 [DOI] [PubMed] [Google Scholar]
- Shafqat S, Velaz-Faircloth M, Henzi VA, Whitney KD, Yang-Feng TL, Seldin MF, Fremeau RTJ (1995) Human brain-specific L-proline transporter: molecular cloning, functional expression and chromosomal localization of the gene in human and mouse genomes. Mol Pharmacol 48:219–229 [PubMed] [Google Scholar]
- Shapiro MB, Senapathy P (1987) RNA splice junctions of different classes of eukaryotes: sequence statistics and functional implication in gene expression. Nucl Acids Res 15:7155–7175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shprintzen RJ, Goldberg R, Golding-Kushner KJ, Marion RW (1992) Late-onset psychosis in the velo-cardio-facial syndrome. Am J Med Genet 42:141–142 [DOI] [PubMed] [Google Scholar]
- Sibani S, Leclerc D, Weisberg IS, O’Ferrall E, Watkins D, Artigas C, Rosenblatt DS, Rozen R (2003) Characterization of mutations in severe methylenetetrahydrofolate reductase deficiency reveals an FAD-responsive mutation. Hum Mutat 21:509–520 [DOI] [PubMed] [Google Scholar]
- Takagi H, Sakai K, Morida K, Nakamori S (2000) Proline accumulation by mutation or disruption of the proline oxidase gene improves resistance to freezing and disiccation stresses in Saccharomyces cerevisiae. FEMS Microbiol Lett 184:103–108 [DOI] [PubMed] [Google Scholar]
- Turner MA, Simpson A, McInnes RR, Howell PL (1997) Human argininosuccinate lyase: a structural basis for intragenic complementation. Proc Natl Acad Sci USA 94:9063–9068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valle D, Downing S, Harris S, Phang J (1973) Proline biosynthesis: multiple defects in Chinese hamster ovary cells. Biochem Biophys Res Commun 53:1130–1136 [DOI] [PubMed] [Google Scholar]
- Valle DL, Goodman SI, Applegarth DA, Shih VE, Phang JM (1976) Type II hyperprolinemia: Δ1-pyrroline-5-carboxylic acid dehydrogenase deficiency in cultured skin fibroblasts and circulating lymphocytes. J Clin Invest 58:598–603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velaz-Faircloth M, Guadano-Ferraz A, Henzi VA, Fremeau RT Jr (1995) Mammalian brain-specific L-proline transporter. J Biol Chem 270:15755–15761 [DOI] [PubMed] [Google Scholar]
- Walker DC, Christodoulou J, Craig HJ, Simard LR, Ploder L, Howell PL, McInnes RR (1997) Intragenic complementation at the human argininosuccinate lyase locus. J Biol Chem 272:6777–6783 [DOI] [PubMed] [Google Scholar]
- Williams HJ, Williams N, Spurlock G, Norton N, Ivanov D, McCreadie RG, Preece A, Sharkey V, Jones S, Zammit S, Nikolov I, Kehaiov I, Thapar A, Murphy KC, Kirov G, Owen MJ, O’Donovan MC (2003a) Association between PRODH and schizophrenia is not confirmed. Mol Psychiatry 8:644–645 [DOI] [PubMed] [Google Scholar]
- Williams HJ, Williams N, Spurlock G, Norton N, Zammit S, Kirov G, Owen MJ, O’Donovan MC (2003b) Detailed analysis of PRODH and PsPRODH reveals no association with schizophrenia. Am J Med Genet 120:42–46 [DOI] [PubMed] [Google Scholar]
- Williams NM, Norton N, Williams H, Ekholm B, Hamshere ML, Lindblom Y, Chowdari KV, Cardno AG, Zammit S, Jones LA, Murphy KC, Sanders RD, McCarthy G, Gray MY, Jones G, Holmans P, Nimgaonkar V, Adolfson R, Ösby U, Terenius L, Sedvall G, O’Donovan MC, Owen MJ (2003c) A systematic genomewide linkage study in 353 sib pairs with schizophrenia. Am J Hum Genet 73:1355–1367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshiba Y, Kiyosue T, Nakashima K, Yamaguchi-Shinozaki K, Shinozaki K (1997) Regulation of levels of proline as an osmolyte in plants under water stress. Plant Cell Physiol 38:1095–1102 [DOI] [PubMed] [Google Scholar]
- Zhang M, White TA, Scheuermann JP, Baban BA, Becker DF, Tanner JJ (2004) Structures of the Escherichia coli PutA proline dehydrogenase domain in complex with competitive inhibitors. Biochemistry 43:12539–12548 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.