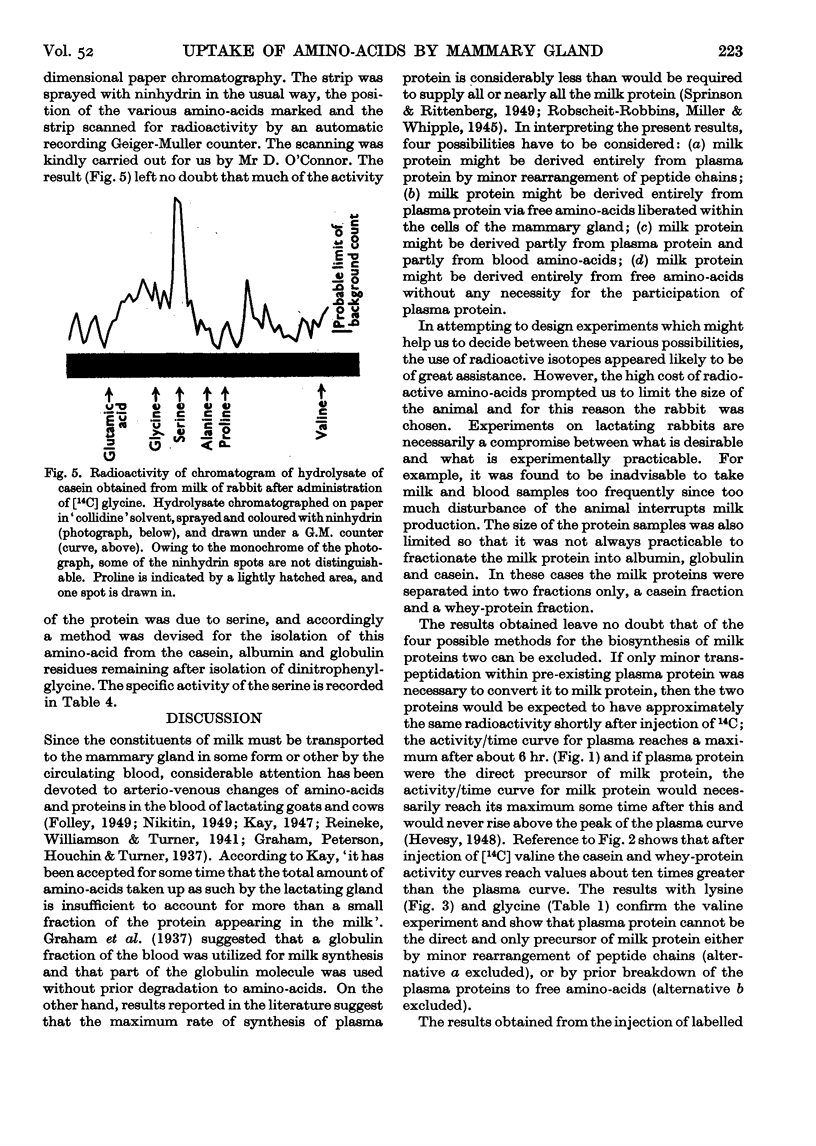
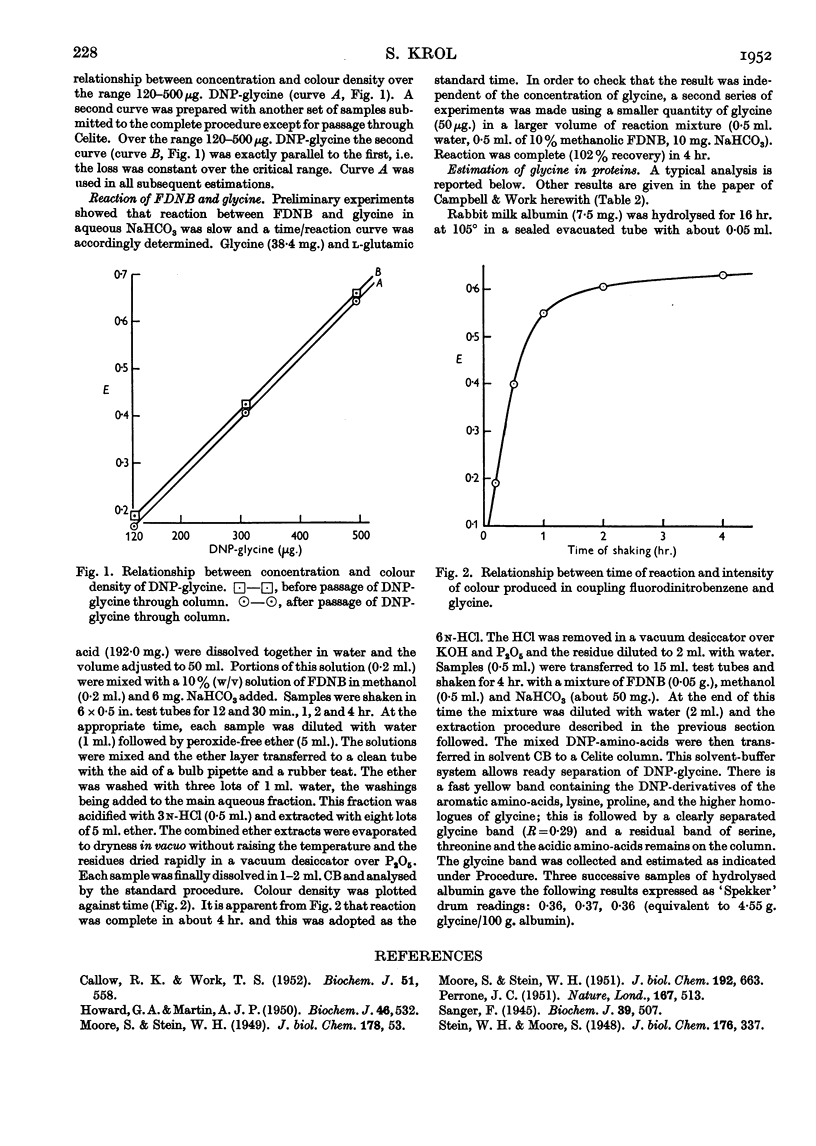
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CALLOW R. K., WORK T. S. Antibiotic peptides from Bacillus licheniformis; licheniformins A, B and C. Biochem J. 1952 Jul;51(4):558–568. doi: 10.1042/bj0510558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CALLOW R. K., WORK T. S. Antibiotic peptides from Bacillus licheniformis; licheniformins A, B and C. Biochem J. 1952 Jul;51(4):558–568. doi: 10.1042/bj0510558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAMPBELL P. N., WORK T. S. Fractionation of the nitrogenous water-soluble constituents of liver. I. The isolation of glycerylphosphorylethanolamine and of taurine. Biochem J. 1952 Feb;50(4):449–454. doi: 10.1042/bj0500449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHRISTENSEN H. N. Peptide wastage consequent to the infusion of two protein hydrolysates. J Nutr. 1950 Oct;42(2):189–193. doi: 10.1093/jn/42.2.189. [DOI] [PubMed] [Google Scholar]
- COULSON E. J., STEVENS H. The serological relationship of bovine whey albumin to serum albumin. J Biol Chem. 1950 Nov;187(1):355–363. [PubMed] [Google Scholar]
- DUNN M. S., McCLURE L. E. The response of Lactobacillus casei to partial hydrolysates of protein. J Biol Chem. 1950 May;184(1):223–233. [PubMed] [Google Scholar]
- Dent C. E. Studies on the absorption of proteins: the amino-acid pattern in the portal blood. Biochem J. 1949;44(3):318–335. [PMC free article] [PubMed] [Google Scholar]
- Elliott D. F., Neuberger A. The irreversibility of the deamination of threonine in the rabbit and rat. Biochem J. 1950 Feb;46(2):207–210. doi: 10.1042/bj0460207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRUTON J. S., JOHNSTON R. B., FRIED M. Elongation of peptide chains in enzyme-catalyzed transamidation reactions. J Biol Chem. 1951 May;190(1):39–53. [PubMed] [Google Scholar]
- FRUTON J. S. The role of proteolytic enzymes in the biosynthesis of peptide bonds. Yale J Biol Med. 1950 Jan;22(3):263–271. [PMC free article] [PubMed] [Google Scholar]
- Fischer A. Amino-acid metabolism of tissue cells in vitro. Biochem J. 1948;43(4):491–497. doi: 10.1042/bj0430491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HANES C. S., HIRD F. J. R., ISHERWOOD F. A. Enzymic transpeptidation reactions involving gamma-glutamyl peptides and alpha-amino-acyl peptides. Biochem J. 1952 Apr;51(1):25–35. doi: 10.1042/bj0510025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HANES C. S., HIRD F. J. R. Synthesis of peptides in enzymic reactions involving glutathione. Nature. 1950 Aug 19;166(4216):288–292. doi: 10.1038/166288a0. [DOI] [PubMed] [Google Scholar]
- HARRIS J. I., WORK T. S. The synthesis of peptides related to gramicidin S and the significance of optical configuration in antibiotic peptides; pentapeptides. Biochem J. 1950 May;46(5):582–589. doi: 10.1042/bj0460582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOWARD G. A., MARTIN A. J. P. The separation of the C12-C18 fatty acids by reversed-phase partition chromatography. Biochem J. 1950 May;46(5):532–538. doi: 10.1042/bj0460532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOHNSTON R. B., MYCEK M. J., FRUTON J. S. Catalysis of transamidation reactions by proteolytic enzymes. J Biol Chem. 1950 Aug;185(2):629–641. [PubMed] [Google Scholar]
- JOHNSTON R. B., MYCEK M. J., FRUTON J. S. Catalysis of transpeptidation reactions by chymotrypsin. J Biol Chem. 1950 Nov;187(1):205–211. [PubMed] [Google Scholar]
- KLUNGSØYR M., SIRNY R. J., ELVEHJEM C. A. Effect of incomplete hydrolysis on microbiological determination of amino acids. J Biol Chem. 1951 Apr;189(2):557–569. [PubMed] [Google Scholar]
- LOWTHER A. G. Identification of N-2: 4-dinitrophenylamino-acids. Nature. 1951 May 12;167(4254):767–768. doi: 10.1038/167767b0. [DOI] [PubMed] [Google Scholar]
- MALIN R. B., CAMIEN M. N., DUNN M. S. Response of lactic acid bacteria to amino acid derivatives. II. Glycine. Arch Biochem Biophys. 1951 Jun;32(1):106–112. doi: 10.1016/0003-9861(51)90243-3. [DOI] [PubMed] [Google Scholar]
- MOORE S., STEIN W. H. Chromatography of amino acids on sulfonated polystyrene resins. J Biol Chem. 1951 Oct;192(2):663–681. [PubMed] [Google Scholar]
- MORTON R. K. Separation and purification of enzymes associated with insoluble particles. Nature. 1950 Dec 30;166(4235):1092–1095. doi: 10.1038/1661092a0. [DOI] [PubMed] [Google Scholar]
- PERRONE J. C. Separation of amino-acids as dinitrophenyl derivatives. Nature. 1951 Mar 31;167(4248):513–515. doi: 10.1038/167513a0. [DOI] [PubMed] [Google Scholar]
- PERRONE J. C. Separation of amino-acids as dinitrophenyl derivatives. Nature. 1951 Mar 31;167(4248):513–515. doi: 10.1038/167513a0. [DOI] [PubMed] [Google Scholar]
- POLIS B. D., SHMUKLER H. W., CUSTER J. H. Isolation of a crystalline albumin from milk. J Biol Chem. 1950 Nov;187(1):349–354. [PubMed] [Google Scholar]
- Popják G., McCarthy E. F. Osmotic pressures of experimental and human lipaemic sera. Evaluation of albumin-globulin ratios with the aid of electrophoresis. Biochem J. 1946;40(5-6):789–803. [PMC free article] [PubMed] [Google Scholar]
- Sanger F. The free amino groups of insulin. Biochem J. 1945;39(5):507–515. doi: 10.1042/bj0390507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F. The free amino groups of insulin. Biochem J. 1945;39(5):507–515. doi: 10.1042/bj0390507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmonds S., Fruton J. S. A Microorganism Exhibiting a Growth Requirement for Peptides. Science. 1949 Jun 3;109(2840):561–562. doi: 10.1126/science.109.2840.561. [DOI] [PubMed] [Google Scholar]
- TAYLOR S. P., Jr, SIMMONDS S., FRUTON J. S. Utilization of methionine derivatives by a mutant strain of Escherichia coli. J Biol Chem. 1950 Dec;187(2):613–620. [PubMed] [Google Scholar]
- WALEY S. G., WATSON J. Rearrangement of the amino-acid residues in peptides by the action of proteolytic enzymes. Nature. 1951 Mar 3;167(4244):360–361. doi: 10.1038/167360a0. [DOI] [PubMed] [Google Scholar]