Abstract
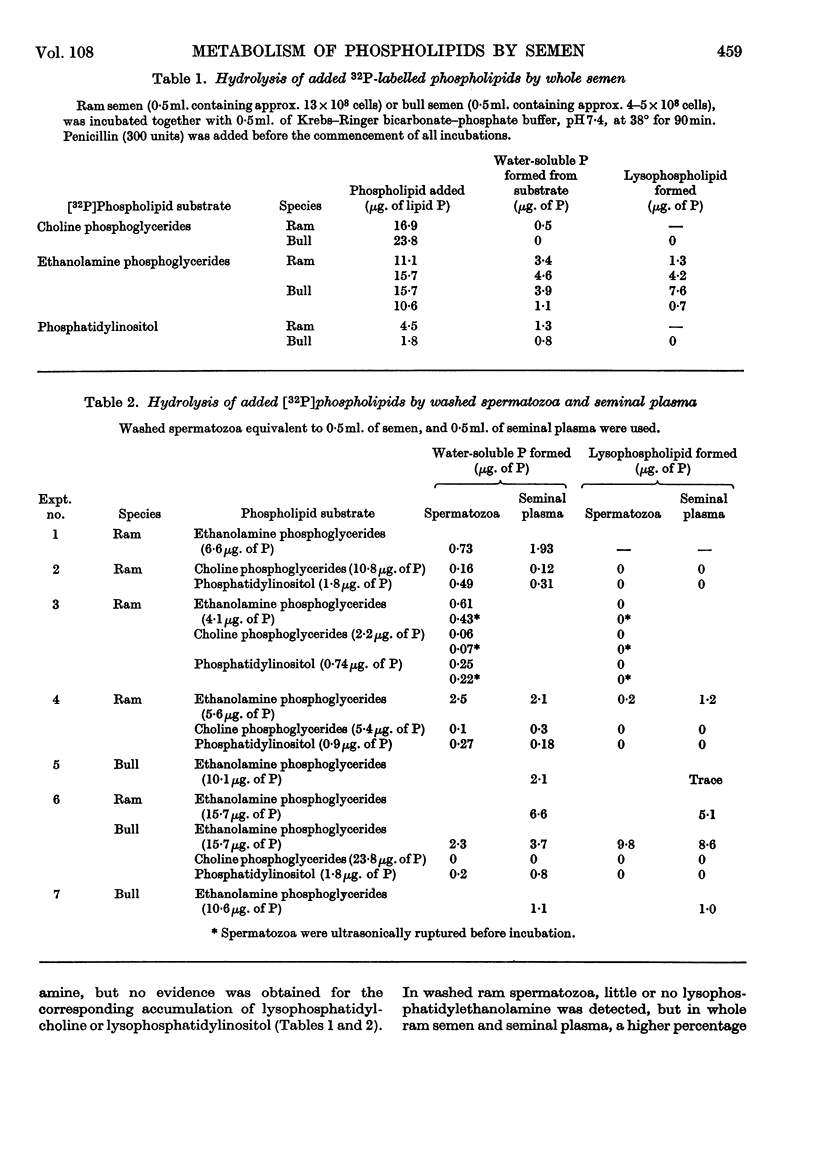
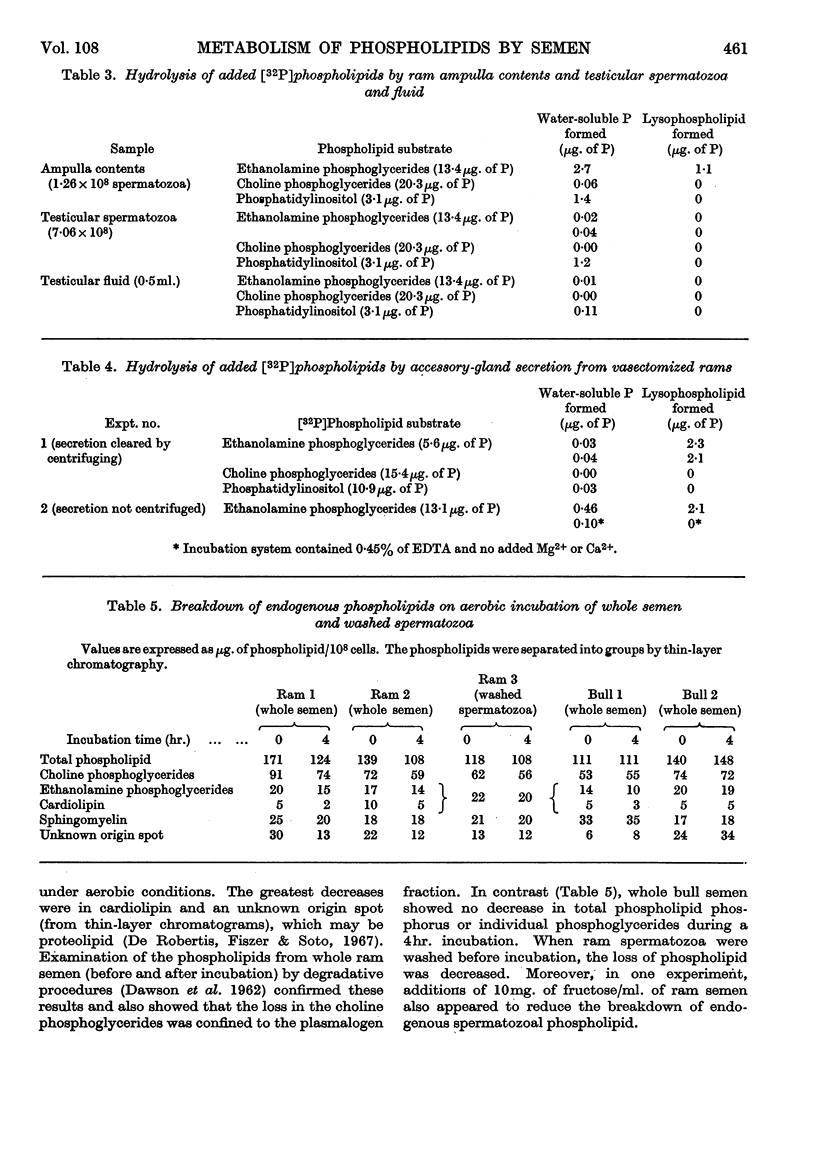
1. The hydrolysis of added 32P-labelled phospholipids by whole ram and bull semen and the separated spermatozoal and plasma components was examined. 2. The ethanolamine phosphoglycerides were rapidly attacked by washed spermatozoa, forming predominantly glycerylphosphorylethanolamine, but with whole semen and seminal plasma a lysophosphatidylethanolamine was also detected. 3. The hydrolysis of lecithin by spermatozoa and plasma was very slow, and glycerylphosphorylcholine was the sole product detected. 4. Ram testicular spermatozoa were comparatively inactive in metabolizing both phospholipids, but ampulla contents showed the same activity as ejaculated semen. 5. Phosphatidylinositol was metabolized by spermatozoa obtained from any portion of the ram reproductive tract and also by seminal plasma. With testicular components, ampulla contents and washed ejaculated spermatozoa, inositol monophosphate, an unidentified phosphate ester and inorganic phosphate were the main products. In contrast, with whole semen and seminal plasma, glycerylphosphorylinositol was the predominant water-soluble phosphate ester. 6. Accessory-gland secretion obtained from vasectomized rams showed a pronounced phospholipase A activity towards ethanolamine phosphoglyceride. 7. On aerobic incubation of whole ram semen there was a decrease in the concentration of all phospholipid classes, although cardiolipin showed the greatest percentage decrease. In the choline phosphoglyceride fraction, this loss was confined to the plasmalogen component. This breakdown of phospholipids was decreased considerably when the spermatozoa were washed, and was not observed when whole bull semen was incubated under similar conditions.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- BOMSTEIN R. A., STEBERL E. A. The utilization of phospholipides by bovine spermatozoa. Exp Cell Res. 1957 Apr;12(2):254–264. doi: 10.1016/0014-4827(57)90140-4. [DOI] [PubMed] [Google Scholar]
- Bjornstad P. Phospholipase activity in rat-liver microsomes studied by the use of endogenous substrates. Biochim Biophys Acta. 1966 Jun 1;116(3):500–510. [PubMed] [Google Scholar]
- Crone H. D. The calcium-stimulated incorporation of ethanolamine and serine into the phospholipids of the housefly Musca domestica. Biochem J. 1967 Aug;104(2):695–704. doi: 10.1042/bj1040695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAWSON R. M., HEMINGTON N., DAVENPORT J. B. Improvements in the method of determining individual phospholipids in a complex mixture by successive chemical hydrolyses. Biochem J. 1962 Sep;84:497–501. doi: 10.1042/bj0840497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAWSON R. M., MANN T., WHITE I. G. Glycerylphosphorylcholine and phosphorylcholine in semen, and their relation to choline. Biochem J. 1957 Apr;65(4):627–634. doi: 10.1042/bj0650627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAWSON R. M. Studies on the enzymic hydrolysis of monophosphoinositide by phospholipase preparations from P. notatum and ox pancreas. Biochim Biophys Acta. 1959 May;33(1):68–77. doi: 10.1016/0006-3002(59)90499-8. [DOI] [PubMed] [Google Scholar]
- DITTMER J. C., LESTER R. L. A SIMPLE, SPECIFIC SPRAY FOR THE DETECTION OF PHOSPHOLIPIDS ON THIN-LAYER CHROMATOGRAMS. J Lipid Res. 1964 Jan;5:126–127. [PubMed] [Google Scholar]
- De Robertis E., Fiszer S., Soto E. F. Cholinergic binding capacity of proteolipids from isolated nerve-ending membranes. Science. 1967 Nov 17;158(3803):928–929. doi: 10.1126/science.158.3803.928. [DOI] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- HARTREE E. F., MANN T. Phospholipids in ram semen: metabolism of plasmalogen and fatty acids. Biochem J. 1961 Sep;80:464–476. doi: 10.1042/bj0800464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARTREE E. F., MANN T. Plasmalogen in ram semen, and its role in sperm metabolism. Biochem J. 1959 Mar;71(3):423–434. doi: 10.1042/bj0710423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim S. A., Sanders H., Thompson R. H. The action of phospholipase A on purified phospholipids, plasma and tissue preparations. Biochem J. 1964 Dec;93(3):588–594. doi: 10.1042/bj0930588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KEMP P., HUBSCHER G., HAWTHORNE J. N. Phosphoinositides. 3. Enzymic hydrolysis of inositol-containing phospholipids. Biochem J. 1961 Apr;79:193–200. doi: 10.1042/bj0790193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PATTERSON M. S., GREENE R. C. MEASUREMENT OF LOW ENERGY BETA-EMITTERS IN AQUEOUS SOLUTION BY LIQUID SCINTILLATION COUNTING OF EMULSIONS. Anal Chem. 1965 Jun;37:854–857. doi: 10.1021/ac60226a017. [DOI] [PubMed] [Google Scholar]
- Scott T. W., Jay S. M., Freinkel N. Further studies on the action of pituitary thyrotropin on the individual phosphatides of thyroid tissue. Endocrinology. 1966 Sep;79(3):591–600. doi: 10.1210/endo-79-3-591. [DOI] [PubMed] [Google Scholar]
- Scott T. W., Voglmayr J. K., Setchell B. P. Lipid composition and metabolism in testicular and ejaculated ram spermatozoa. Biochem J. 1967 Feb;102(2):456–461. doi: 10.1042/bj1020456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voglmayr J. K., Scott T. W., Setchell B. P., Waites G. M. Metabolism of testicular spermatozoa and characteristics of testicular fluid collected from conscious rams. J Reprod Fertil. 1967 Aug;14(1):87–99. doi: 10.1530/jrf.0.0140087. [DOI] [PubMed] [Google Scholar]


