Abstract
Associations of Helicobacter pylori genotypes with disease differ between Western countries and Asia. Therefore, we directly compared histopathological and in vitro responses to clinical isolates with similar genotypes. Sixty-three cagA+ vacAs1/m1 H. pylori isolates (United States, n = 24; Japan, n = 39) and eight cagA-negative vacAs2/m2 strains were incubated with AGS cells, and supernatants were assayed for interleukin-8 (IL-8) and for DNA fragmentation. CagA tyrosine phosphorylation in AGS cells and the sequence of the putative HP0638 (oipA) signal sequence region were determined for 22 representative strains. HP0638 and/or cag island mutant strains were created and examined in IL-8 and CagA tyrosine phosphorylation assays. Levels of IL-8 induction and DNA fragmentation were similar in the U.S. and Japanese cagA+ vacAs1/m1 isolates. All 10 of the isolates with the highest IL-8 induction and 8 of the 10 isolates with the lowest IL-8 induction had an in-frame oipA open reading frame, and all 10 of the isolates with the highest IL-8 induction and 7 of the 10 isolates with the lowest IL-8 induction induced CagA tyrosine phosphorylation in AGS cells. Eight isolates from gastric ulcer patients induced significantly more apoptosis in vitro, and more severe gastritis and atrophy in vivo, than other Japanese isolates. Disruption of HP0638 did not affect IL-8 induction or CagA tyrosine phosphorylation. Thus, H. pylori cagA+ vacAs1/m1 isolates from the United States and Japan induce similar IL-8 and apoptosis levels. Inactivation of HP0638 does not alter epithelial responses mediated by the cag island in vitro. Assessment of apoptosis in vitro identified a group of H. pylori isolates that induce more severe gastric inflammation and atrophy.
Carriage of Helicobacter pylori increases the risk for developing peptic ulcer disease and gastric cancer (14). Persistent colonization results in mucosal release of chemotactic factors that attract neutrophils and mononuclear cells (21, 35, 39), and H. pylori induces release of the neutrophil chemoattractant chemokines from gastric epithelial cells in vitro (20, 22). In particular, interleukin-8 (IL-8) recruits and activates neutrophils (2), but mucosal IL-8 levels vary considerably among colonized individuals (5). There are several nonconserved markers of H. pylori virulence, including particular vacuolating cytotoxin genotypes (vacA genotypes); the presence of cagA, which is a marker for the cag pathogenicity island; the iceA1 allele; and the ability to directly activate neutrophils (11, 16, 17, 25, 47, 58, 63). In vivo, cagA+ strains are associated with higher levels of mucosal IL-8 than are cagA-negative strains (20, 46). In vitro, IL-8 production from gastric epithelial cells following coculture with cagA+ strains is dependent upon particular cag island genes (up-regulating or down-regulating IL-8 synthesis) (19, 32, 59) and possibly on HP0638 (oipA), which encodes a putative outer membrane protein (67). Attachment of H. pylori to epithelial cells in vitro induces another cellular phenotype, tyrosine phosphorylation of the CagA protein, after CagA injection into cells via a type IV secretion system (42). Mutations in several genes of the cag pathogenicity island interfere with tyrosine phosphorylation as well as IL-8 induction (15, 32, 54). H. pylori colonization also affects human gastric epithelial cell apoptosis and proliferation (28, 36, 37, 38, 48). Variation in epithelial cell cycle characteristics appears to be related to particular H. pylori genotypes (30, 37, 50, 51, 66).
In addition to H. pylori allelic differences that are conserved worldwide, certain genotypes are geographically related. In particular, vacA and cagA sequence motifs in strains from the United States and Europe differ from those predominating in East Asia (1, 10, 27, 44, 60, 61, 64). In Western populations, cagA+ vacAs1/m1 H. pylori strains are clearly more highly associated with disease than are cagA-negative vacAs2/m2 strains (7, 63); however, since a high proportion of East Asian strains are cagA+ vacAs1 (34), it has been difficult to detect disease-specific associations in this locale. An alternative hypothesis is that host variation is important in determining population-based differences in disease outcomes (23, 43, 52).
Therefore, we sought to determine whether there are differences in virulence between Western and East Asian H. pylori strains of similar genotype. To do this, we directly compared cagA+ vacAs1/m1 H. pylori strains from the United States and from Japan for their ability to induce IL-8 secretion, apoptosis, and CagA phosphorylation in gastric epithelial cells in vitro. These data then were compared to histological severity of inflammation and injury in the corresponding source patients.
MATERIALS AND METHODS
Study groups and biopsies.
We studied H. pylori strains isolated from 24 U.S. and 39 Japanese patients undergoing gastroscopy for evaluation of upper gastrointestinal tract symptoms, as described previously (6, 9, 46). At the time of endoscopy, three biopsy specimens were obtained from adjacent areas of the gastric antrum: one was for bacterial culture of H. pylori, one was for urease activity (CLO test; Delta West, Bentley, Australia), and one was for routine histological examination (hematoxylin-eosin and Giemsa stains). All biopsies were obtained from an area of endoscopically intact mucosa distant from any focal lesions, such as erosions. At bacterial culture, single colonies were collected and bacteria were identified as H. pylori by Gram stain morphology as well as by urease and oxidase activities. These 63 isolates were selected because their cagA and vacA status had been determined by PCR using established primers (9, 46), and confirmation of cagA and vacA genotypes was established by reverse hybridization using a line probe assay (62). As controls, from the same U.S. population (46, 47), eight cagA-negative strains were included; thus, a total of 71 strains were studied (Table 1). Patients were diagnosed as having duodenal ulcer (DU), gastric ulcer (GU), or nonulcer dyspepsia (NUD), based on endoscopic findings (9, 46). Six patients from Japan had been diagnosed with gastric cancer based on histological examination in addition to endoscopic findings (Table 1) (31).
TABLE 1.
Characteristics of the 71 H. pylori isolates and their respective source patients
| Country, cagA status, and endoscopic diagnosis | No. of patients | Age, yr (mean ± SD) | % Male | No. with the following vacA (s) genotype:
|
|||
|---|---|---|---|---|---|---|---|
| s1a | s1b | s1c | s2 | ||||
| United States | |||||||
| cagA+a | |||||||
| DU | 10 | 58 ± 15 | 100 | 4 | 6 | 0 | 0 |
| GU | 2 | 53 ± 7 | 100 | 1 | 1 | 0 | 0 |
| NUD | 12 | 52 ± 18 | 100 | 2 | 10 | 0 | 0 |
| Total | 24 | 54 ± 16 | 100 | 7 | 17 | 0 | 0 |
| cagA negativeb | 8 | 58 ± 11 | 100 | 0 | 0 | 0 | 8 |
| Japan, cagA+a | |||||||
| DU | 9 | 45 ± 11 | 78 | 0 | 0 | 9 | 0 |
| GU | 17 | 49 ± 6 | 94 | 1 | 0 | 16 | 0 |
| NUD | 7 | 43 ± 11 | 86 | 1 | 0 | 6 | 0 |
| Gastric cancer | 6 | 60 ± 13 | 67 | 0 | 0 | 6 | 0 |
| Total | 39 | 49 ± 11 | 85 | 2 | 0 | 37 | 0 |
For each of these patients, the isolate was cagA+ vacAs1m1.
Four patients had NUD, and one patient had DU; all strains were vacAs2m2.
Histology.
Neutrophil infiltration, mononuclear cell infiltration, glandular atrophy, intestinal metaplasia, and H. pylori density in the gastric biopsies were assessed on a scale of 0 to 3 according to the Sydney system (49), using formalin-fixed biopsy tissues stained with hematoxylin-eosin or Giemsa. All histological evaluations were performed by a single pathologist without knowledge of the experimental results.
AGS cell culture and IL-8 assays.
AGS human gastric epithelial cells (ATCC CRL 1739) were grown in RPMI 1640 (Life Technologies, Inc., Rockville, Md.) supplemented with 10% fetal bovine serum (FBS) and 20 μg of gentamicin per ml in an atmosphere of 5% CO2 at 37°C. For coculture experiments, H. pylori was grown in brucella broth with 5% FBS for 48 h, and cells were harvested by centrifugation (2,000 × g) and resuspended in antibiotic-free RPMI 1640 with 10% FBS to yield a final concentration of 108 CFU/ml. H. pylori was added to AGS cells at a bacteria/cell ratio of 1,000:1 for the IL-8 assays or 100:1 for the apoptosis assays (45, 55). Experiments were performed in antibiotic-free medium with 10% FBS using T-150 flasks (Corning Costar, Cambridge, Mass.) or 96-well polypropylene tissue culture plates (Nunc, Roskilde, Denmark). Levels of IL-8 (picograms per milliliter) in culture supernatants were assayed in duplicate using a specific enzyme-linked immunosorbent assay (ELISA) (Research and Development Systems, Minneapolis, Minn.) according to the manufacturer’s instructions.
Assessment of apoptosis.
DNA fragmentation was quantified using a commercially available ELISA (Boehringer Mannheim Biochemicals, Indianapolis, Ind.) that detects nucleosomal fragments in cytoplasmic fractions of cells undergoing apoptosis but not necrosis. For these experiments, 5 × 103 AGS cells/well in 96-well plates were incubated in duplicate with H. pylori cells (5 × 105 CFU/well) or with RPMI 1640-10% FBS alone for 48 h and lysed. Lysates were centrifuged, and supernatants were used for ELISA. Absorbance measured at 405 nm was compared for AGS cells cultured with H. pylori or with medium alone.
Assessment of HP0638 status.
According to the complete genome sequences of H. pylori strains 26695 and J99, dinucleotide repeats are located in the region carrying the signal sequence of HP0638 (oipA) (4, 57, 67). The signal sequence of HP0638, including the repeat region, was amplified by PCR using primer pair AN9260 (5′-CAAGCGCTTAACAGATAGGC-3′, forward) and AN9261 (5′AAGGCGTTTTCTGCTGAAGC-3′, reverse) as described previously (67). Sequence determinations for amplified DNA fragments were performed at the New York University Medical Center DNA sequencing core facility.
Tyrosine phosphorylation.
AGS cells (5 × 105) were cocultured with H. pylori at a multiplicity of infection of 100 for 4 h. Nonadherent bacteria were removed by washing five times with phosphate-buffered saline (PBS). Cells were suspended in 1 ml of ice-cold cell scraper solution (PBS, 1 mM EDTA, 1 mM orthovanadate, 1 mM phenylmethylsulfonyl fluoride, 1 μM leupeptin, 1 μM pepstatin), collected by centrifugation, and resuspended in 30 μl of PBS. For sodium dodecyl sulfate-polyacrylamide gel electrophoresis, an equal volume of 2× sample buffer (125 mM Tris [pH 6.8], 2% β-mercaptoethanol, 4% sodium dodecyl sulfate, 10% glycerol, 0.006% bromophenol blue) was added to the sample before boiling (5 min). Proteins were separated on a 7% polyacrylamide gel, electrotransferred to a polyvinylidene difluoride membrane, and examined for the presence of CagA using polyclonal rabbit anti-CagA immunoglobulin G (18) or phosphotyrosine (PY20; Transduction Laboratories). The horseradish peroxidase-conjugated antibody was visualized by enhanced chemiluminescence (Amersham Pharmacia Biotech Corp., Piscataway, N.J.) as indicated by the manufacturer.
Disruption of HP0638 in H. pylori strains.
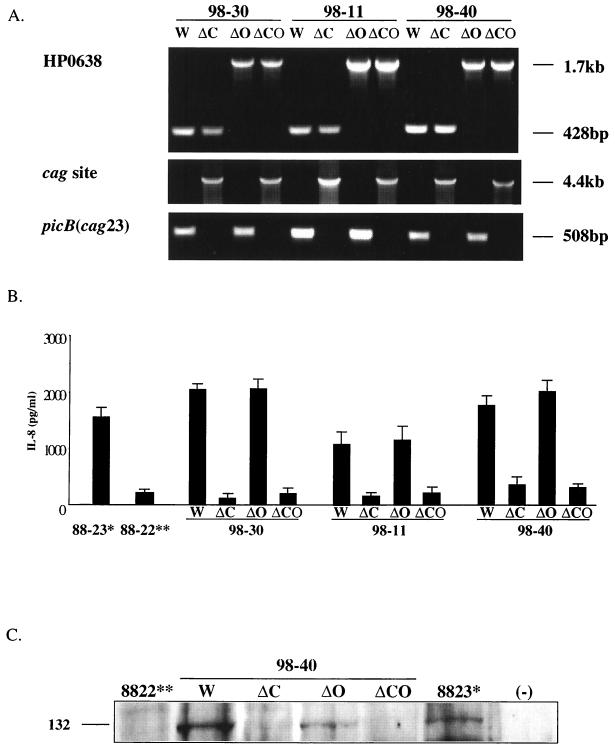
The HP0638 locus of strain 26695 was amplified using primers HP0637F (5′-AAGCGTAAGGGAAG-3′) and HP0638R (5′-ACTTTAAACGCTGGC-3′), and the PCR product was cloned into pGEMT and used to transform Escherichia coli DH5α. A unique EcoRI site was created by inverse PCR with primers HP0638MR (5′-CGGAATTCCGAGCTTTTTTCATGGT-3′) and HP0638MF (5′-CGGAATTCCGTTCTGGCTCCACGCTG-3′). Plasmid pUC4K was digested with EcoRI, after which the Kanr cassette (aphA) was ligated into the inverse PCR product to disrupt the HP0638 open reading frame (ORF), creating pAN0638. H. pylori strains 98-30, 98-11, and 98-40, which were cagA+ and had HP0638 in frame, were transformed to kanamycin resistance with pAN0638 to create 98-30-638::aphA, 98-11-638::aphA, and 98-40-638::aphA, respectively. Chromosomal DNA was isolated from these strains, and the insertion of the aphA cassette within HP0638 in the transformants was confirmed by PCR using primers AN9260 and AN9261, as described above (see Fig. 3A).
FIG. 3.
Effect of mutagenizing HP0638 and the cag island on AGS cell responses. (A) Confirmation of mutations interrupting HP0638 and/or deleting the cag island. PCR was performed with primers AN9260 and AN9261 (HP0638), OP1690 and OP1691 (cag site), and C3814 and C3815 (picB) to ascertain mutant construction. W, wild type; ΔC, cag island deleted; ΔO, HP0638 interrupted; ΔCO, HP0638 interrupted and cag island deleted. (B) IL-8 levels in culture supernatants from AGS cells coincubated with H. pylori wild-type and mutant strains. Data represent the means and standard deviations from three experiments. *, cagA+ control strain 88-23, **, cagA-negative control strain 88-22. (C) Induction of tyrosine-phosphorylated CagA protein by H. pylori wild-type and mutant strains in AGS cells.
cag island deletion.
cag island deletion mutants were generated in strains 98-30, 98-11, and 98-40 and in the HP0638::aphA mutants of these strains by allelic exchange, using the cat cassette (45). Isogenic cag deletion mutants were selected with chloramphenicol (10 μg/ml). Chromosomal DNA was isolated from mutant strains, and cag island allelic exchange with replacement by cat was confirmed by PCR using primers OP1690 (5′-CCAATTTCACTCGCTATGACGGCATG-3′) and OP1691 (5′-AAGCTTTGTCTATTCTAAAATGCAAC-3′). The picB (cag23) locus also was amplified by PCR using primers C3814 (5′-TTGAAAACTTCAAGGATAGGATAGAGC-3′) and C3815 (5′-GCCTAGCGTAATATCACCATTACCC-3′) to confirm the presence or absence of the cag island.
Statistical analysis.
Statistical analysis was performed by the χ2, Fisher’s exact, paired t, or Mann-Whitney U test, depending on the data characteristics. A P value of <0.05 was considered to be statistically significant.
RESULTS
Assessment of H. pylori cagA and vacA status.
PCR and line probe assay were performed on H. pylori strains isolated from symptomatic U.S. and Japanese patients to determine the genotype (Table 1). We confirmed that 63 strains (United States, n = 24; Japan, n = 39) were cagA+ vacAs1/m1. Most of the Japanese strains were vacA subtype s1c, while the U.S. strains were vacAs1a or vacAs1b. Thus, although not identical, the U.S. and Japanese strains had the same cagA and vacAs1/m1 status. We also confirmed that the eight control U.S. strains were cagA negative and vacAs2/m2.
Histological findings.
Comparison of the intensities of antral histological findings (infiltration with mononuclear or polymorphonuclear cells, atrophy, metaplasia, and H. pylori density) among the U.S. and Japanese H. pylori-positive patients indicated that for all five histological features, a greater proportion of persons harboring cagA+ strains showed higher scores than did cagA-negative patients (data not shown). No significant differences were observed in any of the histological scores between the U.S. and Japanese patients colonized with cagA+ vacAs1/m1 isolates.
IL-8 induction in AGS cells.
Gastric mucosal IL-8 levels are significantly correlated with extent of inflammatory cellular infiltration in gastric tissues of H. pylori-positive persons (5). To assess strain-specific cytokine induction, each of the 68 H. pylori isolates was incubated with AGS cells and 24-h supernatants were assayed for IL-8 release. There were no significant differences in IL-8 secretion between the U.S. (median, 1,073 pg/ml; range, 237 to 2,898 pg/ml) and the Japanese (median, 1,259 pg/ml; range, 151 to 2,590 pg/ml) cagA+ vacAs1/m1 isolates (Fig. 1A), but both groups induced significantly (P < 0.001) higher levels than the cagA-negative vacAs2/m2 isolates (median, 302 pg/ml; range, 203 to 472 pg/ml). We next stratified the isolates on the basis of clinical outcome. Among U.S. strains, IL-8 induction did not vary significantly according to clinical group (Fig. 1B). Among Japanese strains, those isolated from DU patients (median, 1,711 pg/ml; range, 726 to 2,081 pg/ml) induced significantly (P = 0.04) higher IL-8 levels than those induced by GU strains (median, 954 pg/ml; range, 151 to 2,590 pg/ml), but their ranges overlapped substantially (Fig. 1B).
FIG. 1.
IL-8 levels in culture supernatants from AGS cells coincubated with H. pylori cells. (A) Sixty-three cagA+ vacAs1/m1 and eight control cagA-negative vacAs2/m2 isolates were studied. IL-8 secretion by AGS cells was not significantly different between U.S. (n = 24) and Japanese (n = 39) cagA+ vacAs1/m1 isolates, but both were significantly (P < 0.001) greater than that for the cagA-negative vacAs2/m2 isolates. Horizontal lines indicate median values. (B) IL-8 levels according to disease outcome. Among the Japanese strains, those isolated from patients with DU induced the highest IL-8 values, but this trend was not present among U.S. strains. Horizontal lines indicate median values.
AGS cell DNA fragmentation.
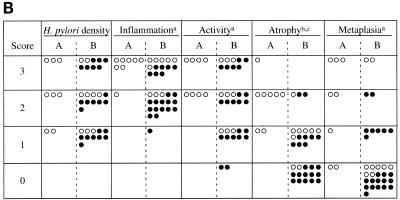
AGS cell DNA fragmentation was no different for U.S. (median, 1.2-fold increase in DNA fragmentation relative to control; range, 0.3 to 3.4) and Japanese (median, 1.3; range, 0.5 to 5.8) cagA+ vacAs1/m1 isolates. DNA fragmentation was significantly higher following coculture with the 17 isolates from Japanese patients with GU (median, 2.2; range, 0.7 to 5.8) than with isolates from Japanese patients with DU (median, 1.0; range, 0.5 to 2.8), NUD (median, 1.3; range, 0.6 to 1.9), or gastric cancer (median, 0.9; range, 0.6 to 1.7) (P < 0.01 for each) (Fig. 2A). Among the 17 Japanese GU strains, 8 induced more than a fourfold increase in DNA fragmentation relative to controls, values higher than in any group of patients (Fig. 2A, box). The demographic characteristics and vacA and iceA genotypes of these eight strains were no different from those of the isolates from the other 31 Japanese patients (Table 2), nor was IL-8 induction (median, 881 pg/ml; range, 151 to 2,590 pg/ml) significantly different from that of the other 31 Japanese isolates (median, 1,416 pg/ml; range, 335 to 2,123 pg/ml). The locations of GU in the Japanese patients harboring the eight high-apoptosis-inducing strains (body, 5; antrum, 2; fundus, 1) were similar to those in the nine Japanese patients colonized with the other strains (body, 5; antrum, 2; fundus, 2). However, histological examination of gastric biopsies from the eight GU patients from whom these strains were isolated showed significantly (P < 0.05) more severe mononuclear cell and neutrophil infiltration, atrophy, and intestinal metaplasia than in the other 31 Japanese patients (Fig. 2B). Comparison of these eight isolates to the nine other isolates from Japanese GU patients revealed no differences in IL-8 induction or patient demographic characteristics. However, the eight GU patients with high-apoptosis-inducing H. pylori isolates had significantly (P < 0.05) more atrophy on gastric biopsy than the other nine GU patients (Fig. 2B).
FIG. 2.
(A) Apoptosis of AGS cells following coculture with H. pylori cells, according to clinical diagnosis of patients from whom strains were isolated. Induction of DNA fragmentation was not significantly different between U.S. (n = 24) and Japanese (n = 39) cagA+ vacAs1/m1 isolates. Fragmentation was highest following coculture with Japanese isolates from GU patients (P < 0.005 for all comparisons with other isolates from Japanese patients). Horizontal lines indicate median values. For further analyses, the values for the eight isolates from GU patients that induced the highest apoptosis scores are boxed. (B) Comparison of histological findings present in the eight Japanese patients with GU from whom the isolates induced the highest apoptosis scores (columns A) and the other 31 Japanese patients (columns B), including nine other GU patients. ○, strain isolated from a patient with GU; •, strain isolated from a patient without GU. a and b, comparison of group A patients and group B patients (a, P < 0.05; b, P < 0.001). c, comparison of group A patients and group B GU patients (P < 0.01).
TABLE 2.
Characteristics of eight H. pylori isolates with high in vitro apoptosis scores from Japanese patients with GU
| Strain | Patient age (yr)/sexa | Apoptosis scoreb | Genotype
|
HP0638 frame status | IL-8 induction (pg/ml)c | P-Tyrd | ||
|---|---|---|---|---|---|---|---|---|
| cagA | vacAs | vacAm | ||||||
| 98-38 | 51/M | 5.8 | + | slc | ml | In | 817 | + |
| 98-35 | 42/M | 4.8 | + | slc | ml | In | 1,929 | + |
| 98-41 | 46/M | 4.8 | + | slc | ml | In | 944 | + |
| 98-39 | 48/M | 4.7 | + | slc | ml | In | 954 | + |
| 98-36 | 46/M | 4.4 | + | slc | ml | In | 287 | + |
| 98-37 | 50/F | 4.4 | + | slc | ml | In | 287 | + |
| 98-32 | 36/M | 4.1 | + | slc | ml | In | 151 | − |
| 98-40 | 65/M | 4.1 | + | slc | ml | In | 2,590 | + |
M, male; F, female.
Fold increase in DNA fragmentation of AGS cells relative to control cells.
Means from two replicate assays.
Presence of tyrosine-phosphorylated CagA protein within epithelial cells, determined as described in Materials and Methods (+, present; −, absent).
Relationship between IL-8 induction, HP0638 frame status, and CagA phosphorylation.
Yamaoka et al. reported that HP0638 (oipA) reading frame status affects the ability of H. pylori strains to induce IL-8 secretion (67). To further evaluate this hypothesis, we sequenced the 439-bp region that includes the putative HP0638 signal sequence for the five cagA+ vacAs1/m1 strains with the highest and the five cagA+ vacAs1/m1 strains with the lowest IL-8 induction from the United States and Japan and for two cagA-negative control strains. All 10 Japanese and 8 of the 10 U.S. strains possessed an in-frame ORF, whereas 2 of the 5 strains from the United States that least induced IL-8 had an out-of-frame HP0638 ORF (Table 3). The HP0638 ORF was out of frame in both cagA-negative control strains. For all 17 Japanese GU isolates, the HP0638 ORF was in frame (data not shown). We also examined the ability of the same strains to induce CagA phosphorylation in AGS cells. All 10 high-IL-8 inducing strains strongly induced tyrosine-phosphorylated CagA in AGS cells, compared with 7 of 10 low-IL-8 inducing strains (Table 3). There was no relationship to HP0638 frame status. As expected, neither cagA-negative control strain induced CagA tyrosine phosphorylation in AGS cells. Seven of the eight GU isolates with the highest apoptosis induction induced CagA phosphoprotein in AGS cells (Table 2).
TABLE 3.
HP0638 frame status of H. pylori isolates that induce either high or low levels of IL-8
| Country and H. pylori isolates | Strain | IL-8 inductiona (pg/ml) | HP0638 frame status | P-Tyrb |
|---|---|---|---|---|
| United States cagA+vacAs1m1 isolates | ||||
| High IL-8 inducers | 97-141 | 1,936 | In | + |
| 97-31 | 2,023 | In | + | |
| 97-511 | 2,381 | In | + | |
| 97-112 | 2,452 | In | + | |
| 95-58 | 2,898 | In | + | |
| Low IL-8 inducers | 98-238 | 265 | In | + |
| 95-79 | 305 | Out | − | |
| 96-32 | 350 | In | − | |
| 96-62 | 362 | In | + | |
| 96-71 | 620 | Out | + | |
| cagA negative vacAs2m2 isolates | ||||
| J188 | 290 | Out | − | |
| J292 | 366 | Out | − | |
| Japan, cagA+vacAs1m1 isolates | ||||
| High IL-8 inducers | 98-15 | 2,051 | In | + |
| 98-30 | 2,053 | In | + | |
| 98-14 | 2,081 | In | + | |
| 98-46 | 2,123 | In | + | |
| 98-40 | 2,590 | In | + | |
| Low IL-8 inducers | 98-32 | 151 | In | − |
| 98-36 | 287 | In | + | |
| 98-37 | 287 | In | + | |
| 98-33 | 335 | In | + | |
| 98-28 | 574 | In | + |
IL-8 levels in supernatants from AGS cells cocultured with specified H. pylori isolates; means from duplicate determinations.
Presence of tyrosine-phosphorylated CagA protein within epithelial cells, determined as described in Materials and Methods (+, present; −, absent).
AGS cell IL-8 induction and CagA tyrosine phosphorylation by mutant strains.
To directly assess the role of HP0638 and the cag island in IL-8 induction, we generated HP0638 mutants, cag mutants, and HP0638 and cag mutants of strains 98-30, 98-11, and 98-40, all of which were strong IL-8 inducers (Fig. 3A). Each of the HP0638 mutant strains induced an IL-8 level similar to that of its wild-type strain. In contrast, each cag mutant strain induced significantly lower IL-8 levels than its wild-type or HP0638 mutant strain (Fig. 3B). There was no difference in IL-8 induction between cag mutant strains and HP0638 and cag mutant strains. Next, we examined the effects of loss of the cag island and/or HP0638 status on CagA tyrosine phosphorylation using wild-type and mutant strains of 98-30, 98-11, and 98-40. All wild-type and HP0638 mutant strains strongly induced tyrosine phosphorylation of the CagA protein in AGS cells, whereas the cagmutant and HP0638 and cag mutant strains did not stimulate CagA tyrosine phosphorylation (Table 4; Fig. 3C).
TABLE 4.
Induction of CagA tyrosine phosphorylation by wild-type and mutant H. pylori strains
| H. pylori strain | Presence of phosphotyrosine-CagAa for:
|
|||
|---|---|---|---|---|
| Wild type | cag island deleted | HP0638 disrupted | cag island deleted and HP0638 disrupted | |
| 98-30 | + | − | + | − |
| 98-11 | + | − | + | − |
| 98-40 | + | − | + | − |
Presence of tyrosine-phosphorylated CagA protein within AGS epithelial cells, determined as described in Materials and Methods (+, present; −, absent).
DISCUSSION
Among H. pylori-positive patients, serological detection of infection with cagA+ strains is at present the best clinically available assay for virulence differences (8). Although cagA+ vacAs1/m1 H. pylori strains are common in both East Asian and Western populations (10, 61, 64) and these strains are associated with atrophic gastritis and gastric cancer (7, 63), the difference in prevalence of gastric cancers between the two locales is not proportional to the differential prevalence of these strains. Since virulence assays have been done with strains from either the United States or Japan, our study sought to examine comparable strains from the two locales in parallel in the same assays done in a single laboratory. However, at least in part due to the enormous diversity of H. pylori strains (56), while we studied similar organisms (all were cagA+ vacAs1/m1), the U.S. and Japanese groups were not identical. For example, most Japanese isolates were s1c, whereas most from the United States were s1b. However, cagA status and vacAs1 status appear to be the most important known determinants of differences in inflammatory responses to H. pylori (12, 40, 65). The substantial overlap in the range of histological responses and IL-8 induction between the U.S. and Japanese isolates and the large differences from the U.S. cagA-negative vacAs2/m2 strains confirm the centrality of that distinction. Yamaoka et al. also reported that when compared among Japanese strains, the levels of IL-8 mRNA expression in gastric mucosa were significantly higher in cagA-positive than in cagA-negative specimens (68).
Nevertheless, IL-8 levels were variable among the cagA+ vacAs1/m1 isolates, regardless of origin. IL-8 induction by the Japanese DU strains is higher than that by Japanese GU strains or U.S. DU strains. Although our results do not suggest that differences in HP0638 underlie these findings, it is possible that differences in gene expression within the cag island and/or differences in cag genetic composition may contribute to these findings. Techniques such as DNA microarray hybridization have recently been utilized to more comprehensively characterize genetic content between H. pylori isolates, and differences in cag island content are directly related to induction of epithelial cell responses such as IL-8 production (26, 53). Application of such techniques to these strains is currently under way in our laboratory. Yamaoka et al. (67) suggested that the HP0638 frame status affects the ability of H. pylori strains to induce gastric epithelial cell IL-8 secretion. When we tested representative strains for HP0638 frame status, most (90%) were in frame, regardless of IL-8 induction (Table 3). In addition, experimental disruption of HP0638 had no effect on IL-8 induction or CagA tyrosine phosphorylation in AGS cells. Thus, in the strain population we studied, HP0638 status does not directly affect IL-8 induction, in contrast to the report by Yamaoka et al. (67). This may be due to the fact that we studied different strains. Whether or not HP0638 affects IL-8 induction cannot be assessed solely from these two studies, and therefore, further independent studies will be required to examine this relationship. While the cag island is largely inherited as a single 27-gene block, strains possessing partial cag islands have been identified (3, 13, 33, 41, 53). Recently, we reported that a cagA+ vacAs1/m1 strain with a large deletion of the cag island induced lower IL-8 levels than a vacAs1/m1 strain with an intact cag island and that partial or complete cag island disruption attenuated IL-8 induction in vitro and significantly decreased gastric inflammation in vivo (26). The fact that certain cagA+ vacAs1/m1 strains induce only low levels of IL-8 (Table 3) and do not induce CagA tyrosine phosphorylation in AGS cells suggests the presence of partial cag island deletions, which might also explain the previous findings of Yamaoka et al. (67).
Although the ability of cagA+ vacAs1/m1 H. pylori strains to induce AGS cell apoptosis was not significantly different between U.S. and Japanese isolates, this study identified eight isolates that induced very high levels of apoptosis. These high-apoptosis-inducing isolates were associated with more severe mononuclear cell and neutrophil tissue infiltration, atrophy, and intestinal metaplasia. However, we used only one biopsy for histological analysis, and since H. pylori colonization may be patchy, sampling errors may affect the results. Therefore, further studies should be done using multiple samples to confirm the correlation between apoptosis and histological features of the gastric mucosa. The fact that all eight strains were from Japanese patients with GU suggests that they may represent a unique group of isolates with enhanced pathogenic potential. Comparison of these isolates to those from other GU patients showed no differences in cagA, vacA, or HP0638 status or in IL-8 induction levels. Taken together, these eight isolates appear to have a new phenotype: high induction of apoptosis in vitro coupled with enhanced atrophy in vivo in their human hosts. Although the genetic bases of this phenomenon are not known, these strains may represent a group of H. pylori strains with enhanced virulence. Testing this hypothesis in suitable animal models will be of great interest. Although we hypothesize that apoptosis is related to development of gastric cancer, the isolates from gastric cancer patients did not induce high levels of apoptosis in vitro. One hypothesis to explain these events is that colonizing strains change in their ability to induce epithelial cell responses, such as apoptosis, as an adaptation to changing conditions within the host milieu. Alternatively, our findings may not represent the true relationship between induction of apoptosis in vitro and gastric cancers, as we examined only six strains isolated from patients with gastric adenocarcinoma. To more completely address this question, apoptosis studies should be done using H. pylori isolates obtained from more gastric cancer patients at different stages of disease.
Apoptosis induction by the U.S. strains in the present study was lower than we have previously reported (45). There are several possibilities for this apparent discordance. First, the U.S. H. pylori strains used in this study differ from those in the previous study, and differences in expression of known virulence determinants (i.e., vacA and picB) and/or unidentified genetic differences may have contributed to the variation in apoptosis induction. Second, in the present study, we used a DNA fragmentation ELISA, while in the previous study, flow cytometry was used not only to assess apoptosis but also to determine the cell cycle distribution following coculture with clinical strains. Finally, apoptosis in the present study was assessed after 48 h of coincubation, while the 72-h time point was chiefly used in our previous study. Collectively, variation in the strain population and these methodologic differences could have led to the differences in apoptosis levels seen in the two studies.
Gastroduodenal illnesses associated with H. pylori also may be related to inappropriately regulated gastric immune responses (24). Clearly, host differences between persons in the United States and Japan may play a role in the different clinical outcomes in H. pylori-positive persons. For example, particular polymorphisms of the human IL-1β gene promoter are risk factors for both atrophic gastritis and gastric adenocarcinoma among H. pylori-positive persons (23). Host differences in other cytokine (IL-8 and GROα) responses to H. pylori also may be important (43), as well as the age at which H. pylori is acquired (29).
In conclusion, H. pylori cagA+ vacAs1/m1 strains from the United States and Japan induce similar ranges of IL-8 secretion and DNA fragmentation in vitro. In contrast to a previous report (67), we show that HP0638 frame status does not affect epithelial cell responses related to pathogenesis. The identification of strains from GU patients that induce unusually high levels of apoptosis in vitro and that are associated with enhanced atrophy in vivo suggests a novel virulence-related phenotype that could be pursued in future studies.
Acknowledgments
This work was supported by NIH grant RO1 DK 53707 and by the Medical Research Service of the Department of Veterans Affairs.
REFERENCES
- 1.Achtman, M., T. Azuma, D. E. Berg, Y. Ito, G. Morelli, Z. J. Pan, S. Suerbaum, S. A. Thompson, A. van der Ende, and L. J. van Doorn. 1999. Recombination and clonal groupings within Helicobacter pylori from different geographical regions. Mol. Microbiol. 32:459–470. [DOI] [PubMed] [Google Scholar]
- 2.Akkoyunlu, M., S. E. Malawista, J. Anguita, and E. Fikrig. 2001. Exploitation of interleukin-8-induced neutrophil chemotaxis by the agent of human granulocytic ehrlichiosis. Infect. Immun. 69:5577–5588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akopyants, N. S., S. W. Clifton, D. Kersulyte, J. E. Crabtree, B. E. Youree, C. A. Reece, N. O. Bukanov, E. S. Drazek, B. A. Roe, and D. E. Berg. 1998. Analyses of the cag pathogenicity island of Helicobacter pylori. Mol. Microbiol. 28:37–53. [DOI] [PubMed] [Google Scholar]
- 4.Alm, R. A., L. S. Ling, D. T. Moir, B. L. King, E. D. Brown, P. C. Doig, D. R. Smith, B. Noonan, B. C. Guild, B. L. deJonge, G. Carmel, P. J. Tummino, A. Caruso, M. Uria-Nickelsen, D. M. Mills, C. Ives, R. Gibson, D. Merberg, S. D. Mills, Q. Jiang, D. E. Taylor, G. F. Vovis, and T. J. Trust. 1999. Genomic-sequence comparison of two unrelated isolates of the human gastric pathogen Helicobacter pylori. Nature 397:176–180. [DOI] [PubMed] [Google Scholar]
- 5.Ando, T., K. Kusugami, M. Ohsuga, M. Shinoda, M. Sakakibara, H. Saito, A. Fukatsu, S. Ichiyama, and M. Ohta. 1996. Interleukin-8 activity correlates with histological severity in Helicobacter pylori-associated antral gastritis. Am. J. Gastroenterol. 91:1150–1156. [PubMed] [Google Scholar]
- 6.Ando, T., G. I. Perez-Perez, K. Kusugami, M. Ohsuga, K. C. Bloch, and M. J. Blaser. 2000. Anti-CagA immunoglobulin G responses correlate with interleukin-8 induction in human gastric mucosal biopsy culture. Clin. Diagn. Lab. Immunol. 7:803–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Atherton, J. C. 1997. The clinical relevance of strain types of Helicobacter pylori. Gut 40:701–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Atherton, J. C. 1998. H. pylori virulence factors. Br. Med. Bull. 54:105–120. [DOI] [PubMed] [Google Scholar]
- 9.Atherton, J. C., P. Cao, R. M. Peek, M. K. Tummuru, M. J. Blaser, and T. L. Cover. 1995. Mosaicism in vacuolating cytotoxin alleles of Helicobacter pylori. Association of specific vacA types with cytotoxin production and peptic ulceration. J. Biol. Chem. 270:17771–17777. [DOI] [PubMed] [Google Scholar]
- 10.Atherton, J. C., P. M. Sharp, T. L. Cover, G. Gonzalez-Valencia, R. M. Peek, S. A. Thompson, C. J. Hawkey, and M. J. Blaser. 1999. Vacuolating cytotoxin (vacA) alleles of Helicobacter pylori comprise two geographically widespread types, m1 and m2, and have evolved through limited recombination. Curr. Microbiol. 39:211–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Atherton, J. C., R. M. Peek, K. T. Tham, T. L. Cover, and M. J. Blaser. 1997. Clinical and pathological importance of heterogeneity in vacA, the vacuolating cytotoxin gene of Helicobacter pylori. Gastroenterology 112:92–99. [DOI] [PubMed] [Google Scholar]
- 12.Audibert, C., B. Janvier, B. Grignon, L. Salaun, C. Burucoa, J. C. Lecron, and J. L. Fauchere. 2000. Correlation between IL-8 induction, cagA status and vacA genotypes in 153 French Helicobacter pylori isolates. Res. Microbiol. 151:191–200. [DOI] [PubMed] [Google Scholar]
- 13.Audibert, C., C. Burucoa, B. Janvier, and J. L. Fauchere. 2001. Implication of the structure of the Helicobacter pylori cag pathogenicity island in induction of interleukin-8 secretion. Infect. Immun. 69:1625–1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blaser, M. J. 1999. The changing relationships of Helicobacter pylori and humans: implications for health and disease. J. Infect. Dis. 179:1523–1530. [DOI] [PubMed] [Google Scholar]
- 15.Censini, S., C. Lange, Z. Xiang, J. E. Crabtree, P. Ghiara, M. Borodovsky, R. Rappuoli, and A. Covacci. 1996. cag, a pathogenicity island of Helicobacter pylori, encodes type I specific and disease-associated virulence factors. Proc. Natl. Acad. Sci. USA 93:14648–14653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Covacci, A., S. Censini, M. Bugnoli, R. Petracca, D. Burroni, G. Macchia, A. Massone, E. Papini, Z. Xiang, N. Figura, and R. Rappuoli. 1993. Molecular characterization of the 128-kDa immunodominant antigen of Helicobacter pylori associated with cytotoxicity and duodenal ulcer. Proc. Natl. Acad. Sci. USA 90:5791–5795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cover, T. L., C. P. Dooley, and M. J. Blaser. 1990. Characterization of and human serologic response to proteins in Helicobacter pylori broth culture supernatants with vacuolizing cytotoxin activity. Infect. Immun. 58:603–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cover, T. L., Y. Glupczynski, A. P. Lage, A. Burette, M. K. Tummuru, G. I. Perez-Perez, and M. J. Blaser. 1995. Serologic detection of infection with cagA+ Helicobacter pylori strains. J. Clin. Microbiol. 33:1496–1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Crabtree, J. E., D. Kersulyte, S. D. Li, I. J. Lindley, and D. E. Berg. 1999. Modulation of Helicobacter pylori induced interleukin-8 synthesis in gastric epithelial cells mediated by cag PAI encoded VirD4 homologue. J. Clin. Pathol. 52:653–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Crabtree, J. E., S. M. Farmery, I. J. Lindley, N. Figura, P. Peichl, and D. S. Tompkins. 1994. CagA/cytotoxic strains of Helicobacter pylori and interleukin-8 in gastric epithelial cell lines. J. Clin. Pathol. 47:945–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Craig, P. M., M. C. Territo, W. E. Karnes, and J. H. Walsh. 1992. Helicobacter pylori secretes a chemotactic factor for monocytes and neutrophils. Gut 33:1020–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Crowe, S. E., L. Alvarez, M. Dytoc, R. H. Hunt, M. Muller, P. Sherman, J. Patel, Y. Jin, and P. B. Ernst. 1995. Expression of interleukin 8 and CD54 by human gastric epithelium after Helicobacter pylori infection in vitro. Gastroenterology 108:65–74. [DOI] [PubMed] [Google Scholar]
- 23.El-Omar, E. M., M. Carrington, W. H. Chow, K. E. McColl, J. H. Bream, H. A. Young, J. Herrera, J. Lissowska, C. C. Yuan, N. Rothman, G. Lanyon, M. Martin, J. F. Fraumeni, and C. S. Rabkin. 2000. Interleukin-1 polymorphisms associated with increased risk of gastric cancer. Nature 404:398–402. [DOI] [PubMed] [Google Scholar]
- 24.Ernst, P. B., and B. D. Gold. 2000. The disease spectrum of Helicobacter pylori: the immunopathogenesis of gastroduodenal ulcer and gastric cancer. Annu. Rev. Microbiol. 54:615–640. [DOI] [PubMed] [Google Scholar]
- 25.Hansen, P. S., P. H. Madsen, S. B. Petersen, and H. Nielsen. 2000. Inflammatory activation of neutrophils by Helicobacter pylori; a mechanism insensitive to pertussis toxin. Clin. Exp. Immunol. 123:73–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Israel, D. A., N. Salama, C. N. Arnold, S. F. Moss, T. Ando, H. P. Wirth, K. T. Tham, M. Camorlinga, M. J. Blaser, S. Falkow, and R. M. Peek. 2001. Helicobacter pylori strains associated with differential gastric cancer risks reconstitute human pathologic responses in experimental models of carcinogenesis. J. Clin. Investig. 107:611–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ito, Y., T. Azuma, S. Ito, H. Suto, H. Miyaji, Y. Yamazaki, Y. Kohli, and M. Kuriyama. 1998. Full-length sequence analysis of the vacA gene from cytotoxic and noncytotoxic Helicobacter pylori. J. Infect. Dis. 178:1391–1398. [DOI] [PubMed] [Google Scholar]
- 28.Jones, N. L., P. T. Shannon, E. Cutz, H. Yeger, and P. M. Sherman. 1997. Increase in proliferation and apoptosis of gastric epithelial cells early in the natural history of Helicobacter pylori infection. Am. J. Pathol. 151:1695–1703. [PMC free article] [PubMed] [Google Scholar]
- 29.Kikuchi, S., O. Wada, T. Nakajima, T. Nishi, O. Kobayashi, T. Konishi, and Y. Inaba. 1995. Serum anti-Helicobacter pylori antibody and gastric carcinoma among young adults. Research Group on Prevention of Gastric Carcinoma among Young Adults. Cancer 75:2789–2793. [DOI] [PubMed] [Google Scholar]
- 30.Knipp, U., S. Birkholz, W. Kaup, and W. Opferkuch. 1996. Partial characterization of a cell proliferation-inhibiting protein produced by Helicobacter pylori. Infect. Immun. 64:3491–3496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lauren, P. 1965. The two histological main types of gastric carcinoma. An attempt at a histo-clinical classification. Acta Pathol. Microbiol. Scand. 64:31–49. [DOI] [PubMed] [Google Scholar]
- 32.Li, S. D., D. Kersulyte, I. J. Lindley, B. Neelam, D. E. Berg, and J. E. Crabtree. 1999. Multiple genes in the left half of the cag pathogenicity island of Helicobacter pylori are required for tyrosine kinase-dependent transcription of interleukin-8 in gastric epithelial cells. Infect. Immun. 67:3893–3899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maeda, S., H. Yoshida, T. Ikenoue, K. Ogura, F. Kanai, N. Kato, Y. Shiratori, and M. Omata. 1999. Structure of cag pathogenicity island in Japanese Helicobacter pylori isolates. Gut 44:336–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maeda, S., K. Ogura, H. Yoshida, F. Kanai, T. Ikenoue, N. Kato, Y. Shiratori, and M. Omata. 1998. Major virulence factors, VacA and CagA, are commonly positive in Helicobacter pylori isolates in Japan. Gut 42:338–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mai, U. E., G. I. Perez-Perez, J. B. Allen, S. M. Wahl, M. J. Blaser, and P. D. Smith. 1992. Surface proteins from Helicobacter pylori exhibit chemotactic activity for human leukocytes and are present in gastric mucosa. J. Exp. Med. 175:517–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mannick, E. E., L. E. Bravo, G. Zarama, J. L. Realpe, X. J. Zhang, B. Ruiz, E. T. Fontham, R. Mera, M. J. Miller, and P. Correa. 1996. Inducible nitric oxide synthase, nitrotyrosine, and apoptosis in Helicobacter pylori gastritis: effect of antibiotics and antioxidants. Cancer Res. 56:3238–3243. [PubMed] [Google Scholar]
- 37.Moss, S. F., E. M. Sordillo, A. M. Abdalla, V. Makarov, Z. Hanzely, G. I. Perez-Perez, M. J. Blaser, and P. R. Holt. 2001. Increased gastric epithelial cell apoptosis associated with colonization with cagA+ Helicobacter pylori strains. Cancer Res. 61:1406–1411. [PubMed] [Google Scholar]
- 38.Moss, S. F., J. Calam, B. Agarwal, S. Wang, and P. R. Holt. 1996. Induction of gastric epithelial apoptosis by Helicobacter pylori. Gut 38:498–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nielsen, H., and L. P. Andersen. 1992. Chemotactic activity of Helicobacter pylori sonicate for human polymorphonuclear leucocytes and monocytes. Gut 33:738–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nogueira, C., C. Figueiredo, F. Carneiro, A. T. Gomes, R. Barreira, P. Figueira, C. Salgado, L. Belo, A. Peixoto, J. C. Bravo, L. E. Bravo, J. L. Realpe, A. P. Plaisier, W. G. Quint, B. Ruiz, P. Correa, and L. J. van Doorn. 2001. Helicobacter pylori genotypes may determine gastric histopathology. Am. J. Pathol. 158:647–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Occhialini, A., A. Marais, M. Urdaci, R. Sierra, N. Munoz, A. Covacci, and F. Megraud. 2001. Composition and gene expression of the cag pathogenicity island in Helicobacter pylori strains isolated from gastric carcinoma and gastritis patients in Costa Rica. Infect. Immun. 69:1902–1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Odenbreit, S., J. Puls, B. Sedlmaier, E. Gerland, W. Fischer, and R. Haas. 2000. Translocation of Helicobacter pylori CagA into gastric epithelial cells by type IV secretion. Science 287:1497–1500. [DOI] [PubMed] [Google Scholar]
- 43.Ohsuga, M., K. Kusugami, K. Ina, T. Ando, H. Yamaguchi, A. Imada, Y. Nishio, M. Shimada, T. Tsuzuki, M. Noshiro, T. Konagaya, and H. Kaneko. 2000. Comparison between in vivo and in vitro chemokine production in Helicobacter pylori infection. Aliment. Pharmacol. Ther. 14(Suppl.1):205–215. [DOI] [PubMed] [Google Scholar]
- 44.Pan, Z. J., D. E. Berg, R. W. van der Hulst, W. W. Su, A. Raudonikiene, S. D. Xiao, J. Dankert, G. N. Tytgat, and A. van der Ende. 1998. Prevalence of vacuolating cytotoxin production and distribution of distinct vacA alleles in Helicobacter pylori from China. J. Infect. Dis. 178:220–226. [DOI] [PubMed] [Google Scholar]
- 45.Peek, R. M., Jr., M. J. Blaser, D. J. Mays, M. H. Forsyth, T. L. Cover, S. Y. Song, U. Krishna, and J. A. Pietenpol. 1999. Helicobacter pylori strain-specific genotypes and modulation of the gastric epithelial cell cycle. Cancer Res. 59:6124–6131. [PubMed] [Google Scholar]
- 46.Peek, R. M., G. G. Miller, K. T. Tham, G. I. Perez-Perez, X. Zhao, J. C. Atherton, and M. J. Blaser. 1995. Heightened inflammatory response and cytokine expression in vivo to cagA+ Helicobacter pylori strains. Lab. Investig. 71:760–770. [PubMed] [Google Scholar]
- 47.Peek, R. M., S. A. Thompson, J. P. Donahue, K. T. Tham, J. C. Atherton, M. J. Blaser, and G. G. Miller. 1998. Adherence to gastric epithelial cells induces expression of a Helicobacter pylori gene, iceA, that is associated with clinical outcome. Proc. Assoc. Am. Physicians 110:531–544. [PubMed] [Google Scholar]
- 48.Peek, R. M., S. F. Moss, K. T. Tham, G. I. Perez-Perez, S. Wang, G. G. Miller, J. C. Atherton, P. R. Holt, and M. J. Blaser. 1997. Helicobacter pylori cagA+ strains and dissociation of gastric epithelial cell proliferation from apoptosis. J. Natl. Cancer Inst. 89:863–868. [DOI] [PubMed] [Google Scholar]
- 49.Price, A. B. 1991. The Sydney System: histological division. J. Gastroenterol. Hepatol. 6:209–222. [DOI] [PubMed] [Google Scholar]
- 50.Ricci, V., C. Ciacci, R. Zarrilli, P. Sommi, M. K. Tummuru, C. Del Vecchio Blanco, C. B. Bruni, T. L. Cover, M. J. Blaser, and M. Romano. 1996. Effect of Helicobacter pylori on gastric epithelial cell migration and proliferation in vitro: role of VacA and CagA. Infect. Immun. 64:2829–2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rokkas, T., S. Ladas, C. Liatsos, E. Petridou, G. Papatheodorou, S. Theocharis, A. Karameris, and S. Raptis. 1999. Relationship of Helicobacter pylori CagA status to gastric cell proliferation and apoptosis. Dig. Dis. Sci. 44:487–493. [DOI] [PubMed] [Google Scholar]
- 52.Sakagami, T., J. Vella, M. F. Dixon, J. O’Rourke, F. Radcliff, P. Sutton, T. Shimoyama, K. Beagley, and A. Lee. 1997. The endotoxin of Helicobacter pylori is a modulator of host-dependent gastritis. Infect. Immun. 65:3310–3316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Salama, N., K. Guillemin, T. K. McDaniel, G. Sherlock, L. Tompkins, and S. Falkow. 2000. A whole-genome microarray reveals genetic diversity among Helicobacter pylori strains. Proc. Natl. Acad. Sci. USA 97:14668–14673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Segal, E. D., C. Lange, A. Covacci, L. S. Tompkins, and S. Falkow. 1997. Induction of host signal transduction pathways by Helicobacter pylori. Proc. Natl. Acad. Sci. USA 94:7595–7599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sharma, S. A., M. K. Tummuru, G. G. Miller, and M. J. Blaser. 1995. Interleukin-8 response of gastric epithelial cell lines to Helicobacter pylori stimulation in vitro. Infect. Immun. 63:1681–1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Suerbaum, S., J. M. Smith, K. Bapumia, G. Morelli, N. H. Smith, E. Kunstmann, I. Dyrek, and M. Achtman. 1998. Free recombination within Helicobacter pylori. Proc. Natl. Acad. Sci. USA 95:12619–12624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tomb, J. F., O. White, A. R. Kerlavage, R. A. Clayton, G. G. Sutton, R. D. Fleischmann, K. A. Ketchum, H. P. Klenk, S. Gill, B. A. Dougherty, K. Nelson, J. Quackenbush, L. Zhou, E. F. Kirkness, S. Peterson, B. Loftus, D. Richardson, R. Dodson, H. G. Khalak, A. Glodek, K. McKenney, L. M. Fitzegerald, N. Lee, M. D. Adams, E. K. Hickey, D. E. Berg, J. D. Gocayne, T. R. Utterback, J. D. Peterson, J. M. Kelley, M. D. Cotton, J. M. Weidman, C. Fujii, C. Bowman, L. Watthey, E. Wallin, W. S. Hayes, M. Borodovsky, P. D. Karp, H. O. Smith, C. M. Fraser, and J. C. Venter. 1997. The complete genome sequence of the gastric pathogen Helicobacter pylori. Nature 388:539–547. [DOI] [PubMed] [Google Scholar]
- 58.Tummuru, M. K. R., T. L. Cover, and M. J. Blaser. 1993. Cloning and expression of a high molecular mass major antigen of Helicobacter pylori: evidence of linkage to cytotoxin production. Infect. Immun. 61:1799–1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tummuru, M. K., S. A. Sharma, and M. J. Blaser. 1995. Helicobacter pylori picB, a homologue of the Bordetella pertussis toxin secretion protein, is required for induction of IL-8 in gastric epithelial cells. Mol. Microbiol. 18:867–876. [DOI] [PubMed] [Google Scholar]
- 60.van der Ende, A., Z. J. Pan, A. Bart, R. W. van der Hulst, M. Feller, S. D. Xiao, G. N. Tytgat, and J. Dankert. 1998. cagA-positive Helicobacter pylori populations in China and The Netherlands are distinct. Infect. Immun. 66.:1822–1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.van Doorn, L. J., C. Figueiredo, F. Megraud, S. Pena, P. Midolo, D. M. Queiroz, F. Carneiro, B. Vanderborght, M. D. Pegado, R. Sanna, W. De Boer, P. M. Schneeberger, P. Correa, E. K. Ng, J. Atherton, M. J. Blaser, and W. G. Quint. 1999. Geographic distribution of vacA allelic types of Helicobacter pylori. Gastroenterology 116:823–830. [DOI] [PubMed] [Google Scholar]
- 62.van Doorn, L. J., C. Figueiredo, R. Rossau, G. Jannes, M. van Asbroek, J. C. Sousa, F. Carneiro, and W. G. Quint. 1998. Typing of Helicobacter pylori vacA gene and detection of cagA gene by PCR and reverse hybridization. J. Clin. Microbiol. 36:1271–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.van Doorn, L. J., C. Figueiredo, R. Sanna, A. Plaisier, P. Schneeberger, W. de Boer, and W. Quint. 1998. Clinical relevance of the cagA, vacA, and iceA status of Helicobacter pylori. Gastroenterology 115:58–66. [DOI] [PubMed] [Google Scholar]
- 64.van Doorn, L. J., C. Figueiredo, R. Sanna, M. J. Blaser, and W. G. Quint. 1999. Distinct variants of Helicobacter pylori cagA are associated with vacA subtypes. J. Clin. Microbiol. 37:2306–2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.van Doorn, L. J., P. M. Schneeberger, N. Nouhan, A. P. Plaisier, W. G. Quint, and W. A. de Boer. 2000. Importance of Helicobacter pylori cagA and vacA status for the efficacy of antibiotic treatment. Gut 46:321–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wagner, S., W. Beil, J. Westermann, R. P. Logan, C. T. Bock, C. Trautwein, J. S. Bleck, and M. P. Manns. 1997. Regulation of gastric epithelial cell growth by Helicobacter pylori: evidence for a major role of apoptosis. Gastroenterology 113:1836–1847. [DOI] [PubMed] [Google Scholar]
- 67.Yamaoka, Y., D. H. Kwon, and D. Y. Graham. 2000. A M(r) 34,000 proinflammatory outer membrane protein (oipA) of Helicobacter pylori. Proc. Natl. Acad. Sci. USA 97:7533–7538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yamaoka, Y., M. Kita, T. Kodama, N. Sawai, and J. Imanishi. 1996. Helicobacter pylori cagA gene and expression of cytokine messenger RNA in gastric mucosa. Gastroenterology 110:1744–1752. [DOI] [PubMed] [Google Scholar]