Abstract
1. Single- and double-tracer experiments were performed in cats to investigate the relationship between the aortic cells and the cell bodies accumulating 5-hydroxytryptamine (5-HT) in the nodose ganglion. In one series of experiments, horseradish peroxidase (HRP) was applied to the central end of the aortic nerve, anterogradely transported and accumulated in ganglionar perikarya. The distribution of HRP-positive neurones was reconstructed in serial sections through the nodose ganglion. In a second series of experiments, the distribution of [3H]5-HT-accumulating cell bodies was assessed following incubation of the nodose ganglion in [3H]5-HT. The third series of experiments combined the treatments of the preceding ones: anterograde transport of HRP in the aortic nerve followed by incubation of the nodose ganglion in [3H]5-HT.
2. The results from these experiments provide more information with regard to (i) the topographical relationship between the aortic and [3H]5-HT-accumulating cell bodies in the same ganglion and (ii) the distribution and number of double-labelled neurones, giving further indications about histochemical components of the aortic nerve.
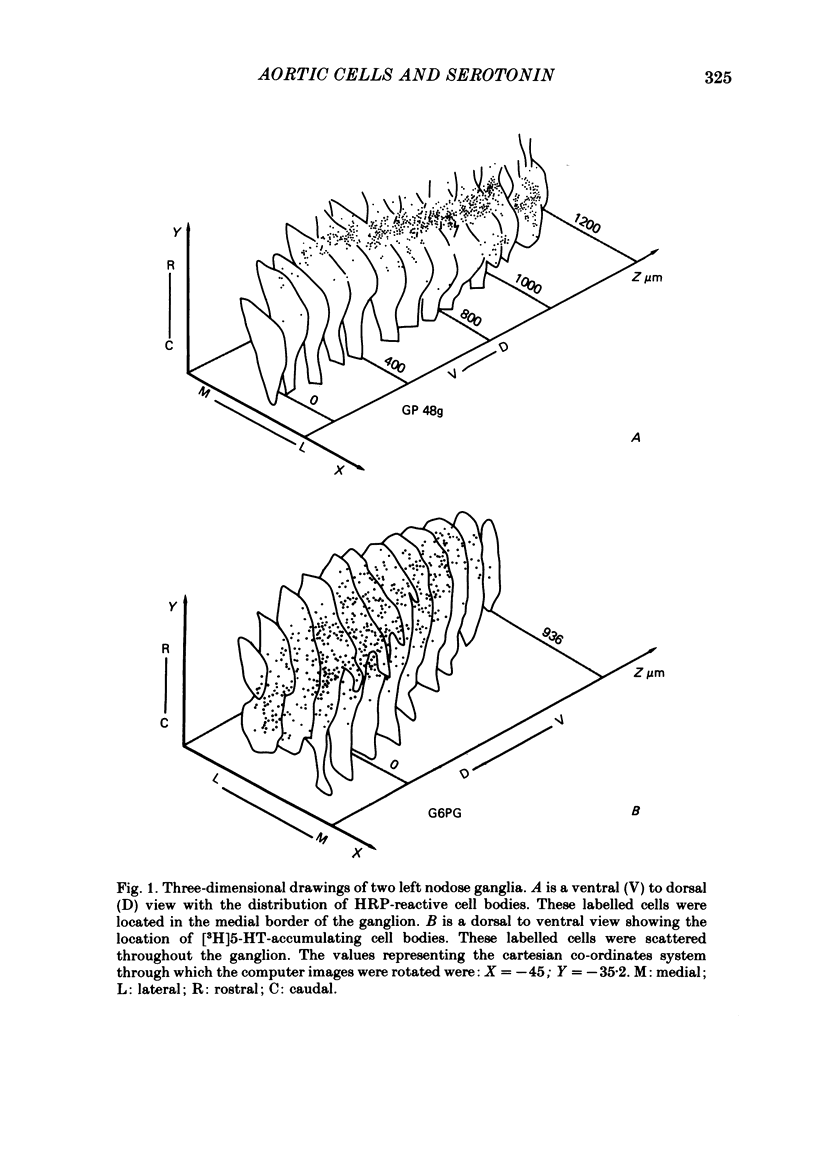
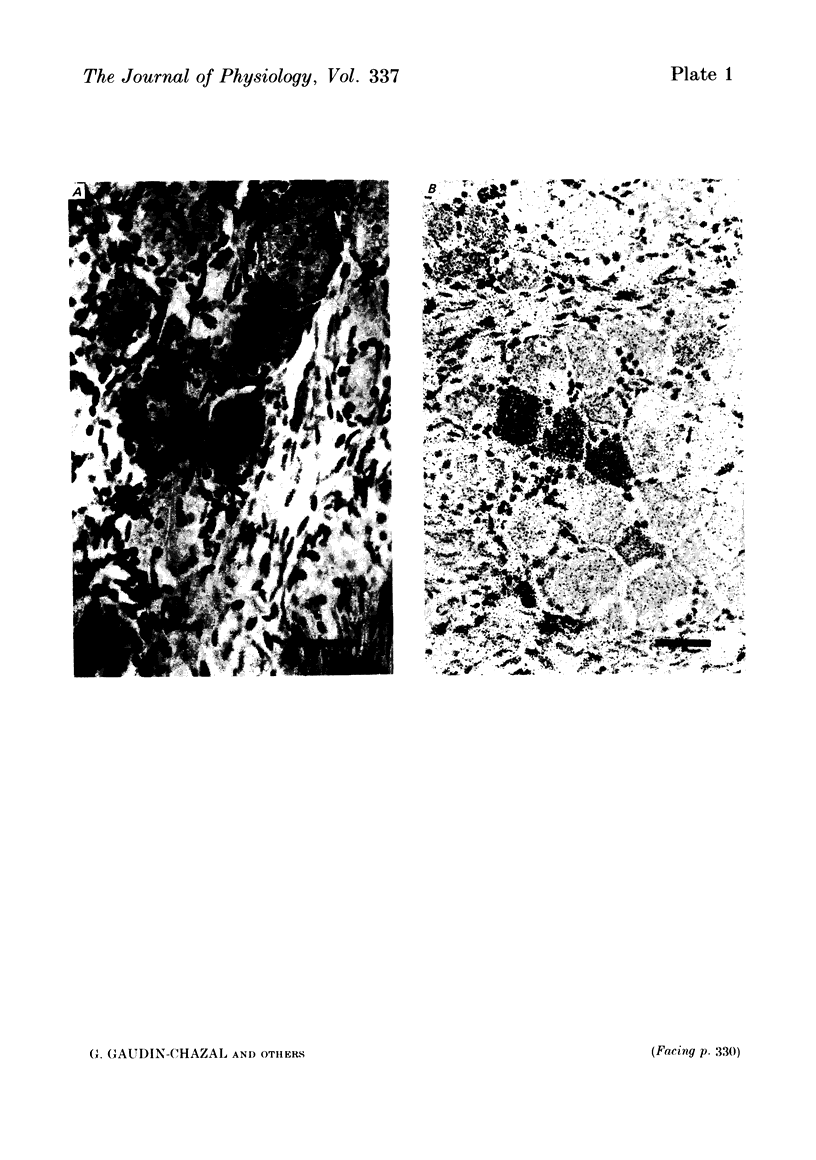
3. The HRP experiments demonstrated that HRP-positive cells show a preferential pattern of topographical organization. They were mostly located in the medial border of the ganglion where the laryngeal and aortic nerves enter. On the other hand, [3H]5-HT-accumulating neurones were scattered throughout the ganglion.
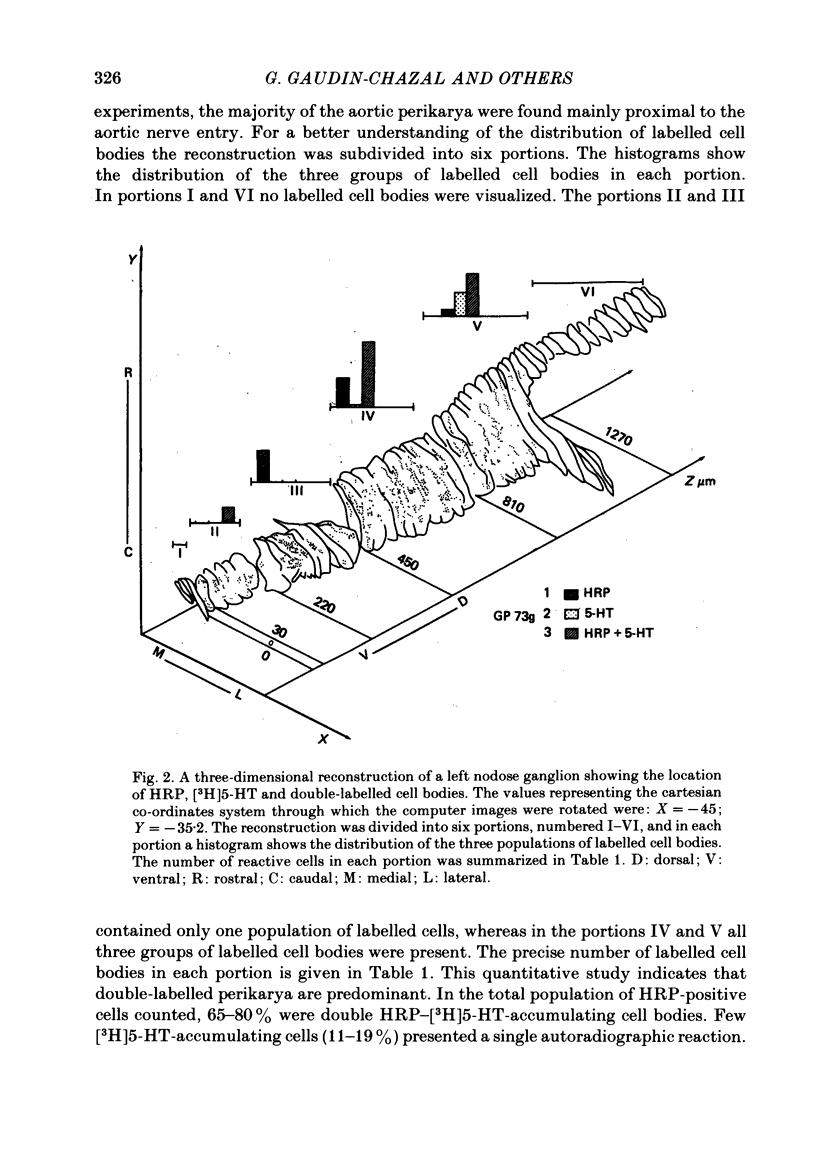
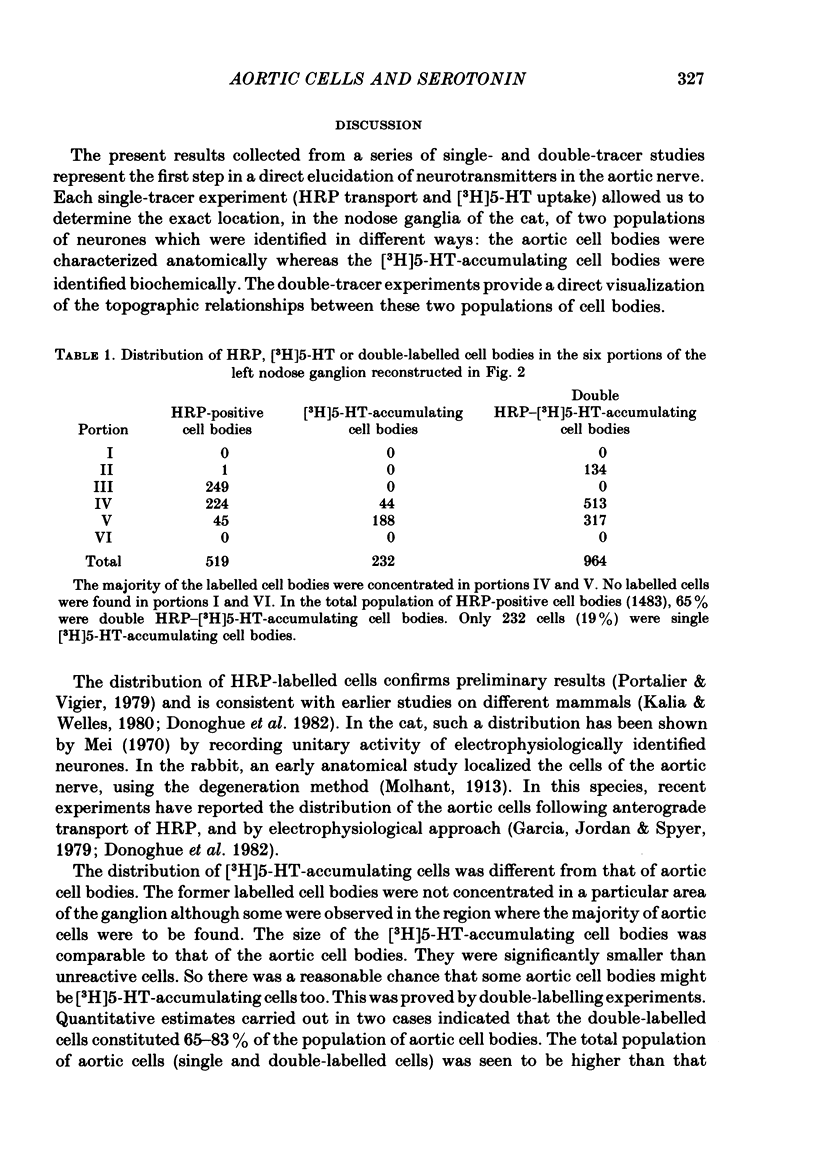
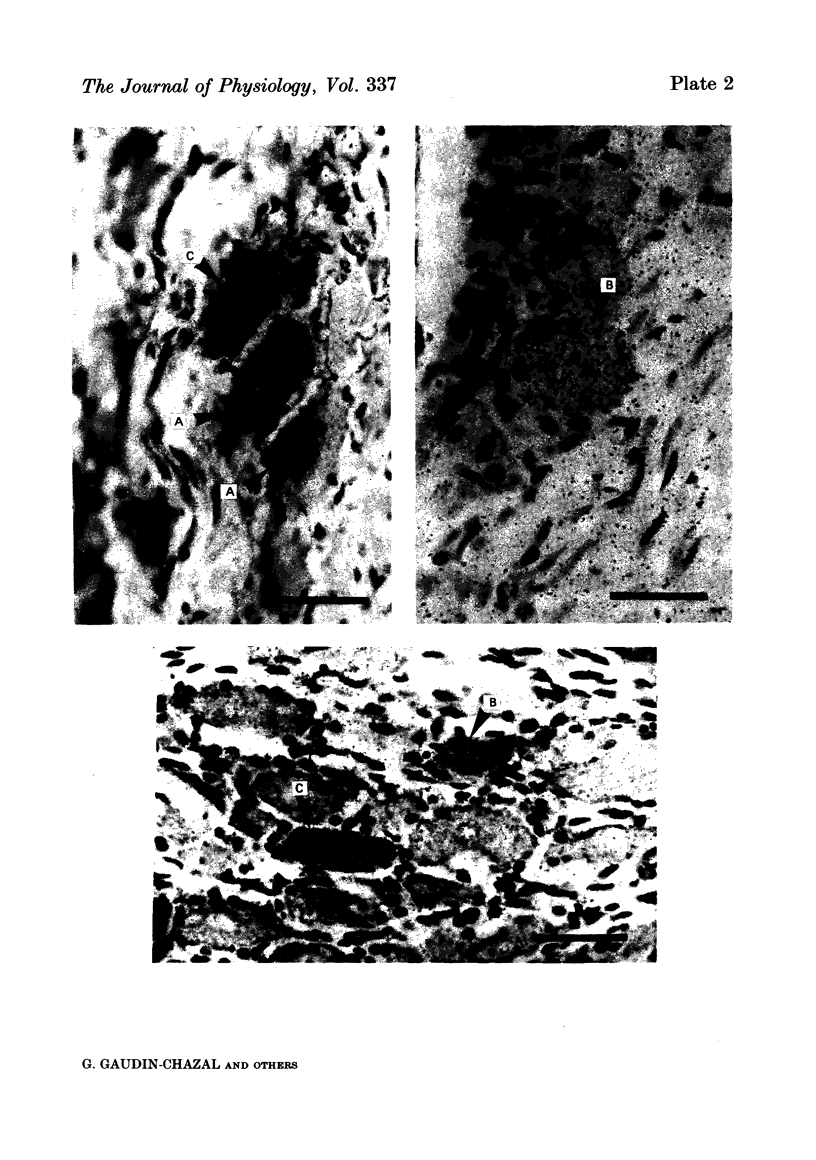
4. In double-tracer experiments, three populations of labelled cell bodies were distinguished in the same nodose ganglion: (1) single HRP-cells; (2) single [3H]5-HT-accumulating cells and (3) double-labelled cells. The distribution of the latter population exhibited no preferential localization. Quantitative estimates indicated that double-labelled neurones constituted 65-85% of the population of HRP-positive cell bodies.
5. These results show that most aortic neurones are able to take up exogenous serotonin and may be serotonergic neurones. They suggest that serotonin may be involved in physiological effects mediated via the aortic nerves.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AGOSTONI E., CHINNOCK J. E., DE DALY M. B., MURRAY J. G. Functional and histological studies of the vagus nerve and its branches to the heart, lungs and abdominal viscera in the cat. J Physiol. 1957 Jan 23;135(1):182–205. doi: 10.1113/jphysiol.1957.sp005703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COTTLE M. K. DEGENERATION STUDIES OF PRIMARY AFFERENTS OF IXTH AND XTH CRANIAL NERVES IN THE CAT. J Comp Neurol. 1964 Jun;122:329–345. doi: 10.1002/cne.901220304. [DOI] [PubMed] [Google Scholar]
- Chan-Palay V. Indoleamine neurons and their processes in the normal rat brain and in chronic diet-induced thiamine deficiency demonstrated by uptake of 3H-serotonin. J Comp Neurol. 1977 Dec 15;176(4):467–493. doi: 10.1002/cne.901760402. [DOI] [PubMed] [Google Scholar]
- Descarries L., Beaudet A., Watkins K. C. Serotonin nerve terminals in adult rat neocortex. Brain Res. 1975 Dec 26;100(3):563–588. doi: 10.1016/0006-8993(75)90158-4. [DOI] [PubMed] [Google Scholar]
- Donoghue S., Garcia M., Jordan D., Spyer K. M. Identification and brain-stem projections of aortic baroreceptor afferent neurones in nodose ganglia of cats and rabbits. J Physiol. 1982 Jan;322:337–352. doi: 10.1113/jphysiol.1982.sp014040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FUXE K. EVIDENCE FOR THE EXISTENCE OF MONOAMINE NEURONS IN THE CENTRAL NERVOUS SYSTEM. IV. DISTRIBUTION OF MONOAMINE NERVE TERMINALS IN THE CENTRAL NERVOUS SYSTEM. Acta Physiol Scand Suppl. 1965:SUPPL 247–247:37+. [PubMed] [Google Scholar]
- FUXE K., HOEKFELT T., NILSSON O. A FLUORESCENCE AND ELECTRONMICROSCOPIC STUDY ON CERTAIN BRAIN REGIONS RICH IN MONOAMINE TERMINALS. Am J Anat. 1965 Jul;117:33–45. doi: 10.1002/aja.1001170104. [DOI] [PubMed] [Google Scholar]
- Garcia M., Jordan D., Spyer K. M. Identification of aortic nerve cell bodies in the nodose ganglion of the rabbit and their central projections [proceedings]. J Physiol. 1979 May;290(2):23P–24P. [PubMed] [Google Scholar]
- Gaudin-Chazal G., Seyfritz N., Araneda S., Vigier D., Puizillout J. J. Selective retrograde transport of 3H-serotonin in vagal afferents. Brain Res Bull. 1982 May;8(5):503–509. doi: 10.1016/0361-9230(82)90008-9. [DOI] [PubMed] [Google Scholar]
- Hanker J. S., Yates P. E., Metz C. B., Rustioni A. A new specific, sensitive and non-carcinogenic reagent for the demonstration of horseradish peroxidase. Histochem J. 1977 Nov;9(6):789–792. doi: 10.1007/BF01003075. [DOI] [PubMed] [Google Scholar]
- KERR F. W. Facial, vagal and glossopharyngeal nerves in the cat. Afferent connections. Arch Neurol. 1962 Apr;6:264–281. doi: 10.1001/archneur.1962.00450220006003. [DOI] [PubMed] [Google Scholar]
- Kalia M., Mesulam M. M. Brain stem projections of sensory and motor components of the vagus complex in the cat: I. The cervical vagus and nodose ganglion. J Comp Neurol. 1980 Sep 15;193(2):435–465. doi: 10.1002/cne.901930210. [DOI] [PubMed] [Google Scholar]
- Kalia M., Welles R. V. Brain stem projections of the aortic nerve in the cat: a study using tetramethyl benzidine as the substrate for horseradish peroxidase. Brain Res. 1980 Apr 21;188(1):23–32. doi: 10.1016/0006-8993(80)90553-3. [DOI] [PubMed] [Google Scholar]
- Key B. J., Mehta V. H. Changes in electrocortical activity induced by the perfusion of 5-hydroxytryptamine into the nucleus of the solitary tract. Neuropharmacology. 1977 Feb;16(2):99–106. doi: 10.1016/0028-3908(77)90055-7. [DOI] [PubMed] [Google Scholar]
- MOHIUDDIN A. Vagal preganglionic fibres to the alimentary canal. J Comp Neurol. 1953 Oct;99(2):289–317. doi: 10.1002/cne.900990205. [DOI] [PubMed] [Google Scholar]
- Mei N., Condamin M., Boyer A. The composition of the vagus nerve of the cat. Cell Tissue Res. 1980;209(3):423–431. doi: 10.1007/BF00234756. [DOI] [PubMed] [Google Scholar]
- Mei N. Disposition anatomique et propriétés électrophysiologiques des neurones sensitifs vagaux chez le chat. Exp Brain Res. 1970;11(5):465–479. [PubMed] [Google Scholar]
- Palkovits M., Brownstein M., Saavedra J. M. Serotonin content of the brain stem nuclei in the rat. Brain Res. 1974 Nov 15;80(2):237–249. doi: 10.1016/0006-8993(74)90688-x. [DOI] [PubMed] [Google Scholar]
- Portalier P., Vigier D. Localization of aortic cells in the nodose ganglion by HRP retrograde transport in the cat. Neurosci Lett. 1979 Jan;11(1):7–11. doi: 10.1016/0304-3940(79)90047-8. [DOI] [PubMed] [Google Scholar]
- Puizillout J. J., Foutz A. S. Characteristics of the experimental reflex sleep induced by vago-aortic nerve stimulation. Electroencephalogr Clin Neurophysiol. 1977 Apr;42(4):552–563. doi: 10.1016/0013-4694(77)90219-x. [DOI] [PubMed] [Google Scholar]
- Sayadi A. K., Gaudin-Chazal G., Seyfritz N., Puizillout J. J. Effet hypnogène de la sérotonine par mise en jeu de la voie vago-aortique afférente. C R Seances Acad Sci D. 1980 Oct 13;291(6):569–572. [PubMed] [Google Scholar]
- Segu L., Gaudin-Chazal G., Seyfritz N., Puizillout J. J. A serotoninergic system in the nodose ganglia of the cat: radioautographic studies. J Physiol (Paris) 1981;77(2-3):187–189. [PubMed] [Google Scholar]
- Steinbusch H. W. Distribution of serotonin-immunoreactivity in the central nervous system of the rat-cell bodies and terminals. Neuroscience. 1981;6(4):557–618. doi: 10.1016/0306-4522(81)90146-9. [DOI] [PubMed] [Google Scholar]
- Wolf W. A., Kuhn D. M., Lovenberg W. Blood pressure responses to local application of serotonergic agents in the nucleus tractus solitarii. Eur J Pharmacol. 1981 Jan 29;69(3):291–299. doi: 10.1016/0014-2999(81)90475-1. [DOI] [PubMed] [Google Scholar]