Abstract
Sweet potato (Ipomoea batatas L.) is a nutrient-dense tuber often used in traditional diabetic treatment. This research compares the antidiabetic potential of three sweet potato varieties: orange-fleshed (OFSP), purple-peel white-fleshed (PPWSP), and white-peel white-fleshed (WPWSP), utilising in vitro and in vivo techniques. Sweet potatoes (OFSP, PPWSP, and WPWSP) boiled at 100°C for 20 minutes were incorporated into formulated diets and administered to streptozotocin-induced diabetic rats for 14 days. Aqueous extracts of the diets were tested in vitro for antioxidants and phytochemicals. Glycaemic control parameters, lipid profiles, oxidative stress indicators, and pancreatic histology were investigated. Gene expression analysis was performed on critical diabetes-related pathways. OFSP showed significant strong anti-diabetic benefits, including better glycemic control, weight maintenance, lower HOMA-IR scores, and lowered α-amylase and α-glucosidase activity. OFSP-fed rats had higher insulin, glycogen, and hexokinase activity than those given PPWSP and WPWSP. OFSP decreased mRNA expression of DPP-4 while increasing GLP-1 expression. OFSP also improved lipid profiles, increasing HDLc while decreasing LDLc and triglycerides more than other varieties. Histopathological examination revealed restorative effects in pancreatic beta cells. OFSP demonstrated more pronounced antidiabetic effects compared to PPWSP and WPWSP, particularly in terms of glycemic control, insulin regulation, and lipid profile improvement. These findings suggest that OFSP may offer significant potential for diabetes management. However, further clinical studies are needed to validate these results and explore the practical dietary applications of OFSP in diabetes control.
Keywords: Sweet potato, Diabetes, Streptozotocin, Antioxidants, Dyslipidemia
1. Introduction
Diabetes mellitus is a chronic disease that currently burdens a significant portion of the global population. It results from impaired insulin production or poor cell utilization of insulin [1]. Type-2 diabetes (T2DM) has become a major health challenge worldwide, leading to a growing number of cases and deaths each year. As of 2021, over 500 million individuals are living with T2DM, spanning all age groups [2], [3]. Projections indicate that the incidence of T2DM will continue to rise sharply in the coming decades [3], underscoring the urgent need to explore effective and sustainable therapeutic solutions.
T2DM is primarily characterized by insulin resistance and pancreatic beta-cell dysfunction, which is often exacerbated by oxidative stress and inflammation [4], [5], [6]. These factors hinder glucose uptake by cells, leading to persistent hyperglycemia. In addition to high blood sugar levels, T2DM patients frequently exhibit dyslipidemia, with elevated cholesterol and triglyceride levels, further increasing the risk of cardiovascular diseases [7]. The excessive production of free radicals contributes to oxidative damage, weakening the body’s antioxidant defense systems and promoting tissue damage [8], [9], [10].
Despite the availability of conventional antidiabetic drugs, managing diabetes remains challenging due to drug side effects, high costs, and limited accessibility in low-income regions. Many synthetic medications focus on symptom control rather than addressing the underlying metabolic dysfunctions of the disease. Consequently, there is a growing interest in identifying natural alternatives, particularly functional foods rich in bioactive compounds, which may offer sustainable strategies for diabetes prevention and management.
This study investigates the comparative antidiabetic properties of three varieties of sweet potato—Orange-Fleshed Sweet Potato (OFSP), Purple-Peel White-Fleshed Sweet Potato (PPWSP), and White-Peel White-Fleshed Sweet Potato (WPWSP)—using streptozotocin-induced diabetic rat models. Sweet potatoes (Ipomoea batatas L.) are economically significant plants with a wide range of flesh colors, including white, purple, orange, and yellow [11]. Rich in nutrients and bioactive compounds, sweet potatoes are known for their potential health benefits, including antiobesity, antidiabetic, antilipidemic, and antioxidant properties [12], [13], [14], [15]. These benefits make sweet potatoes an attractive candidate for traditional medicine, especially in African and Asian countries, where they play a key role in local diets [14], [16]. Notably, sweet potato consumption is also promoted in some parts of Japan as a natural therapy for diabetes [17], [18], [19], [20]. Almost all sweet potato parts may be helpful as food, although their respective nutritional compositions vary from one part to the other [14].
However, it is important to consider the potential side effects of OFSP consumption, especially with long-term use. While OFSP is generally considered safe and beneficial, excessive intake of certain bioactive compounds, such as carotenoids, can lead to mild conditions like carotenemia, where the skin may take on an orange tint. Additionally, the high fiber content of sweet potatoes could cause digestive discomfort, such as bloating or gas, in some individuals if consumed in large amounts. Though rare, these side effects highlight the need for balanced consumption. Furthermore, any potential interactions with pre-existing medical conditions, such as kidney disease or glucose management issues, should be considered. The current study explores these aspects while evaluating the health benefits of OFSP as an affordable alternative to conventional treatments.
The rising global prevalence of diabetes, along with the associated complications, emphasizes the need for affordable and effective alternatives to traditional antidiabetic drugs. Functional foods, particularly those rich in bioactive phytochemicals, are gaining attention for their potential to modulate various therapeutic targets involved in the pathogenesis of diabetes [21]. Sweet potato, especially OFSP, has emerged as a promising candidate due to its high polyphenolic content, which contributes to its medicinal properties. Previous studies have highlighted the antidiabetic potential of sweet potato [22], [23], [24], but there is limited research comparing the efficacy of different varieties. OFSP, PPWSP, and WPWSP have each shown promise in exhibiting antidiabetic, antilipidemic, and antioxidant effects [25], [26], [27], but a direct comparison of their relative therapeutic potentials remains unexplored.
Prior studies have demonstrated the potential of sweet potatoes in managing diabetes, particularly due to their rich content of bioactive compounds such as anthocyanins, carotenoids, and phenolics. Research has shown that the consumption of various sweet potatoes can significantly lower blood glucose levels, improve insulin sensitivity, and reduce oxidative stress in diabetic models [28], [29]. Solihah et al. [30] reported the hypoglycemic effect of purple sweet potato leaf fractions in diabetic rats. However, comparative studies focusing on the effectiveness of different sweet potato varieties, especially OFSP, in diabetic treatment remain limited, providing a strong rationale for the current investigation.
This study seeks to fill this gap by comparing the antidiabetic activities of OFSP, PPWSP, and WPWSP, providing valuable insights into which variety may offer the greatest health benefits. Such knowledge would be beneficial to the scientific community, healthcare professionals, and the food industry, as it could guide the selection of sweet potato varieties for potential use as affordable and effective antidiabetic therapies.
By incorporating both in vitro and in vivo approaches, we aim to evaluate the effects of these sweet potato varieties on glucose and lipid metabolism, as well as antioxidant status. The saponin, anthocyanin, and carotenoid contents of these varieties will also be quantified to correlate their bioactive compound content with observed therapeutic effects. Ultimately, this investigation aligns with the global need for practical, sustainable, and cost-effective solutions to combat the rising incidence of type-2 diabetes.
2. Materials and methods
2.1. Processing of the plant materials
Three varieties of sweet potato (Ipomoea batatas L.) tubers (orange-fleshed sweet potato (OFSP), purple-peel white-fleshed sweet potato (PPWSP) and white-peel white-fleshed sweet potato (WPWSP) were obtained from farmland in Warri, Delta State, southern Nigeria in May 2023 at maturity (Fig. 1). The tubers were identified at the Department of Plant Science and Biotechnology, Ekiti State University, Ado Ekiti, with voucher specimen numbers UHAE 2023029, UHAE 2023030 and UHAE 2023031 for the PPWSP, WPWSP and OFSP, respectively. Commercial corn starch (12.12 % moisture content, 0.3 % protein, and 0.9 % fibre) was purchased from a merchant.
Fig. 1.
Purple-peel-white-fleshed sweet potato (PPWSP), orange- fleshed sweet potato (OFSP), and white-peel-white-fleshed sweet potato (WPWSP).
2.2. Preparation of sweet potato samples
The tubers from each variety were washed with water and boiled at 100°C for 20 minutes. Afterwards, the boiled potatoes were peeled and cut into thin portions, subsequently air-dried at room temperature. A local pulverizer was used to process the potatoes into a dry powder stored in sealed bags. Since uncooked cornstarch has a low glycemic index, it was chosen as the control diet.
2.3. Dietary formulation ingredients
Rice husks were obtained from a rice mill in Igbemo-Ekiti, Ekiti State. Soybeans were purchased from Ado Ekiti, Ekiti State, Nigeria. The branded soybean oil was purchased from Boram Foods in Nigeria, while the multivitamin premix was purchased from Zagro Industries Pte Ltd., Bukit Timah, Singapore.
2.4. Chemicals, drugs and assay kits
Acarbose was purchased from Strides Pharma. Inc. New Jersey, USA. Streptozotocin and all the chemicals used were products of Sigma Chemical Company, St. Louis, MO, USA. All the assay kits used were Randox Laboratories Co-Artrim, United Kingdom products.
2.5. Preparation of aqueous extracts from the formulated diets
This study employed the method of [31] to prepare aqueous extracts from experimental diets. One hundred millilitres of distilled water extracted 10 g of each powdered sample for 24 hours. After filtration of the resulting mixture, the filtrate was freeze-dried, reconstituted and stored until analysis.
2.6. Determination of polyphenolic contents and in vitro antioxidant contents of the formulated diets
The total anthocyanin, saponin, total carotenoid, total phenolic, and total flavonoid contents were determined as previously described [32], [33], [34], [35], [36]. The 1,1-diphenyl-2 picrylhydrazyl (DPPH), 2,2-azinobis (3-ethylbenzo-thiazoline-6-sulfonate) ABTS* , Fe2 +-chelating ability, hydroxyl radical scavenging ability, nitric oxide, FRAP, and lipid peroxidation were evaluated as previously described [37], [38], [39], [40], [41], [42], [43], [44], [45], [46].
2.7. Laboratory animals
Eighty (80) adult rats (Wistar strain) were used for this study. The average weight of the rats was 253 ± 22 g, and they were approximately three to four months old. The rats were sourced from a breeding pack from an animal facility at the University of Ilorin, Nigeria (Department of Biochemistry). The animals were acclimatized for two weeks and cared for according to the guidelines for the care and use of experimental animals.
2.8. Induction of diabetes via streptozotocin (STZ)/ animal grouping
After the rats were acclimatized, they were sorted and placed on two dietary regimens (control and high-fat diet; HFD). After feeding for two weeks, the HFD-fed rats were induced with streptozotocin (STZ). After an overnight fast, the HFD-fed rats were injected (intraperitoneally) with 40 mg/kg body weight freshly prepared STZ (dissolved in citrate buffer; pH 4.5, 0.1 M) to model type 2 diabetes mellitus (T2DM) [45]. The initial blood glucose levels of the rats were recorded (Accu-check glucometer, Roche Diabetes Care, Inc., USA) before the induction of diabetes. Food was removed from the rats approximately 12 hours before blood glucose levels were measured. After 72 hours, a test was conducted to assess the blood glucose levels after blood samples were collected through a caudal vein puncture. Blood glucose readings of 250 mg/dl and above were used to identify diabetic rats who were subsequently used for the present study. The normoglycemic and diabetic rats were subsequently reallocated into different groups, as shown below:
• Group 1: Healthy rats (nondiabetic rats) fed a cornstarch-based diet (basal diet), designated the control (CTRL).
• Group 2: Healthy rats (nondiabetic rats) fed a cornstarch-based diet, administered 1 ml of 0.1 mol/L citrate buffer i.p once, designated the vehicle control (VEH CTRL).
• Group 3: healthy rats (nondiabetic rats) fed an OFSP-based diet (OFSP).
• Group 4: healthy rats (nondiabetic rats) fed a PPWSP-based diet (PPWSP).
• Group 5: healthy rats (nondiabetic rats) fed a WPWSP-based diet (WPWSP).
• Group 6: diabetic control rats fed a basal diet; designated STZ-induced untreated (STZ).
• Group 7: diabetic rats fed an OFSP-based diet (STZ + OFSP).
• Group 8: diabetic rats fed a PPWSP-based diet (STZ + PPWSP).
• Group 9: diabetic rats fed a WPWSP-based diet (STZ + WPWSP).
• Group 10: diabetic rats fed basal diet and orally administered ACA (25 mg/kg body weight), designated STZ + ACA.
Every three days during the trial (days 1, 4, 7, 10, and 14), the rats' weight and blood glucose levels were checked while their feed consumption was checked daily. Table 1 displays the compounded diets for various animal groupings. The rats were fed according to the designated dietary regimen allocated to their respective groups for two weeks. After that, all the rats were humanely sacrificed via ketamine and xylazine euthanasia. EDTA bottles were used for blood collection through the puncture of the cardiac muscles. The liver, small intestine, and pancreatic tissues were carefully isolated, cleansed with cold saline, and blotted with filter paper. The samples were homogenized in phosphate buffer (pH 7.4, 0.1 M). The resulting homogenates were subjected to centrifugation. Biochemical assays were conducted on the resulting clear supernatant.
Table 1.
Diet formulation for different groups of animals (g/100 g).
| Group 1 | Group 2 | Group 3 | Group 4 | Group 5 | Group 6 | Group 7 | Group 8 | Group 9 | Group 10 | |
|---|---|---|---|---|---|---|---|---|---|---|
| Drug/ingredients | CTRL | VEH CTRL | OFSP | PPWSP | WPWSP | STZ | STZ + OFSP | STZ + PPWSP | STZ + WPWSP | STZ + ACA |
| UCF | 58.60 | 58.60 | - | - | - | 58.60 | - | - | - | 58.60 |
| OFSP | - | - | 58.60 | - | - | - | 58.60 | - | - | - |
| PPFSP | - | - | - | 58.60 | - | - | - | 58.60 | - | - |
| WFSP | - | - | - | - | 58.60 | - | - | - | 58.60 | - |
| Citrate buffer i.p | - | 1 ml | - | - | - | - | - | - | - | - |
| ACA | - | - | - | - | - | - | - | - | - | 25 mg/kg BW |
| Cellulose | 6.00 | 6.00 | 6.00 | 6.00 | 6.00 | 6.00 | 6.00 | 6.00 | 6.00 | 6.00 |
| Soybean | 25.00 | 25.00 | 25.00 | 25.00 | 25.00 | 25.00 | 25.00 | 25.00 | 25.00 | 25.00 |
| Soybean oil | 6.00 | 6.00 | 6.00 | 6.00 | 6.00 | 6.00 | 6.00 | 6.00 | 6.00 | 6.00 |
| Premix | 4.00 | 4.00 | 4.00 | 4.00 | 4.00 | 4.00 | 4.00 | 4.00 | 4.00 | 4.00 |
| D-methionine | 0.40 | 0.40 | 0.40 | 0.40 | 0.40 | 0.40 | 0.40 | 0.40 | 0.40 | 0.40 |
| Total (g) | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
CTRL, control; STZ, streptozotocin; UCF, uncooked corn flour; OFSP, orange-fleshed sweet potato flour, PPWSP, purple-peel-white fleshed sweet potato flour; WPWSP, white peel-white-fleshed sweet potato flour; ACA, acarbose. The vitamin premix (mg or IU/g) was the following composition: 3200 IU vitamin A, 600 IU vitamin D3, 2.8 mg vitamin E, 0.6 mg vitamin K3, 0.8 mg vitamin B1, 1 mg vitamin B2, 6 mg niacin, 2.2 mg pantothenic acid, 0.8 mg vitamin B6, 0.004 mg vitamin B12, 0.2 mg folic acid, 0.1 mg biotin H2, 70 mg choline chloride, 0.08 mg cobalt, 1.2 mg copper, 0.4 mg iodine, 8.4 mg iron, 16 mg manganese, 0.08 mg selenium, 12.4 mg zinc, and 0.5 mg antioxidant. Group 1(control) included normal control rats fed an uncooked corn flour-based diet; Group 2 included normal rats fed an orange-fleshed sweet potato (OFSP)-based diet; Group 3 included normal rats fed a purple-peel with white fleshed sweet potato (PPFSP)-based diet; Group 4 included normal rats fed a white-fleshed sweet potato (WPWSP)-based diet; Group 5 included diabetic control rats fed an uncooked corn flour (UCF)-based diet; Group 6 included diabetic rats fed an orange-fleshed sweet potato (OFSP)-based diet; Group 7 included diabetic rats fed a purple-peel with white fleshed sweet potato (PPWSP)-based diet; Group 8 included diabetic rats fed a white-fleshed sweet potato (WPWSP)-based diet; and Group 9 included diabetic rats fed an uncooked corn flour-based diet and treated with acarbose orally/day (25 mg/kg body wt.). Group 2 was given 1 ml of 0.1 mol/L citrate buffer i.p once.
2.9. Assessment of food intake and rat body weight
A weighing scale was used to measure the amount of food consumed by the animals daily throughout the experiment. The body weight of each rat was also measured with a measuring scale.
2.10. Organ weights
The rat kidneys, pancreas, and liver weights were measured after excision from the rats. The relative weight was calculated via the following formula:
2.10.1. In vivo assays
2.10.1.1. α-Amylase activity
The method of [46] was used to assay the activity of α-amylase. The pancreatic homogenate (50 µL) was mixed with buffer (sodium phosphate; pH 6.9; 0.006 mol/L NaCl) and added to the starch solution. Dinitrosalicylic acid (200 µL) was added to the resulting mixture after incubation for 10 minutes at 25 oC. This mixture was also subjected to another round of incubation under the same conditions. Distilled water was added after the solution was cooled, and the absorbance values were measured (540 nm) with a UV-spectrophotometer. The activity of α-amylase was calculated and recorded in units/mg protein.
2.10.1.2. α-Glucosidase activity
By the method of [47], α-glucosidase activity was assayed in the small intestine. The homogenate of the small intestines (50 µL) was mixed with buffer (sodium phosphate; pH 6.9; 0.006 mol/L NaCl) and added to glutathione (3 mmol/L). P-nitrophenyl-α-d-glucopyranoside (40 µL, 5 mmol/L) was added to the resulting mixture after incubation for 10 minutes at 37 oC. This mixture was also subjected to another round of incubation under the same conditions before introducing sodium carbonate (2 ml). The absorbance values were measured (405 nm) using a UV-spectrophotometer. The activity of α-glucosidase was calculated and recorded in units/mg protein.
2.11. Measurement of total antioxidant capacity (TAC)
A myoglobin solution (50 μL, 18 μM) and an ABTS solution (3 mM) were mixed with phosphate-buffered saline (90 μL, 10 mM, pH 7.2) into which the tissue homogenate was added to a microplate (96 wells). The resulting solution was mixed adequately for 3 mins at 26 oC. The solution was incubated after hydrogen peroxide (20 μL, 250 μM) was added to each well. The absorbance values were recorded at 600 nm via a spectrophotometer (Molecular Devices). The values for total antioxidant capacity were expressed as µmol/mg of protein [48].
2.12. Lipid peroxidation assay
The formation of thiobarbituric acid reactive substances (TBARS) was used to determine lipid peroxidation. The tissue homogenate was mixed with a combined solution containing standard malondialdehyde (0.03 mM), thiobarbituric acid (500 ml; 0.8 %), sodium dodecyl sulfate (200 ml, 8.1 %), and acetic acid (pH 3.4; 2.5 M) and heated for 1 hour. The absorbance was measured at 532 nm, and the concentration of TBARS was recorded as micromoles of malondialdehyde produced per mg of protein [49].
2.13. Assay of antioxidant enzyme and insulin concentrations
Superoxide dismutase activity was measured by determining the inhibition of epinephrine autooxidation (at 30 oC, pH 10.2) [50]. The unit activity of superoxide dismutase refers to the amount of the enzyme required to inhibit the autooxidation of epinephrine by 50 %. Catalase activity was also measured in the homogenate by the rate of hydrogen peroxide absorbance (at 25 oC, 240 nm, pH 7.0) as described previously [51]. The insulin concentration was determined by immunoassay via an Elisa ELX405TM kit. HOMA-IR and HOMA-β scores were calculated at the end of the intervention according to the following formulas:
Conversion factor: Insulin (1 U/L = 7.174 pmol/l) and blood glucose (1 mmol/l = 18 mg/dl)
2.14. Measurement of total thiol levels
A prepared reaction mixture containing potassium phosphate buffer (0.1 M, pH 7.4) and 5,5-dithiobis-2-nitrobenzoic acid (10 ml, 10 mM), totalling 200 ml, was added to the heart homogenate (40 ml). The absorbance values were measured at 412 nm after the solution was incubated for 30 minutes at room temperature. Cysteine was used as a standard, and the calculations were extrapolated from a standard curve. The total thiol levels were recorded as µmol/mg protein [52].
2.15. Measurement of non-protein thiol levels
A previously described procedure for the assay of nonprotein thiol (NPSH) levels was employed in this study [52]. Trichloroacetic acid (10 %) was mixed with a portion of the heart homogenate (1:1). This was followed by centrifugation (10,000 rpm, 3 g, 5 min), after which the clear supernatant was assayed for the available sulfhydryl groups present following protein precipitation. 5,5-Dithiobis-2-nitrobenzoic acid (1.5 ml, 0.1 mM), heart homogenate (50 ml), and phosphate buffer (450 ml) were incubated at 37 oC for 10 mins. NPSH values were recorded as µmol/mg of protein.
2.16. Evaluation of lipid profiles, markers and carbohydrate-metabolizing enzymes
Plasmatic VLDLC, LDLC, HDLC, TG, TC, fructose-1,6-bisphosphatase, glucose-6-phosphatase, and hexokinase activities and glycogen content were estimated via standard colorimetric kits (CORMAY, Łomianki, POLAND) according to the commercial instructions for the kits. Serum albumin was used as a standard, and a Coomassie blue-based protocol [53] was used for the protein assay.
2.17. Gene expression
The mRNA levels of dipeptidyl-peptidase 4 (DPP-4), protein tyrosine phosphatase 1B (PTP1B), and glucagon-like peptide-1 (GLP-1) were also monitored via reverse transcriptase-PCR analysis (RT-PCR).
2.18. Total RNA isolation and cDNA conversion
Cold TRIzol (TRI) reagent served as a homogenizing medium for the samples. Chloroform was used to partition the total RNA in the sample, after which centrifugation was carried out (15,000 rpm; 15 mins). Extraction of RNA was achieved by adding isopropanol, after which the precipitated RNA pellets were cleansed in ethanol (70 %; 70 ml absolute ethanol) and nuclease-free water (30 ml). The pellets were added to sodium citrate (pH 6.4, 1 mM) buffer after air drying for 5 mins. The quality and quantity of RNA were measured prior to conversion to cDNA. Treatment with deoxyribonuclease I (NEB) removes any DNA contaminants, after which reverse transcription converts 100 ng of the RNA (DNA-free) to complementary DNA. The reaction was performed at ambient temperature, and reverse transcriptase (M-MuLV) was inactivated at 65 oC for 20 mins [54].
2.19. PCR amplification
The primers (100 pmol, 2 µL) displayed in Table 2 were used for the polymerase chain reaction (PCR) amplification stage. Other reacting components included 2 µL of cDNA, nuclease-free water (8.5 µL) and master mix (Ready Mix Taq PCR). The annealing procedure was performed for 30 seconds after denaturation for 30 seconds (at 95 oC), and the sample was extended for 1 minute. A final extension was carried out at 72 oC for 10 mins. Negative control was created that did not contain any cDNA [54].
Table 2.
List of primers.
| Primer | Accession number | Forward Primer Sequence (5′-3′) | Reverse primer sequence (3′-5′) | Melting Temp. (oC) | Optimum amplicon size | ||
|---|---|---|---|---|---|---|---|
| PTP1B | NM_013821.3 | TCCCGCCATGGAAATGGAGA | TCTCGGTACCTGTTCCGGTT | 59°C | 188 bp | ||
| DPP−4 | NM_010476.3 | GTGATGGCGATCTGCGACTA | AGGCCTAATCTTCCGCACC | 61°C | 151 bp | ||
| GLP−1 | NM_011485.5 | ACCGTTTACATCGTGGCTGG | CCCTGTGAATGGCGTTTCTC | 59°C | 191 bp | ||
| GAPDH | NM_031144.3 | CTGGCTCCTAGCACCATGAA | CGCAGCTCAGTAACAGTCCG | 61°C | 192 bp | ||
2.20. Pancreatic tissue histopathology
Pancreatic samples were stored in formalin (10 %) in preparation for clearing and tissue dehydration. After thin sample layers were embedded in paraffin, ultrathin slices were excised via a microtome. Eosin and hematoxylin were used as stainers during examination with a microscope. The morphometric and morphological features of the samples were evaluated.
2.21. Statistical analysis
The data collected during this study were subjected to a one-way analysis of variance (ANOVA). All the results were analyzed and expressed in terms of the mean and standard deviation. Duncan multiple range test was carried out to test the significance level between the means, with the level of significance recorded at p < 0.05.
3. Results and discussion
3.1. Phytochemical contents and in vitro antioxidant parameters
Saponins, carotenoids, and anthocyanins are commonly investigated phytochemicals. Saponins are complex compounds with soap-like characteristics known to possess antilipidemic properties because they lower cholesterol and triglyceride levels in dyslipidemic conditions [55]. Saponins can also lower blood glucose, as previously reported by authors [55], [56]. Carotenoids are widely known for their antioxidant, anticancer, antihyperglycemic, and anti-inflammatory activities [57], whereas anthocyanins are a class of flavonoids known to possess potent antioxidant and antidiabetic properties [58]. The results demonstrated that the saponin and carotenoid contents of the PPWSP were significantly greater than those of the OFSP and WPWSP (p < 0.05) (Table 3). Additionally, the anthocyanin content was more significant in OFSP than in PPWSP and WPWSP. These findings suggest that the PPWSP may display better antilipidemic activity than OFSP and WPWSP. OFSP may also possess better antioxidant potential than PPWSP and WPWSP.
Table 3.
Phenolic, anthocyanin, saponin, and flavonoid contents, IC50 values (mg/ml) of in vitro free radical scavenging abilities of aqueous extracts of the compounded diets.
| Parameters | A | B | C | D |
|---|---|---|---|---|
| Saponins (µg Diosgenin Eqv./g) | 0.15 ± 0.03a | 0.16 ± 0.04b | 0.15 ± 0.01a | 0.19 ± 0.08c |
| Carotenoids (mg β-carotene Eqv./g) | 88.44 ± 0.33a | 112.28 ± 0.45b | 122.28 ± 0.45c | 94.87 ± 0.35d |
| Anthocyanins (mg/100 g) | 73.98 ± 0.78a | 95.60 ± 0.37b | 91.84 ± 0.66c | 120.65 ± 0.47d |
| Total phenolic content (mg GAE/g) | 6.25 ± 0.02a | 7.58 ± 0.01b | 12.62 ± 0.01c | 10.64 ± 0.01d |
| Total flavonoid content (mg QE/g) | 3.24 ± 0.01a | 5.86 ± 0.01b | 10.94 ± 0.00c | 7.46 ± 0.01d |
| Inhibition of lipid peroxidation in rat's pancreas | 2.50 ± 0.02a | 1.84 ± 0.00b | 0.31 ± 0.04c | 0.63 ± 0.03d |
| FRAP | 0.76 ± 0.01a | 0.54 ± 0.02b | 0.24 ± 0.02c | 0.29 ± 0.03c |
| DPPH radical | 7.20 ± 0.05a | 0.77 ± 0.02b | 0.35 ± 0.03c | 0.72 ± 0.04d |
| OH* radical | 0.42 ± 0.02a | 0.37 ± 0.01b | 0.23 ± 0.00c | 0.31 ± 0.00b |
| NO* radical | 0.35 ± 0.00a | 0.31 ± 0.01a | 0.25 ± 0.00b | 0.28 ± 0.00c |
| Iron chelation | 0.44 ± 0.00a | 2.37 ± 0.00b | 1.94 ± 0.00c | 2.43 ± 0.02d |
| ABTS* radical | 0.28 0.01a | 0.24 ± 0.00a | 1.88 ± 0.00b | 2.21 ± 0.01c |
A = corn starch-based diet; B = WPWSP-based diet; C = OFSP-based diet; D = PPWSP-based diet. Each value is the mean of three determinations ± SD. Values with different superscripts across the rows are significantly different (P < 0.05). ND = not detected. White-peel-white-fleshed sweet potato (WPWSP)-based diet, purple-peel-white-fleshed sweet potato (PPWSP)-based diet, orange-fleshed sweet potato (OFSP)-based diet. GAE - gallic acid equivalent; QE - quercetin equivalent. FRAP - ferric reducing antioxidant power; NO - nitric oxide; OH - hydroxy radical; DPPH - 2,2-diphenyl-1-picrylhydrazyl, ABTS* - 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid).
This study also examined the total phenolic content, total flavonoid content, and in vitro antioxidant abilities of OFSP, PPWSP, and WPWSP-based diets. Iron-chelating ability, ferric-reducing antioxidant power (FRAP), inhibition of hydroxyl radicals (OH*), nitric oxide radicals (NO*), DPPH radicals and ABTS radicals were also assessed (Table 3). Compared with the PPWSP and WPWSP samples, the total phenolic and flavonoid content of the OFSP samples was higher (p < 0.05). This observation could result from environmental factors, origin, or genetic variation. The difference could also be attributed to the structural makeup of most phenolics and flavonoids, as they share common structural properties such as aromatic rings and hydroxyl groups [59], [60], [61]. WPWSP had the highest values for FRAP. Compared with the PPWSP and WPWSP, the OFSP had the lowest IC50 value in the rat pancreas, whereas the PPWSP had the highest OH* radical inhibition value. NO* radicals were most inhibited by the WPWSP, whereas the PPWSP most inhibited ABTS* radicals. The iron-chelation properties of the PPWSP were greater than those of the OFSP and WPWSP. Previous studies have correlated free radical scavenging properties with phenolics [62], [63]. Thus, the phenolic contents of the WPWSP, OFSP, and PPWSP may be responsible for their free radical scavenging abilities.
3.2. Body weight
Weight management is considered a crucial factor in type-2 diabetes management. According to the literature, a weight loss management strategy may be helpful for type-2 diabetic patients [64]. Thus, the prevention of excessive weight gain and the ability to buffer weight change within a physiologically acceptable limit are good attributes that must be introduced by potential antidiabetic solutions [65], [66]. Table 4 displays the weights of rats before and after the study. A considerable percentage of weight gain (p < 0.05) was recorded for all the normal rats fed PPWSP, WPWSP, and OFSP. Among all the normal rats fed sweet potato diets, those fed the PPWSP presented the lowest % weight gain compared with the control rats and those fed other sweet potato-based diets.
Table 4.
Effects of different varieties of sweet potato-based diets on the body weights (g) of normal and streptozotocin-induced diabetic rats.
| Treatment groups | Initial weight (g) | Final weight (g) | %Weight Change |
|---|---|---|---|
| CTRL | 230.25 ± 0.86a | 276.75 ± 1.50a | 16.8↑ |
| VEH CTRL | 234.00 ± 1.73b | 277.00 ± 1.23a | 15.52↑ |
| OFSP | 244.25 ± 0.86c | 277.25 ± 1.11a | 11.9↑ |
| PPWSP | 274.66 ± 2.50d | 289.50 ± 1.50b | 5.13↑ |
| WPWSP | 214.60 ± 1.73e | 257.50 ± 1.23c | 16.66↑ |
| STZ | 258.00 ± 1.73 f | 218.33 ± 1.23d | 18.16↓ |
| STZ + OFSP | 245.00 ± 1.73 g | 240.00 ± 1.23c | 2.08↓ |
| STZ + PPWSP | 264.16 ± 0.86 h | 257.83 ± 1.50e | 2.45↓ |
| STZ + WPWSP | 251.00 ± 1.75j | 233.00 ± 1.28 f | 7.72↓ |
| STZ + ACA | 241.60 ± 0.86k | 225.80 ± 1.11 g | 6.62↓ |
The data are expressed as the means ± SDs (n = 8). a-kValues with different letters along a column for a given parameter are significantly different (P < 0.05) from each other. Weight loss ( ↓); Weight gain ( ↑)
The diabetic rats induced with STZ presented the most significant weight loss percentage. However, the weight loss percentage was lower in the rats fed the OFSP- and PPWSP-based diets than in the rats fed the WPWSP-based diet. Thus, sweet potato-based diets could sustain weight change over time. These findings indicate that the OFSPP, PPWSP, and WPWSP may be considered potentially effective alternatives for weight management in diabetic patients.
3.3. Organ weights
There is a minimal correlation between caloric intake and fat accumulation in the body [67], especially in visceral organs. Moreover, obesity has been reported to influence visceral organs' weight [68] negatively. Excessive weight gain is accompanied by consuming excess calories through diets that abound in fats and carbohydrates [67]. Owing to these facts, organs such as the liver and pancreas are at risk of abnormal weight gain owing to an inability to filter excess fat produced over time. On the other hand, low food intake may cause severe organ weight loss due to a lack of nutrient supply, which may affect the muscles of the liver and kidneys if persistent. Adequate dietary intervention may be helpful to resolve negative changes in the weight of organs. Table 5 shows the effects of the OFSP, PPWSP, and WPWSP-based diets on the weights of the pancreas, kidneys, and liver of normal and diabetic rats. In the normal rats fed the OFSP, PPWSP, and WPWSP-based diets, the diets slightly increased the weights of the pancreas, kidneys, and liver (p < 0.05). However, a significant decrease in weight was observed across all the groups after induction with STZ (p < 0.05) compared with the normal rats. This weight loss may be attributed to many factors, such as persistent hyperglycemia-induced oxidative stress, the absence of insulin or insulin insensitivity, inflammation [69], [70], and the general loss of muscle mass (protein) due to insufficient nutrients. Favorably, the weights of the pancreas, kidneys, and liver of the rats fed the OFSP, PPWSP, and WPWSP-based diets were normalized in all the diabetic rats. These diets may have exhibited weight management due to the presence of antioxidants (phenolics and flavonoids), which have been linked with weight loss activity [13], [71]. These findings may indicate that the OFSP, PPWSP, and WPWSP may reverse muscle wasting in diabetic rats, further strengthening the potential of these varieties as functional weight management solutions for diabetic subjects.
Table 5.
Effects of different varieties of sweet potato-based diets on organ weights and food intake in normal and streptozotocin-induced diabetic rats.
| Pancreas | Kidney | Liver | ||||||
|---|---|---|---|---|---|---|---|---|
| Absolute weight (g) | Relative weight | Absolute weight (g) | Relative weight | Absolute weight (g) | Relative weight | Food intake (g/rat/14 days) | Weight of rat at sacrificial time (g) | |
| CTRL | 0.41 ± 0.06a | 0.14 ± 0.02a | 1.60 ± 0.43a | 0.56 ± 0.15a | 7.20 ± 1.87a | 2.60 ± 0.67a | 230.78 ± 0.80a | 276.75 ± 1.50a |
| VEH CTRL | 0.42 ± 0.01a | 0.15 ± 0.01a | 1.61 ± 0.20a | 0.57 ± 0.33a | 7.22 ± 0.30a | 2.63 ± 0.36a | 230.14 ± 0.53a | 277.00 ± 1.23a |
| OFSP | 0.54 ± 0.00b | 0.19 ± 0.00b | 1.95 ± 0.19b | 0.70 ± 0.06b | 8.45 ± 0.45b | 3.04 ± 0.97b | 230.71 ± 1.43a | 277.25 ± 1.11a |
| PPWSP | 0.51 ± 0.01b | 0.17 ± 0.00c | 1.85 ± 0.25c | 0.63 ± 0.08c | 10.30 ± 1.31c | 3.55 ± 0.45c | 230.92 ± 1.14a | 289.50 ± 1.50b |
| WPWSP | 0.45 ± 0.0c | 0.17 ± 0.01c | 1.90 ± 0.29b | 0.73 ± 0.20d | 9.85 ± 2.18d | 3.82 ± 0.84d | 230.79 ± 0.80a | 257.50 ± 1.23c |
| STZ | 0.19 ± 0.03d | 0.08 ± 0.00d | 0.82 ± 0.28d | 0.36 ± 0.12e | 4.90 ± 1.05e | 2.17 ± 0.41e | 230.78 ± 1.21a | 218.33 ± 1.23d |
| STZ + OFSP | 0.34 ± 0.01e | 0.12 ± 0.00e | 1.75 ± 0.44e | 0.67 ± 0.17 f | 8.05 ± 1.17 f | 3.12 ± 0.45 f | 230.93 ± 1.54a | 240.00 ± 1.23c |
| STZ + PPWSP | 0.33 ± 0.0e | 0.15 ± 0.00a | 1.93 ± 0.16b | 0.66 ± 0.46 g | 7.53 ± 1.71 g | 2.58 ± 0.18 g | 230.92 ± 1.14a | 257.83 ± 1.50e |
| STZ + WPWSP | 0.21 ± 0.01 f | 0.08 ± 0.00d | 1.66 ± 0.30 f | 0.52 ± 0.24 h | 6.93 ± 1.52 h | 2.16 ± 0.25e | 230.78 ± 0.80a | 233.00 ± 1.28 f |
| STZ + ACA | 0.25 ± 0.01 g | 0.11 ± 0.00 f | 1.66 ± 0.34 f | 0.75 ± 0.14i | 6.45 ± 0.98i | 2.76 ± 0.42 h | 230.14 ± 0.53a | 225.80 ± 1.11 g |
The data are expressed as the means ± SDs (n = 8). a-iValues with different letters along a column for a given parameter are significantly different (P < 0.05) from each other.
3.4. Fasting blood glucose
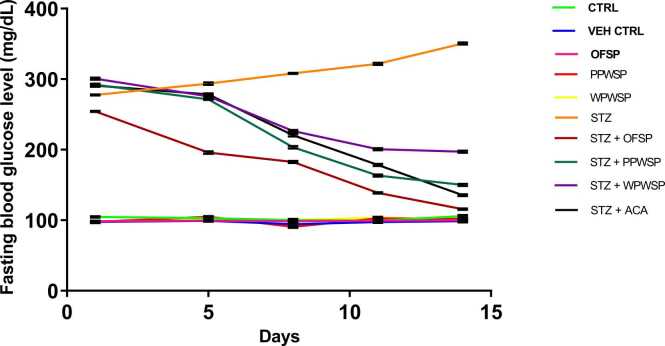
Fasting blood glucose, alternately called fasting glucose, is essential for diagnosing and monitoring diabetes. It refers to the basal concentration of plasma glucose approximately more than 8 hours after a meal, usually an overnight fast. In normoglycemic individuals, fasting glucose should not exceed 100 mg/dL. However, impaired fasting blood glucose may lead to inflated values ranging between 100 and 125 mg/dL [72], [73], [74]. Table 6 and Fig. 2 show the effects of the OFSP, PPWSP, and WPWSP-based diets on fasting blood glucose at different intervals. Seventy-two hours after the rats were induced with STZ, all the rats were confirmed to be diabetic because the average fasting blood glucose readings were above 125 mg/dL: 254.33 mg/dL (OFSP), 292 mg/dL (PPWSP), and 300.60 mg/dL (WPWSP). After 5 days of dietary intervention with the OFSP, PPWSP, and WPWSP-based diets, the average fasting blood glucose values decreased from 254.33 mg/dL to 195.80 mg/dL (OFSP), 292 mg/dL to 271.33 mg/dL (PPWSP), and 300.60 mg/dL to 275.66 mg/dL (WPWSP), respectively. Fasting blood glucose steadily and progressively decreased in all the rats fed the OFSP, PPWSP, and WPWSP-based diets. After 14 days, diabetic rats placed on the OFSP displayed a significant percentage change in fasting blood glucose (-119.89 mg/dL) compared with diabetic rats (STZ group) (p < 0.05). This trend was closely followed by the diabetic rats in the PPWSP (-94.67 mg/dL) and WPWSP (-52.59 mg/dL) groups (p < 0.05). The observed antihyperglycemic effects could be due to essential phytochemicals such as flavonoids, alkaloids, saponins, and polyphenols, which have all been linked to improved glucose metabolism in diabetic subjects [75]. Furthermore, the OFSP-based diet performed better than the acarbose diet, with a % change in fasting glucose of −114.63 mg/dL after 14 days. Therefore, the OFSP may have better blood glucose-regulating potential than the PPWSP, WPWSP, and acarbose.
Table 6.
Effects of different varieties of sweet potato-based diets on fasting blood glucose levels (mg/dL) in normal and streptozotocin-induced diabetic rats.
| FBG value at different days of treatment | |||||||
|---|---|---|---|---|---|---|---|
| Treatment groups |
FBG value before induction |
FBG value 72 hrs after induction |
5 | 8 | 11 | 14 | % Change on day 14 |
| CTRL | 103.42 ± 0.96 | 104.71 ± 0.96 | 103.00 ± 1.63 | 100.00 ± 0.73 | 99.43 ± 1.51 | 106.00 ± 1.50 | + 1.22 |
| VEH CTRL | 93.33 ± 1.73 | 97.66 ± 1.72 | 99.00 ± 0.87 | 94.33 ± 1.70 | 97.33 ± 1.23 | 98.66 ± 1.73 | + 1.01 |
| OFSP | 96.25 ± 0.86 | 98.50 ± 0.86 | 99.25 ± 1.52 | 98.50 ± 1.70 | 99.25 ± 1.73 | 99.75 ± 1.31 | + 1.25 |
| PPWSP | 92.33 ± 1.73 | 97.50 ± 1.72 | 104.83 ± 0.96 | 90.66 ± 1.56 | 102.66 ± 1.53 | 101.83 ± 1.53 | + 4.25 * |
| WPWSP | 92.40 ± 0.86 | 97.75 ± 0.81 | 100.25 ± 1.70 | 100.50 ± 1.73 | 103.75 ± 1.41 | 100.25 ± 1.49 | + 2.74 * |
| STZ | 82.00 ± 0.86 | 277.60 ± 0.87 | 293.60 ± 1.73 | 308.20 ± 0.86 | 321.60 ± 1.50 | 350.60 ± 1.50 | + 20.82 * |
| STZ + OFSP | 89.00 ± 0.86 | 254.33 ± 0.91 | 195.80 ± 1.70 | 182.66 ± 1.52 | 138.83 ± 1.21 | 115.66 ± 0.96 | −119.89# |
| STZ + PPWSP | 96.00 ± 1,53 | 292.00 ± 1.63 | 271.33 ± 0.86 | 203.33 ± 1.73 | 163.33 ± 1.63 | 150.00 ± 1.62 | −94.67# |
| STZ + WPWSP | 85.40 ± 1.73 | 300.60 ± 1.64 | 275.66 ± 1.63 | 226.33 ± 1.74 | 200.66 ± 1.63 | 197.00 ± 1.53 | −52.59# |
| STZ + ACA | 89.00 ± 1.73 | 290.83 ± 1.75 | 278.33 ± 1.43 | 220.50 ± 1.71 | 178.33 ± 1.43 | 135.50 ± 1.38 | −114.63# |
The data are expressed as the means ± SDs (n = 8). The values are significantly different at *p < .05 versus CTRL, #p < .05 versus STZ. FBG = Fasting blood glucose. Decrease (-), increase (+).
Fig. 2.
Effect of different varieties of sweet potato-based diet on blood glucose level (mg/ml) of normal and streptozotocin-induced diabetic rats. Data are expressed as mean ± SD (n = 8).
3.5. Blood lipid profile
Lipid profile anomalies are peculiar to cases of atherosclerosis during diabetes [76]. These anomalies are characterized by concurrent inflation of serum low-density lipoprotein (LDL) and triglyceride levels and a reduction in high-density lipoprotein (HDL) levels. In the present study, we evaluated the influence of OFSP, PPWSP, and WPWSP-based diets on the lipid profile of diabetic rats. The parameters assessed are displayed in Table 7. The diabetic rats in the STZ group presented significant increases in TG, TC, LDL-c, and VLDL-c levels, with corresponding decreases in HDL-c levels (p < 0.05). However, compared with the STZ and acarbose groups, the OFSP, PPWSP, and WPWSP-based groups presented significant decreases in TG, TC, LDL-c, and VLDL-c. Interestingly, all the diets also significantly augmented the levels of HLD-c (p < 0.05). The OFSP-based diet performed better than the PPWSP and WPWSP-based diets in reducing TG, LDL-c, and VLDL-c levels while significantly augmenting HDL-c levels (p < 0.05). According to previous studies, the serum level of LDL is linked with atherogenicity [76], especially in type-2 diabetes patients. In this study, all the sweet potato-based diets demonstrated antilipidemic potential. The results also suggest that OFSP may effectively lower atherogenicity in type-2 diabetes patients because of its ability to lower LDL-c levels better than acarbose. These results may also explain why the OFSP-based diet resulted in better glycemic control than the PPWSP and WPWSP-based diets since previous studies have suggested a positive correlation between glycemic control and the lipid profile [77].
Table 7.
Effects of different varieties of sweet potato-based diets on the lipid profiles of normal and streptozotocin-induced diabetic rats fed sweet potato based diets.
| Groups | PARAMETERS | ||||
|---|---|---|---|---|---|
| TG (mg/dL) | TC (mg/dL) | LDL-c (mg/dL) | HDL-c (mg/dL) | VLDL-c (mg/dL) | |
| CTRL | 36.42 ± 0.26a | 41.42 ± 0.14a | 13.40 ± 0.44a | 23.91 ± 0.33a | 4.32 ± 0.05a |
| VEH CTRL | 36.12 ± 0.25a | 40.86 ± 0.07a | 13.17 ± 0.94a | 23.49 ± 0.14b | 4.22 ± 0.05a |
| OFSP | 31.92 ± 0.67b | 35.59 ± 0.12b | 9.96 ± 0.11b | 29.32 ± 0.78c | 3.38 ± 0.13b |
| PPWSP | 34.37 ± 0.30b | 39.54 ± 0.91c | 10.24 ± 0.18c | 25.46 ± 0.11d | 3.87 ± 0.06a,b |
| WPWSP | 35.61 ± 0.32a | 39.58 ± 0.07c | 10.89 ± 0.29d | 24.60 ± 0.87a,d | 4.12 ± 0.06a |
| STZ | 52.27 ± 0.26c | 63.38 ± 0.49d | 35.56 ± 0.55e | 12.38 ± 1.39e | 15.45 ± 0.05c |
| STZ + OFSP | 36.65 ± 0.04d | 39.15 ± 0.91e | 15.75 ± 0.24 f | 17.89 ± 0.71 f | 5.55 ± 0.24d |
| STZ + PPWSP | 37.92 ± 0.18d | 37.80 ± 0.14 f | 16.63 ± 0.87 f | 15.46 ± 0.81 g | 5.81 ± 0.11d |
| STZ + WPWSP | 38.85 ± 0.86e | 40.47 ± 0.56 g | 19.75 ± 0.24 g | 13.67 ± 1.22 h | 7.17 ± 0.17e |
| STZ + ACA | 36.16 ± 1.85 f | 38.43 ± 0.35 f | 17.63 ± 0.87 f | 15.42 ± 0.31 g | 5.48 ± 0.74d |
The data are expressed as the means ± SDs (n = 8). a-g Values with different letters along a column for a given parameter are significantly different (P < 0.05) from each other. *TC (total cholesterol); TG (total triglyceride); *HDL-c (high-density lipoprotein-cholesterol); *VLDL-c (very low-density lipoprotein-cholesterol); *LDL-c (low-density lipoprotein-cholesterol)
3.6. α-Amylase activity and α-glucosidase activities
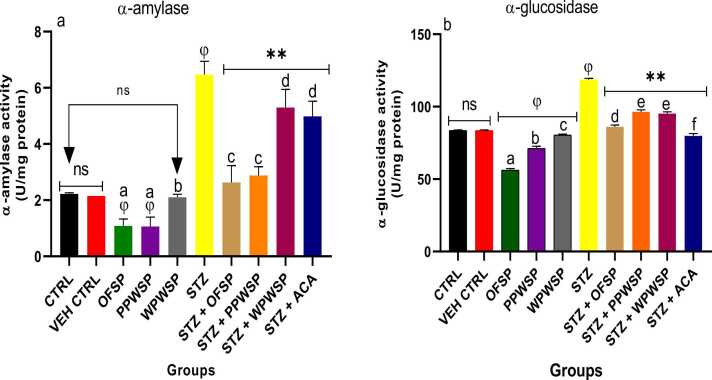
α-Amylase and α-glucosidase are starch hydrolases relevant to the metabolism of carbohydrates (polysaccharides and oligosaccharides) into glucose units for absorption in the small intestine. These enzymes are primary therapeutic targets for antidiabetic interventions [78], [79]. In type-2 diabetes, inhibiting both α-amylase and α-glucosidase is a potent approach for preventing the intestinal absorption of glucose, thereby exerting glycemic control [80], [81]. This study assessed the activities of OFSP, PPWSP, and WPWSP-based diets as potential inhibitors of α-amylase and α-glucosidase activities in vivo (Fig. 3). Compared with the normal rats in the control group, the diabetic rats in the STZ group presented significantly increased α-amylase and α-glucosidase activities (p < 0.05). Compared with the normal control rats, the normal rats fed the OFSP, PPWSP, and WPWSP-based diets presented significantly lower α-amylase and α-glucosidase activities (p < 0.05). Additionally, OFSP, PPWSP, and WPWSP-based diets significantly inhibited the activities of α-amylase and α-glucosidase (p < 0.05). Interestingly, OFSP-based diets inhibited α-amylase activity better than PPWSP, WPWSP, and acarbose in normal and diabetic rats. Furthermore, OFSP-based diets resulted in more excellent α-glucosidase inhibitory activity than PPWSP, and WPWSP-based diets did in both normoglycemic and diabetic rats (p < 0.05).
Fig. 3.
Effect of different varieties of sweet potato-based diet on (a) α-amylase (b) α-glucosidase activities of normal and streptozotocin-induced diabetic rats. Each value is a mean of 8 determinations ± SD. Values are statistically different at ϕp < .05 versus CTRL, * *p < .05 versus STZ, values with different superscript letters are significantly different (p < .05), ns = not statistically different.
The present investigation suggested that the increased α-amylase and α-glucosidase activities exhibited by rats fed OFSP, PPWSP, and WPWSP-based diets may be associated with increased expression of both enzymes at the gene level. A suitable inhibitor of α-amylase and α-glucosidase should also be able to modulate blood glucose by suppressing their activities [82], [83]. Thus, this study suggests that OFSP-based diets display better antidiabetic potential by suppressing α-amylase and α-glucosidase activities.
3.7. Liver glycogen content and serum insulin levels
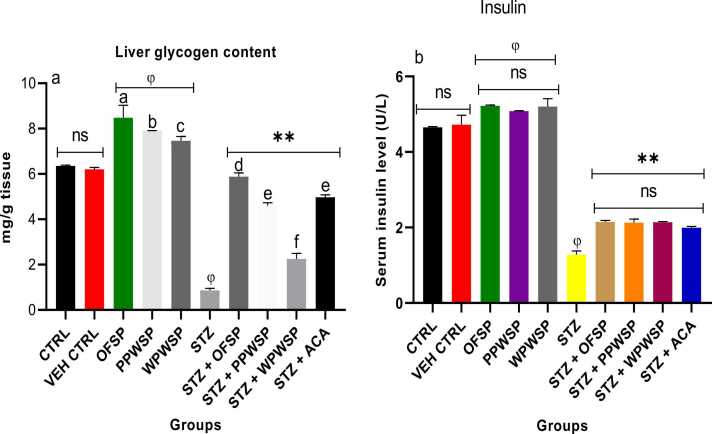
The liver is a vital organ associated with carbohydrate metabolism. It is the site for the storage of glucose units as glycogen. It is sensitive to the presence of insulin, and thus, it plays a vital role in sending signals for glycogen generation. Diabetes is characterized by dysfunctional insulin signalling (insensitivity) and secretion, affecting glycogen metabolism [84], [85]. The effects of different sweet potato-based diets on the liver glycogen content and serum insulin levels of normal rats and streptozotocin-induced diabetic rats are displayed in Fig. 4. The results revealed that the liver glycogen content of normal rats fed the OFSP-based diet was significantly greater than that of normal rats fed the PPWSP and WPWSP-based diets (p < 0.05) compared with the control group.
Fig. 4.
Effect of different varieties of sweet potato-based diet on (a) liver glycogen (b) serum insulin contents of normal and streptozotocin-induced diabetic rats. Each value is a mean of 8 determinations ± SD. Values are statistically different at ϕp < .05 versus CTRL, * *p < .05 versus STZ, values with different superscript letters are significantly different (p < .05), ns = not statistically different.
Additionally, there was an increase in insulin levels in normal rats fed OFSP, PPWSP, and WPWSP-based diets (not significant at p < 0.05). These findings indicate that the diets positively influence liver glycogen content and insulin levels. The results also revealed that diabetic rats in the STZ group presented significantly decreased liver glycogen content and serum insulin levels (p < 0.05). These findings indicate an impairment in glucose and glycogen metabolism in the liver. However, the OFSP and PPWSP-based diets were able to significantly increase the liver glycogen content compared with the STZ group and the acarbose group, with the OFSP-based diet resulting in the highest liver glycogen content compared with the PPWSP and WPWSP-based diets (p < 0.05).
Furthermore, all the sweet potato-based diets presented increased insulin levels compared with those in the acarbose group, although the difference was insignificant. Nonetheless, the insulin levels observed in the diabetic rats fed the OFSP, PPWSP, and WPWSP-based diets were significantly more significant than the diabetic rats in the STZ group. In summary, the results showed that the OFSP-based diet increased the liver glycogen content and insulin levels more efficiently than the PPWSP and WPWSP-based diets. These findings suggest that sweet potato-based diets may exert their antidiabetic effects by increasing insulin levels and liver glycogen content.
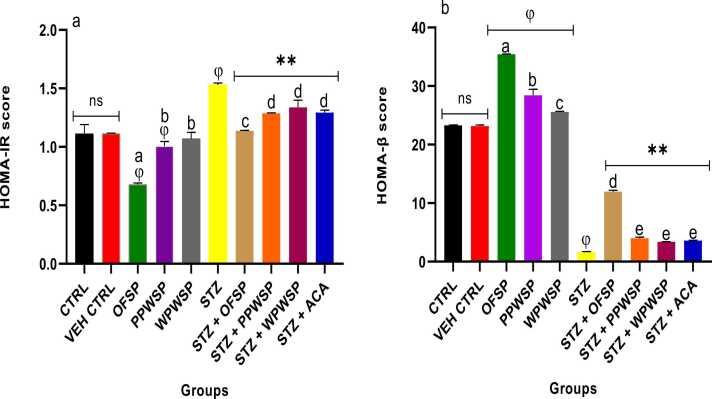
3.8. HOMA-IR and HOMA-β scores
HOMA-IR and HOMA-β are commonly used statistical estimators of insulin resistance and beta cell function in type-2 diabetes [86]. Both indices depend on glucose and insulin fasting levels to measure the efficiency of glucose metabolism and insulin activity [87]. Higher HOMA-IR levels indicate insulin resistance, whereas lower levels of HOMA-β translate to inefficient beta cell functionality. In the present study, the HOMA-IR scores were significantly lower in normal rats fed sweet potato diets (OFSP, PPWSP, and WPWSP), with the OFSP-based diet exhibiting a lower HOMA-IR score than the control diet, PPWSP, and WPWSP-based diets did (p < 0.05) (Fig. 5). Conversely, normal rats fed sweet potato-based diets presented significantly higher HOMA-β scores than the control group.
Fig. 5.
Effect of different varieties of sweet potato-based diet on (a) Homeostasis model assessment-insulin resistance (HOMA-IR) (b) Homeostasis model assessment-β (HOMA- β) scores of normal and streptozotocin-induced diabetic rats. Each value is a mean of 8 determinations ± SD. Values are statistically different at ϕp < .05 versus CTRL, * *p < .05 versus STZ, values with different superscript letters are significantly different (p < .05), ns = not statistically different.
Interestingly, OFSP-based diets presented the highest HOMA-β score compared to the control and other sweet potato-based diets. The STZ group also presented higher HOMA-IR and lower HOMA-β levels (p < 0.05). The results further revealed a trend reversal, as the OFSP, PPWSP, and WPWSP-based diets significantly lowered HOMA-IR levels and significantly increased HOMA-β levels compared with those in the STZ and acarbose groups. Concerning the HOMA-IR and HOMA-β levels, the OFSP-based diet produced good scores, indicating that the OSP-based diet improved beta cell function and attenuated insulin resistance in diabetic rats. This observation could also explain its ability to improve glucose metabolism in the diabetic state.
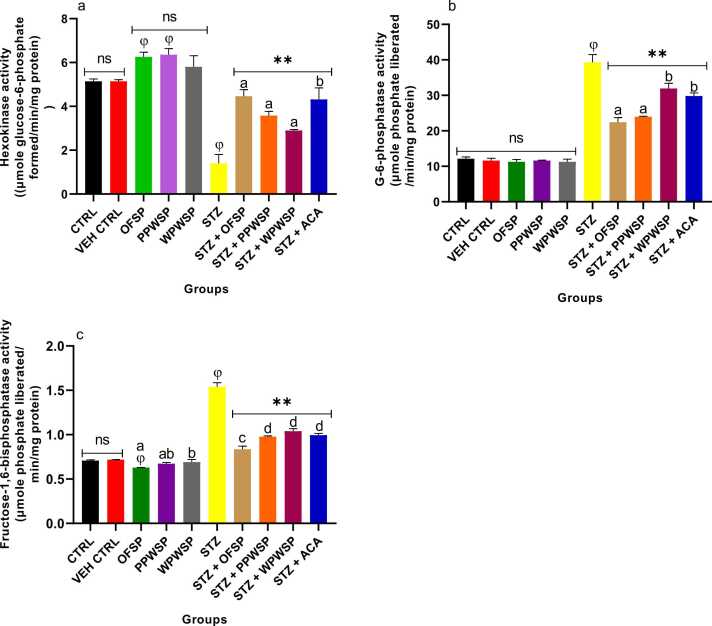
3.9. Hexokinase, glucose-6-phosphatase, and fructose-1,6-bisphosphatase activities
Hexokinase, glucose-6-phosphatase (G6P), and fructose-1,6-bisphosphatase (FBP) are key regulatory enzymes linked with glucose metabolism through the glycolytic pathway. Their indispensable roles in the glycolytic pathway render them credible biomarkers for the efficiency of glucose metabolism. Hexokinase is a glycolytic enzyme that catalyzes the conversion of glucose into its phosphorylated form (glucose-6-phosphate) via ATP. G6P is a substrate for glycolysis and the hexose monophosphate shunt [88], [89], [90]. Under diabetic conditions, hexokinase activity decreases due to the reduced glucose uptake by the cells for metabolic utilization because of decreased hexokinase mRNA and enzyme production [90]. However, G6P and FBP are gluconeogenic enzymes. The gluconeogenesis pathway activities increase due to dysfunctional insulin sensitivity in type-2 diabetes patients. Thus, increased activities of G6P and FBP are associated with insulin insensitivity and reduced glucose utilization in the liver under diabetic conditions [85]. Fig. 6 shows the effects of various sweet potato-based diets on hexokinase, G6P, and FBP activities in normal and streptozotocin-induced rats. Compared with the control group, the normoglycemic group of rats fed the OFSP, PPWSP, and WPWSP-based diets presented improved hexokinase activity (not significant at p < 0.05) and decreased activity of G6P and FBP (significant at p < 0.05).
Fig. 6.
Effect of different varieties of sweet potato-based diet on carbohydrate metabolizing enzymes (a) Hexokinase (b) Glucose-6-phosphatase (c) Fructose-1,6-bisphosphatase activities of normal and streptozotocin-induced diabetic rats. Each value is a mean of 8 determinations ± SD. Values are statistically different at ϕp < .05 versus CTRL, * *p < .05 versus STZ, values with different superscript letters are significantly different (p < .05), ns = not statistically different.
Furthermore, all the diabetic rats in the STZ group presented significantly decreased hexokinase activity and significantly increased G6P and FBP activity (p < 0.05). However, compared with the STZ diet, all the sweet potato-based diets significantly improved hexokinase activity. Additionally, the sweet potato-based diets significantly reduced the activities of G6P and FBP compared with those in the STZ and acarbose groups. Moreover, OFSP-based diets were more effective than acarbose, PPWSP, and WPWSP-based diets. Therefore, the results suggest that the OFSP-based diet is more efficient than the PPWSP and WPWSP-based diets for regulating hexokinase, G6P, and FBP activity under diabetic conditions. This finding also suggests another possible mechanism through which OFSP regulates glucose metabolism better than PPWSP and WPWSP.
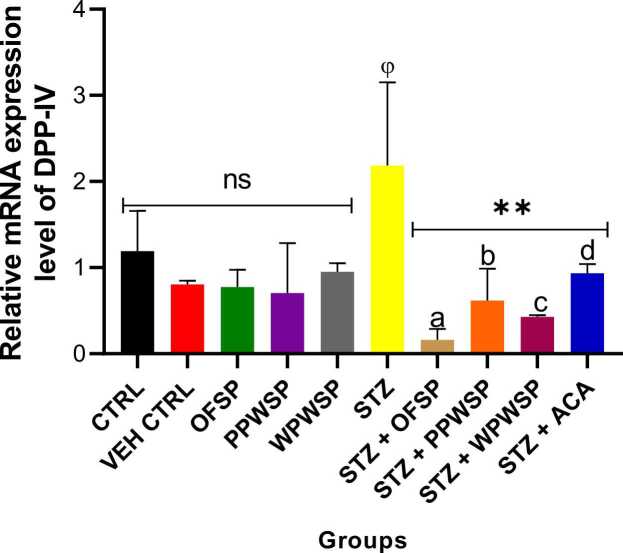
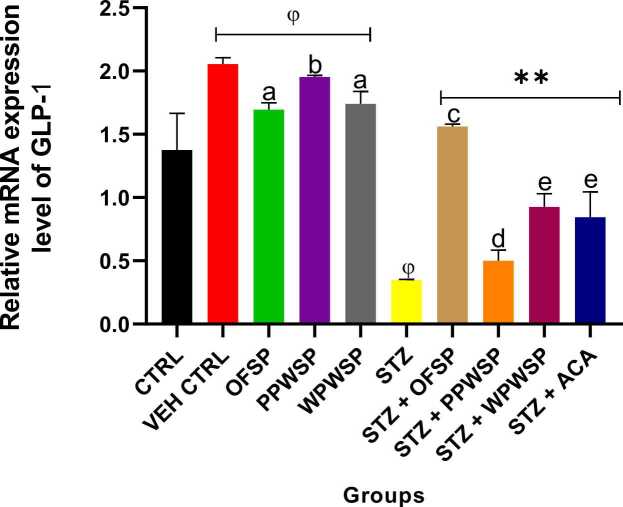
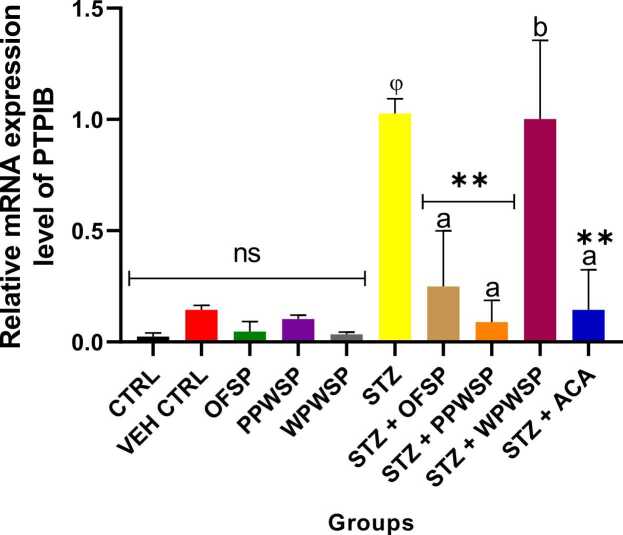
3.10. mRNA expression of the enzymes DPP-4, GLP-1, and PTP1B
DPP-4, GLP-1, and PTP1B are linked with glucose metabolism and are notable in the pathogenesis of diabetes mellitus (type-2). DPP-4 is known for its role in degrading incretins. As such, the levels of GLP-1 are regulated by the levels of DPP-4 [91]. GLP-1 plays a vital role in sensitizing insulin secretion from the beta cells while inhibiting the release of glucagon from alpha cells to regulate blood glucose [92]. It can also support weight management in diabetic patients by inducing satiety. This action of DPP-4 on GLP-1 negatively affects the secretion of insulin and, ultimately, glucose metabolism. Protein tyrosine phosphatase 1B (PTP1B) is known for its negative influence on insulin signaling routes by suppressing insulin sensitivity [93]. The present study assessed the effects of OFSP, PPWSP, and WPWSP-based diets on the expression of the DPP-4, GLP-1, and PTP1B genes (Fig. 7, Fig. 8, Fig. 9). In normal rats placed on the OFSP, PPWSP, or WPWSP, there was no significant change in the expression of the DPP-4 and PTP-1B genes, whereas there was a significant change in the expression of GLP-1 compared with that in the control group (p < 0.05). In the STZ group, the expression of the DPP-4 and PTP1B genes significantly increased, whereas the expression of GLP-1 significantly decreased (p < 0.05). The results also revealed that the OFSP, PPWSP, and WPWSP-based diets significantly downregulated the expression of the DPP-4 and PTP1B genes while concurrently increasing the expression of the GLP-1 gene (p < 0.05). Notably, the OFSP-based diet improved GLP-1 expression and reduced DPP-4 expression better than the PPWSP and WPWSP-based diets. Additionally, the PPWSP-based diet reduced PTP1B gene expression compared with the OFSP and WPWSP-based diets. These observations point to improved glycemic control by sweet potato-based diets. These investigations suggest that the antidiabetic potential of OFSP, PPWSP, and WPWSP may be associated with their ability to downregulate DPP-4 and PTP1B gene expression while upregulating GLP-1 gene expression.
Fig. 7.
Effect of different varieties of sweet potato-based diet on dipeptidyl peptidase IV (DPP-4) mRNA expression of normal and streptozotocin-induced diabetic rats. Each value is a mean of 8 determinations ± SD. Values are statistically different at ϕp < .05 versus CTRL, * *p < .05 versus STZ, values with different superscript letters are significantly different (p < .05), ns = not statistically different.
Fig. 8.
Effect of different varieties of sweet potato-based diet on glucagon-like peptide 1 (GLP-1) mRNA expression of normal and streptozotocin-induced diabetic rats. Each value is a mean of 8 determinations ± SD. Values are statistically different at ϕp < .05 versus CTRL, * *p < .05 versus STZ, values with different superscript letters are significantly different (p < .05), ns = not statistically different.
Fig. 9.
Effect of different varieties of sweet potato-based diet on protein tyrosine phosphatase 1B (PTP-1B) mRNA expression of normal and streptozotocin-induced diabetic rats. Each value is a mean of 8 determinations ± SD. Values are statistically different at ϕp < .05 versus CTRL, * *p < .05 versus STZ, values with different superscript letters are significantly different (p < .05), ns = not statistically different.
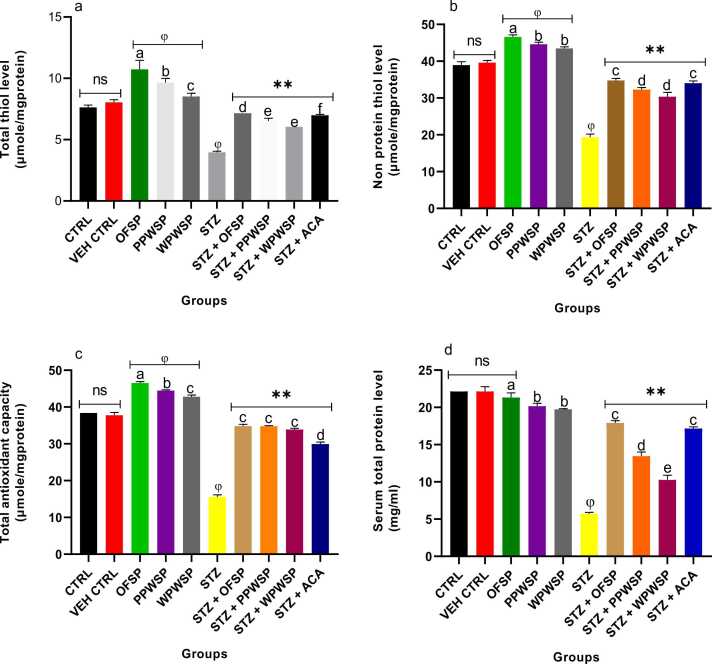
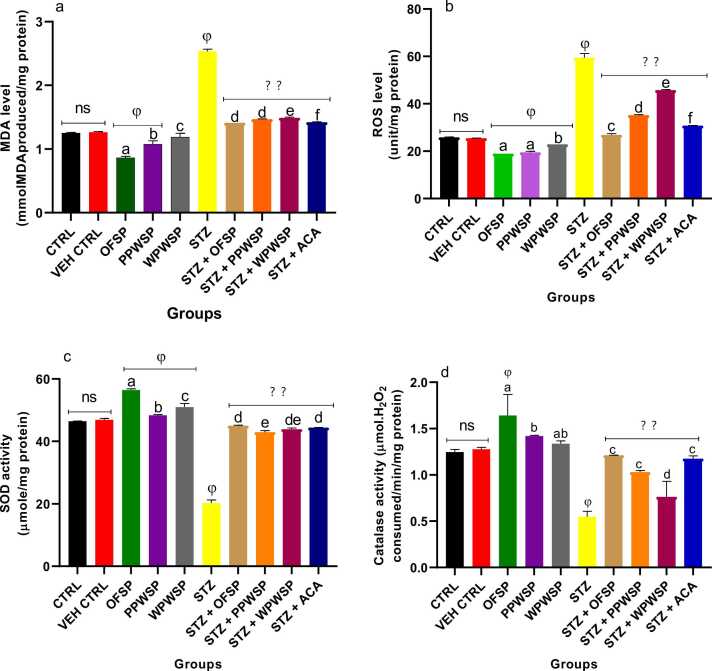
3.11. Antioxidant levels
Antioxidants are potent molecules that ameliorate the negative impact of free radicals in the body. The defensive role of antioxidants is crucial for maintaining homeostasis while preventing the oxidation of structurally important molecules present in body organs [94], [95]. Oxygen facilitates the generation of reactive species, which ultimately damage cells and disrupts normocellular activities [96], [97]. In type-2 diabetes, generating these reactive species is implicated in vascular complications associated with the disease [98]. The excessive generation of reactive oxygen species drastically inhibits the production of essential antioxidants, such as glutathione, glutathione peroxidase, glutathione reductase, catalase, and superoxide dismutase [97]. The sources of free radicals that induce oxidative stress may include protein glycation (nonenzymatic) and excessive autoxidation of lipids (peroxidation) [97], [99]. Hyperglycemia, dyslipidemia, and hyperinsulinemia also trigger notable diseases in the vascular system, which cause exponential production of reactive oxygen species, thereby inducing oxidative stress [97], [100]. In this study, the effects of OFSP, PPWSP, and WPWSP-based diets on total thiol, nonprotein thiol, total antioxidant capacity, total protein, catalase (CAT), and superoxide dismutase (SOD) levels were examined. The levels of reactive oxygen species and malondialdehyde were evaluated (Fig. 10, Fig. 11). The present study's findings revealed that compared with the control diet, the OFSP, PPWSP, and WPWSP-based diets improved the antioxidant levels (p < 0.05). A significant increase in total thiol, nonprotein thiol, total antioxidant capacity, CAT, and SOD activities was observed (p < 0.05), although there was no significant change in total protein levels. Concurrently, there was a significant decrease in the production of malondialdehyde and reactive oxygen species. These findings indicate that OFSP, PPWSP, and WPWSP-based diets will likely protect normal rats by increasing their antioxidant defences.
Fig. 10.
Effect of different varieties of sweet potato-based diet on (a) total thiol (TSH) (b) non protein thiol (NPSH)(c) Total antioxidant capacity (TAC) (d) serum total protein levels of normal and streptozotocin-induced diabetic rats. Each value is a mean of 8 determinations ± SD. Values are statistically different at ϕp < .05 versus CTRL, * *p < .05 versus STZ, a-evalues with different superscript letters are significantly different (p < .05), ns = not statistically different.
Fig. 11.
Effect of different varieties of sweet potato-based diet on (a) malondialdehyde (MDA) level (b) reactive oxygen species (ROS) level(c) superoxide dismutase (SOD) activity (d) catalase activity of normal and streptozotocin-induced diabetic rats. Each value is a mean of 8 determinations ± SD. Values are statistically different at ϕp < .05 versus CTRL, * *p < .05 versus STZ, a-evalues with different superscript letters are significantly different (p < .05), ns = not statistically different.
Furthermore, significant decreases in total thiol, nonprotein thiol, total antioxidant capacity, CAT, and SOD activities were detected in the STZ group, which was also associated with concurrent increases in ROS and malondialdehyde levels (p < 0.05) (Fig. 10, Fig. 11). Interestingly, all the sweet potato-based diets efficiently reversed the oxidative effect of STZ by significantly increasing the levels of antioxidant parameters (total thiol, nonprotein thiol, total antioxidant capacity, CAT, and SOD) in diabetic rats (p < 0.05). This trend was also accompanied by a significant decrease in ROS and malondialdehyde levels (p < 0.05).
OFSP's higher antioxidant content, particularly its rich supply of polyphenolic compounds such as anthocyanins and carotenoids, is believed to play a significant role in its antidiabetic effects. Antioxidants help mitigate oxidative stress, which is a major contributor to the pathophysiology of type-2 diabetes [101]. In diabetic conditions, excessive production of reactive oxygen species (ROS) leads to damage of pancreatic beta cells, impairing insulin secretion, and to the dysfunction of other metabolic processes [101]. The antioxidants in OFSP scavenge these ROS, reducing cellular oxidative damage and thus protecting pancreatic cells. This antioxidant activity not only helps to preserve insulin secretion but also improves insulin sensitivity by modulating key signaling pathways involved in glucose metabolism [102]. Furthermore, antioxidants may reduce inflammation and improve lipid metabolism, both of which are commonly disrupted in diabetic patients [103]. By improving oxidative stress levels and mitigating its damaging effects, OFSP's antioxidant-rich composition provides a potential mechanism for its beneficial impact on blood glucose control and overall metabolic health.
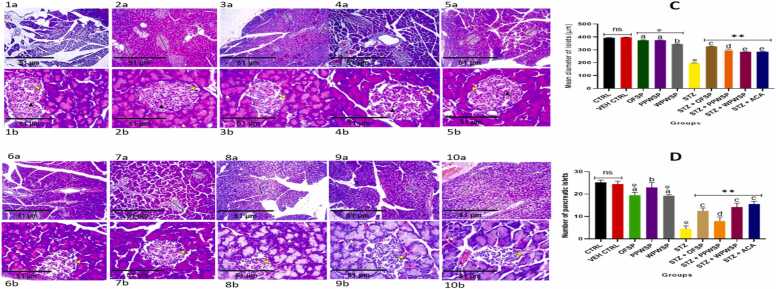
3.12. Histopathological studies
Streptozotocin (STZ) is a potent and commonly used diabetogenic agent because of its selective toxicity to beta cells of the pancreas [104]. Streptozotocin decreases the number of islet cells in the pancreas, leading to hyperglycemia. As such, it is often used in experimental animal models involving type-1 or type-2 diabetes [104]. Fig. 12 shows the effects of the OFSP, PPWSP, and WPWSP-based diets on the structure of the pancreatic islets of normal and STZ-induced diabetic rats. The results revealed a significant decrease in the number and size of pancreatic islets in the STZ group compared with those in the control group (p < 0.05). However, the OFSP, PPWSP, and WPWSP-based diets were able to reverse this effect by causing a significant increase in the number and size of the pancreatic islets. These findings suggest that the OFSP, PPWSP, and WPWSP may possess regenerative properties that help beta cells produce more insulin. The results also revealed that the OFSP-based diet significantly increased the size of the pancreatic islets more than the acarbose, PPWSP, and WPWSP-based diets (p < 0.05). Additionally, no significant difference in the number of pancreatic islets was observed between the OFSP and WPWSP-based diets and the acarbose diet. Thus, the OFSP may be more effective in reversing damage to the pancreas than the PPWSP and WPWSP are.
Fig. 12.
Effect of different varieties of sweet potato-based diet on the structure of pancreatic islets of normal and streptozotocin-induced diabetic rats.Islet cells (yellow arrowhead), capillaries (black arrowhead), acinar cells (white arrowhead), blood vessels (star), long black arrow (Islet diameter). The magnification of parts of all a’s are × 800 and parts of all b’s are × 80. (Scale bar = 51 μm), H&E staining.(C and D) Graphical representation of the number of pancreatic islets mean diameter and pancreatic islets analyzed with the aid of ImageJ software (1.48 V). [1 = healthy rats (Non-diabetic rats) fed corn starch-based diet (basal diet), designated as control (CTRL), 2 = healthy rats (Non-diabetic rats) fed corn starch-based diet but given 1 ml of 0.1 mol L−1 citrate buffer i.p once, designated as vehicle control (VEH CTRL), 3 = healthy rats (Non-diabetic rats) fed OFSP-based diet, designated as (OFSP), 4 = healthy rats (Non-diabetic rats) fed PPWSP-based diet rats, designated as (PPWSP), 5 = healthy rats (Non-diabetic rats) fed WPWSP-based diet, designated as (WPWSP), 6 = Diabetic control rats fed basal diet and were designated as STZ-induced untreated (STZ), 7 = diabetic rats fed OFSP-based diet, designated as (STZ + OFSP), 8 = diabetic rats fed PPWSP-based diet, designated as (STZ + PPWSP), 9 = diabetic rats fed WPWSP-based diet, designated as (STZ + WPWSP), 10 = diabetic rats fed basal diet and administered ACA orally (25 mg/kg body weight), designated as (STZ + ACA)].
4. Conclusion
The current findings presented in this study revealed that OFSP, PPWSP, and WPWSP demonstrated antidiabetic potentials to varying degrees. However, OFSP displayed superior antidiabetic potentials than PPWSP and WPWSP by upregulating the gene expression of GLP-1, downregulating DPP-4, and PTP1B genes, decreasing HOMA-IR score and fasting blood glucose, while improving insulin metabolism, liver glycogen content, fructose-1,6-bisphosphatase, hexokinase, glucose-6-phosphatase activities and HOMA-β score. OFSP also reversed damage to the pancreas by enhancing the size of the pancreatic islets more than PPWSP and WPWSP. Overall, we propose that OFSP may be more effective than PPWSP and WPWSP for antidiabetic therapy. However, future research could further explore the underlying molecular mechanisms responsible for their observed effects on glucose and lipid metabolism. Additionally, it is important to acknowledge that the study primarily relies on animal models. Long-term clinical trials are needed to assess the safety, efficacy, and potential side effects of these sweet potato varieties in human populations, particularly in individuals with type-2 diabetes.
Authors’ statement
We, the authors, hereby confirm that the manuscript titled " Comparative Evaluation of the Antidiabetic potential of three varieties of Ipomoea batatas L." is an original work and has not been published or submitted elsewhere for consideration.
Funding
There was no funding received for this research.
CRediT authorship contribution statement
Adewale Omowumi O.: Writing – review & editing, Visualization, Investigation, Formal analysis, Data curation. Agboola Oluwaseun E.: Writing – review & editing, Visualization, Formal analysis, Data curation. Ajayi Oluwadamilare O.: Writing – original draft, Project administration, Methodology, Formal analysis. Akomolafe Seun: Writing – review & editing, Visualization, Validation, Supervision, Methodology, Investigation, Formal analysis, Data curation, Conceptualization.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We acknowledge the input of all the researchers in the Food and Nutritional Biochemistry Unit of Department of Biochemistry, Ekiti State University.
Ethics approval and consent to participate
The ethical committee of the Office of Research and Development (ORD) of the Ekiti State University, Ado Ekiti, Nigeria authorized the protocol used for the animal study, and it was issued the certifcate number ORD/AD/EAC/23/122. The National Institutes of Health (NIH), USA, published recommendations for the care of experimental animals, were followed.
Consent for publication
Not applicable.
Handling Editor: Prof. L.H. Lash
Data Availability
Data will be made available on request.
References
- 1.Sarwar N., Gao P., Seshasai S.R., Gobin R., Kaptoge S., Angelantonio Di, et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Emerging risk factors collaboration. Lancet. 2010;26(375):2215–2222. doi: 10.1016/S0140-6736(10)60484-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khan M.A., Hashim M.J., King K.K., Govender R.D., Mustafa H., Al Kaabi J. Epidemiology of type 2 diabetes – global burden of disease and forecasted trends. J. Epidemiol. Glob. Health. 2020;10(1):107–111. doi: 10.2991/jegh.k.191028.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reed J., Bain S., Kanamarlapudi V. A review of current trends with type 2 diabetes epidemiology, aetiology, pathogenesis, treatments and future perspectives. Diabetes Metab. Syndr. Obes. 2021;14:3567–3602. doi: 10.2147/DMSO.S319895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saing M., Harahap U., Sitorus P. Combination of purple sweet potato (Ipomea batatas) leaf extract with metformin on blood glucose and total cholesterol levels of rats induced by high-fat diet and streptozotocin. Int. J. Basic Clin. Pharmacol. 2024;13(2):1–5. doi: 10.18203/2319-2003.ijbcp20240001. [DOI] [Google Scholar]
- 5.Bardini G., Rotella C.M., Giannini S. Dyslipidemia and diabetes: reciprocal impact of impaired lipid metabolism and Beta-cell dysfunction on microand macrovascular complications. Rev. Diabet. Stud. 2012;9(2–3):82. doi: 10.1900/RDS.2012.9.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Darenskaya M.A., Kolesnikova L.I., Kolesnikov S.I. Oxidative stress: pathogenetic role in diabetes mellitus and its complications and therapeutic approaches to correction. Bull. Exp. Biol. Med. 2021;171:179–189. doi: 10.1007/s10517-021-05191-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kalra S., Raizada N. Dyslipidemia in diabetes. India Heart J. 2024;76(1):S80–S82. doi: 10.1016/j.ihj.2023.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ighodaro O.M. Molecular pathways associated with oxidative stress in diabetes mellitus. Biomed. Pharm. 2018;108:656–662. doi: 10.1016/j.biopha.2018.09.058. [DOI] [PubMed] [Google Scholar]
- 9.Lima J.E., Moreira N.C., Sakamoto-Hojo E.T. Mechanisms underlying the pathophysiology of type 2 diabetes: from risk factors to oxidative stress, metabolic dysfunction, and hyperglycemia. Mutat. Res. /Genet. Toxicol. Environ. Mutagen. 2022;874 doi: 10.1016/j.mrgentox.2021.503437. [DOI] [PubMed] [Google Scholar]
- 10.Caturano A., D’Angelo M., Mormone A., Russo V., Mollica M.P., Salvatore T., Galiero R., Rinaldi L., Vetrano E., Marfella R., Monda M., Giordano A., Sasso F.C. Oxidative stress in type 2 diabetes: impacts from pathpgenesis to lifestyle modifications. Curr. Issues Mol. Biol. 2023;45(8):6651–6666. doi: 10.3390/cimb45080420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aywa A.K., Nawiri M.P., Nyambaka H.N. Nutrient variation in colored varieties of Ipomea batatas grown in Vihiga County, Western Kenya. Int. Food Res. J. 2013;20(2):819–825. [Google Scholar]
- 12.Solihah I., Herlina M.E., Haryanti H., Amalia M., Rasyid R.S.P. The hypoglycemic effect of purple sweet potato leaf fractions in diabetic rats. J. Adv. Pharm. Educ. Res. 2023;13(3):64–72. [Google Scholar]
- 13.Mu T.H., Li P.G. In: Sweet potato: Chemistry, processing and nutrition. Mu In.T.H., Singh J., editors. Academic Press; 2019. Sweet potato: Origin and production; pp. 5–25. [Google Scholar]
- 14.Alam M.K. A comprehensive review of sweet potato (Ipomea batatas [L.] Lam): revisiting the associated health benefits. Trends in Food Science and Technology. 2021;115:512–529. [Google Scholar]
- 15.Luo D., Mu T., Sun H. Profiling of phenolic acids and flavonoids in sweet potato (Ipomoea batatas L.) leaves and evaluation of their antioxidant & hypoglycemic activities. Food Biosci. 2021;39 doi: 10.1016/j.fbio.2020.100801. [DOI] [Google Scholar]
- 16.Kusano S., Abe H. Antidiabetic activity of white skinned sweet potato (Ipomoea batatas L.) in obese Zucker fatty rats. Biol. Pharm. Bull. 2000;23(15):23–26. doi: 10.1248/bpb.23.23. [DOI] [PubMed] [Google Scholar]
- 17.Kusano S., Abe H., Tamura H. Isolation of antidiabetic components from white-skinned sweet potato (Ipomoea batatas L.) Biosci. Biotechnol. Biochem. 2001;65:109–114. doi: 10.1271/bbb.65.109. [DOI] [PubMed] [Google Scholar]
- 18.Ludvik B., Neuffer B., Pacini G. Efficacy of Ipomoea batatas (Caiapo) on diabetes control in type 2 diabetic subjects treated with diet. Diabetes Care. 2004;27:436–440. doi: 10.2337/diacare.27.2.436. [DOI] [PubMed] [Google Scholar]
- 19.Mohanraj R., Sivasankar S. Sweet potato (Ipomea batatas [L.] Lam) – a valuable medicinal food: A review. J. Med. Food. 2014;17(7):733–741. doi: 10.1089/jmf.2013.2818. DOI: 10.1089/jmf.2013.2818. [DOI] [PubMed] [Google Scholar]
- 20.Bocanegra A., Macho-González A., Garcimartín A., Benedí J., Sánchez-Muniz F.J. Whole alga, algal extracts, and compounds as ingredients of functional foods: composition and action mechanism relationships in the prevention and treatment of type-2 diabetes mellitus. Int. J. Mol. Sci. 2021;22(8):3816. doi: 10.3390/ijms22083816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Akhtar N., Akram M., Daniyal M., Ahmad S. Evaluation of antidiabetic activity of Ipomea batatas L. extract in alloxan-induced diabetic rats. Int. J. Immunopathol. Pharm. 2018;32 doi: 10.1177/2058738418814678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kinoshita A., Nagata T., Furuya F., Nishizawa M., Mukai E. White-skinned potato (Ipomea batatas L.) acutely suppresses postprandial blood glucose elevation by improving insulin sensitivity in normal rats. Heliyon. 2023;9 doi: 10.1016/j.heliyon.2023.e14719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang L., Tu Z.C., Yuan T., Wang H., Xie X., Fu Z.F. Antioxidants and -glucosidase inhibitors from Ipomoea batatas leaves identified by bioassay-guided approach and structure-activity relationships. Food Chem. 2016;208:61–67. doi: 10.1016/j.foodchem.2016.03.079. [DOI] [PubMed] [Google Scholar]
- 24.Arisanti C.I.S., Wirasuta I.M.A.G., Musfiroh I., Ikram E.H.K., Muchtaridi M. Mechanism of anti-diabetic activity from sweet potato (Ipomoea batatas): a systematic review. Foods. 2023;12(14):2810. doi: 10.3390/foods12142810. 10.3390/foods12142810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ayeleso T.B., Ramachela K., Mukwevho E. Aqueous-methanol extracts of orange-fleshed sweet potato (Ipomoea batatas) ameliorate oxidative stress and modulate type 2 diabetes associated genes in insulin resistant C2C12 cells. Molecules. 2018;23:2058. doi: 10.3390/molecules23082058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jiang T., Shuai X., Li J., Yang N., Deng L., Li S., He Y., Guo H., Li Y., He J. Protein-bound anthocyanin compounds of purple sweet potato ameliorate hyperglycemia by regulating hepatic glucose metabolism in high-fat diet/streptozotocin-induced diabetic mice. J. Agric. Food Chem. 2020;68:1596–1608. doi: 10.1021/acs.jafc.9b06916. [DOI] [PubMed] [Google Scholar]
- 27.Fadimu G.J., Farahnaky A., Gill H., Olalere O.A., Gan C., Truong T. In-Silico analysis and antidiabetic effect of α-amylase and α-glucosidase inhibitory peptides from lupin protein hydrolysate: enzyme-peptide interaction study using molecular docking approach. Foods. 2022 doi: 10.3390/foods11213375. doi: 10.3390/foods11213375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kinoshita A., Nagata T., Furuya F., Nishizawa M., Mukai E. White-skinned sweet potato (Ipomoea batatas L.) acutely suppresses postprandial blood glucose elevation by improving insulin sensitivity in normal rats. Heliyon. 2023;9(4):2023. doi: 10.1016/j.heliyon.2023.e14719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chintha P., Sarkar D., Pecota K., Dogramaci M., Hatterman-Valenti H., Shetty K. Phenolic bioactive-linked antioxidant, anti-hyperglycemic, and antihypertensive properties of sweet potato cultivars with different flesh color. Hortic., Environ., Biotechnol. 2023;64(5):877–893. [Google Scholar]
- 30.Solihah I., Herlina E.M., Haryanti H., Amalia M., Puspita R.S. The hypoglycemic effect of purple sweet potato leaf fractions in diabetic rats. J. Adv. Pharm. Educ. Res. 2023;13(3):65. [Google Scholar]
- 31.Oboh G., Puntel R.L., Rocha J.B.T. Hot pepper (Capsicum annuum, Tepin and Capsicum Chinese, Habanero) prevents Fe2+- induced lipid peroxidation in brain – in vitro. Food Chem. 2007;12(1):178–185. doi: 10.1016/j.foodchem.2006.05.048. [DOI] [Google Scholar]
- 32.Shekhar S., Mishra D., Buragohain A.K., Chakraborty S., Chakraborty N. Comparative analysis of phytochemicals and nutrient availability in two contrasting cultivars of sweet potato (Ipomoea batatas L.) Food Chem. 2015;173:957. doi: 10.1016/j.foodchem.2014.09.172. -65. [DOI] [PubMed] [Google Scholar]
- 33.Le V., Parks A.E., Nguyen S.H., Roach D. Improving the vanillin-sulphuric acid method for quantifying total saponins. Technologies. 2018;6(3):84. doi: 10.3390/technologies6030084. [DOI] [Google Scholar]
- 34.Barba A.I.O., Hurtado M.C., Mata M.C.S., Ruiz V.F., Tejada M.L.S. Application of a UV–visible detection-HPLC method for a rapid determination of lycopene and β-carotene in vegetables. Food Chem. 2006;95(2):328–336. doi: 10.1016/j.foodchem.2005.02.028. doi: 10.1016/j.foodchem.2005.02.028. [DOI] [Google Scholar]
- 35.Singleton V.L., Orthofer R., Lamuela-Raventos R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent Methods Enzym. 1999;299:152. doi: 10.1016/S0076-6879(99)99017-1. -79. [DOI] [Google Scholar]
- 36.Meda A., Lamien C.E., Romito M. Determination of the total phenolic, flavonoid and proline contents in Burkina Fasan honey, as well as their radical scavenging activity. Food Chem. 2005;91:571–577. doi: 10.1016/j.foodchem.2004.10.006. [DOI] [Google Scholar]
- 37.Gyamfi M.A., Yonamine M.Y., Aniya Y. Free-radical scavenging action of medicinal herbs from Ghana: thonningiasanguinea on experimentally induced liver injuries. Gen. Pharmacol. 1999;32(6):661–667. doi: 10.1016/S0306-3623(98)00238-9. [DOI] [PubMed] [Google Scholar]
- 38.Re R., Pellegrini N., Proteggente A., Pannala A., Yang M., Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999;26:1231–1237. doi: 10.1016/s0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- 39.Puntel P.R.L., Nogueira C.W., Rocha J.B.T. Krebs cycle intermediates modulate Thiobarbituric Reactive Species (TBARS) production in rat brain in vitro. Neuro Chem. Res. 2005;30(2):225–235. doi: 10.1007/s11064-004-2445-7. [DOI] [PubMed] [Google Scholar]
- 40.Halliwell B., Gutteridge J.M.C. Fomation of a thiobarbituric-acidreactive substance from deoxyribose in the presence of iron salts: the role of superoxide and hydroxyl radicals. FEBS Lett. 1981;128:347–352. doi: 10.1016/0014-5793(81)80114-7. [DOI] [PubMed] [Google Scholar]
- 41.Oyaizu M. Studies on products of browning reaction: antioxidative activity of products of browning reaction prepared from glucosamine. Jpn. J. Nutr. 1986;44(6):307–315. doi: 10.5264/eiyogakuzashi.44.307. [DOI] [Google Scholar]
- 42.Igbinosa O.O., Igbinosa I.H., Chigor V.N., Uzunuigbe O.E., Oyedemi S.O., Odjadjare E.E. Polyphenolic contents and antioxidant potential of stem bark extracts from Jatorpha curcas (Linn) Int J. Mol. Sci. 2011;12:2958–2971. doi: 10.3390/ijms12052958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ohkawa H., Ohishi N., Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979;95:351–358. doi: 10.3390/ijms12052958. [DOI] [PubMed] [Google Scholar]
- 44.Banwart W.L., Porter P.M., Granato T.C., Hassett J.J. HPLC separation and wavelength area ratios of more than 50 phenolic acids and falvaonoids. J. Chem. Ecol. 1985;11(3):383–395. doi: 10.1007/BF01411424. [DOI] [PubMed] [Google Scholar]
- 45.Hasanein P., Shahidi S. Effects of Hypericum perforatum extract on diabetes-induced learning and memory impairment in rats. Phytother. Res. 2011;25(4):544–549. doi: 10.1002/ptr.3298. [DOI] [PubMed] [Google Scholar]
- 46.V. Worthington. Worthington Enzyme Manual; Freehold: 1993. Worthington Biochemical Corp. Alpha amylase; pp. 36–41. [Google Scholar]
- 47.Apostolidis E., Kwon Y.L., Shetty K. Inhibitory potentials of herb, fruit, and fungal-enriched cheese against enzymes linked to type 2 diabetes and hypertension. 2007;8(1):46–54. doi: 10.1016/j.ifset.2006.06.001. [DOI] [Google Scholar]
- 48.Kambayashi Y., Binh N.T., Asakura H.W., Hibino Y., Hitomi Y., Nakamura H., Ogino K. Efficient assay for total antioxidant capacity in human plasma using a 96-well microplate. J. Clin. Biochem. Nutr. 2009;44(1):46–51. doi: 10.3164/jcbn.08-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jentzsch A.M., Bachmann H., Furst P., Biesalski H.K. Improved analysis of malondialdehyde in human body fluids. Free Radic. Biol. Med. 1996;20(2):251–256. doi: 10.1016/0891-5849(95)02043-8. [DOI] [PubMed] [Google Scholar]
- 50.Misra H.P., Fridovich I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J. Biol. Chem. 1972;247:3170–3175. doi: 10.1016/S0021-9258(19)45228-9. [DOI] [PubMed] [Google Scholar]
- 51.Claiborne A. In: CRC Handbook of Methods for Oxygen Radical Research. Greenwald R.A., editor. CRC Press; Boca Raton: 1985. Catalase activity; pp. 283–284. [Google Scholar]
- 52.Ellman G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959;82:70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 53.Bradford M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein – dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 54.Ajayi O.O., Ajayi O.B. Short-term effects of garlic-based diets on MRNA expression of angiotensinogen, angiotensin-1 converting enzyme, and atrial natriuretic peptide in cyclosporine-induced prehypertensive rats. 2023;38(5):32–42. doi: 10.9734/arrb/2023/v38i530586. [DOI] [Google Scholar]
- 55.Lu J., Wang Y., Yan H., Lin P., Gu W., Yu J. Antidiabetic effect of total saponins from polygonatumkingianum in streptozotocin-induced diabetic rats. J. Ethnopharmacol. 2016;179:291–300. doi: 10.1016/j.jep.2015.12.057. [DOI] [PubMed] [Google Scholar]
- 56.Bhatt T., Patel K. Carotenoids: potent to prevent diseases review. Nat. Prod. Bioprospecting. 2020;10:109–117. doi: 10.1007/s13659-020-00244-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang J., Xiao J., Giampieri F., Forbes-Hernandez T.Y., Gasparrini M., Afrin S., Cianciosi D., Reboredo-Rodriguez P., Battino M., Zheng X. J. Berry Reasearch. 2019;9(1):109–123. [Google Scholar]
- 58.Ghasemi Pirbalouti A., Hashemi M., Taherian Ghahfarokhi F. Essential oil and chemical compositions of wild and cultivated Thymus daenensis Celak and Thymus vulgaris L. Ind. Crops Prod. 2013;48:43–48. [Google Scholar]
- 59.Moghaddam M., Mehdizadeh L. Variability of total phenolic, flavonoid androsmarinic acid content among Iranian basil accessions. LWT-Food Sci. Technol. 2015;63:535–540. [Google Scholar]
- 60.Bajalan I., Mohammadi M., Alaei M., Pirbalouti Ghasemi. A. Total phenolic and flavonoid contents and antioxidant activity of extracts from different populations of lavandin. Ind. Crops Prod. 2016;87:255–260. [Google Scholar]
- 61.Nickavar B., Alinaghi A., Kamalinejad M. Evaluation of the antioxidant properties of five Mentha species. Iran. J. Pharm. Res. 2008;7(3):203–209. [Google Scholar]
- 62.Fatiha B., Didier H., Naima G., Khodir M., Martin K., Leocadie K., Caroline S., Mohamed C., Pierre D. Phenolic composition, in vitro antioxidant effects and tyrosinase inhibitory activity of three Algerian Mentha species: M. spicata (L.), M. pulegium (L.) and M. rotundifolia (L.) Huds (Lamiaceae) Ind. Crops Prod. 2015;74:722–730. [Google Scholar]
- 63.Apovian C.M., Okemah J., O’Neil P.M. Body weight considerations in the management of type 2 diabetes. Adv. Ther. 2019;36:44–58. doi: 10.1007/s12325-018-0824-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wing R.R., Lang W., Wadden T.A., et al. Benefits of modest weight loss in improving cardiovascular risk factors in overweight and obese individuals with type 2 diabetes. Diabetes Care. 2011;34:1481–1486. doi: 10.2337/dc10-2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Scheen A.J., Van Gaal L.F. Combating the dual burden: therapeutic targeting of common pathways in obesity and type 2 diabetes. Lancet Diabetes Endocrinol. 2014;2:911–922. doi: 10.1016/S2213-8587(14)70004-X. [DOI] [PubMed] [Google Scholar]
- 66.Roehrig M., Duncan J., Sularz A. In: Encyclopedia of Behavioral Medicine. Gellman M.D., Turner J.R., editors. Springer; New York, NY: 2013. Caloric Intake. [DOI] [Google Scholar]
- 67.Sundararajan Safei M., Driss E.A., Boulila M., Shapi’i W. A. A systematic literature review on obesity: understanding the causes and consequences of obesity and reviewing various machine learning approaches used to predict obesity. Comput. Biol. Med. 2021;136(104754):1–17. doi: 10.1016/j.compbiomed.2021.104754. [DOI] [PubMed] [Google Scholar]
- 68.Kong L.C., Wuillemin P., Bastard J., Sokolovska N., Gougis S., Fellahi F., Bonnefont-Rousselot D., Bittar R., Dore J., Zucker J., Clement K., Rizkalla S. Insulin resistance and inflammation predict kinetic body weight changes in response to dietary weight loss and maintenance in overweight and obese subjects by using a Bayesian network approach. Am. J. Clin. Nutr. 2013;98(6):1385–1394. doi: 10.3945/ajcn.113.058099. [DOI] [PubMed] [Google Scholar]
- 69.Huang C.J., McAllister M.J., Slusher A.L., et al. Obesity-related oxidative stress: the impact of physical activity and diet manipulation. Sports Med – Open. 2015;1:32. doi: 10.1186/s40798-015-0031-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Xie L., Su H., Sun C., Zheng X., Chen W. Recent advances in understanding the obesity activity of anthocyanins and their biosynthesis in microorganisms. Trends Food Sci. Technol. 2018;72:13–24. [Google Scholar]
- 71.Gardner D.G., Shoback D. Greenspan’s Basic And Clinical Endocrinology. 8th ed. McGraw-Hill; New York: 2007. [Google Scholar]
- 72.Kronenber H., Melmed S., Polonsky K., Larsen P.R. Williams textbook of endocrinology. 11th ed. Saunders Elsevier; Philadelphia: 2008. [Google Scholar]
- 73.Carrillo A., Gomez-Meade C. In: Encyclopedia of Behavioral Medicine. Gellman M.D., editor. Springer; Cham: 2020. Fasting Glucose. [DOI] [Google Scholar]
- 74.Chen J., Jin L., Chen M., Xu K., Huang Q., He B. Application of natural compounds in the treatment and prevention of diabetes. Front. Nutr. 2023;10:1301129. doi: 10.3389/fnut.2023.1301129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gordon L.A., Morrison E.Y., McGrowder D.A., Young R., Fraser Y.T.P., Zamora E.M., lexander-Lindo R.L., Irving R.R. Effect of exercise therapy on lipid profile and oxidative stress indicators in patients with type-2 diabetes. 2008;8(21):1–10. doi: 10.1186/1472-6882-8-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Agrawal R.P., Aradhana R., Hussain S., Sabir M., Kochar D.K., Kothari R.P. Influence of yogic treatment on quality of life outcomes, glycemic control and risk factors in diabetes mellitus. Int. J. Diabetes Dev. Ctries. 2003;23(4):130–134. [Google Scholar]
- 77.Papoutsis K., Zhang J., Bowyer M.C., Brunton N., Gibney E.R., Lyng J. Fruit, vegetables, and mushrooms for the preparation of extracts with α-amylase and α-glucosidase inhibition properties: A review. Food Chem. 2021;338 doi: 10.1016/j.foodchem.2020.128119. [DOI] [PubMed] [Google Scholar]
- 78.Cisneros-Yupanqui M., Lante A., Mihaylova D., et al. The α-amylase and α-glucosidase inhibition capacity of grape pomace: a review. Food Bioprocess Technol. 2023;16:691–703. doi: 10.1007/s11947-022-02895-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gin H., Rigalleau V. Postprandial hyperglycemia and diabetes. Diabetes Metab. 2000;26(4):265–272. [PubMed] [Google Scholar]
- 80.Mahnashi M.H., Alqahtani Y.S., Alyami B.A., Alquarni A.O., Alqahl S.A., Ullah F., Sadiq A., Zeb A., Ghufran M., Kuraev A., Nawaz A., Ayaz M. HPLC-DAD phenolics analysis, α-glucosidase, α-amylase inhibitory, molecular docking, and nutritional profiles of Persicaria hydropiper L. BMC Complement. Med. Ther. 2022;22(26):1–20. doi: 10.1186/s12906-022-03510-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Williamson C.R., Khurana S., Nguyen P., Byrne C.J., Tai T.C. Comparative analysis of renin-angiotensin system (RAS)- related gene expression between hypertensive and normotensive rats. Med. Sci. Monit. Basic Res. 2017;23:20–24. doi: 10.12659/MSMBR.901964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Poovitha S., Parani M. In vitro and in vivoα-amylase and α-glucosidase inhibiting activities of the protein extracts from two varieties of bitter gourd (Momordica charantia L. BMC Complement. Altern. Med. 2016;16(1):185. doi: 10.1186/s12906-016-1085-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rossi G. Diagnosis and classification of diabetes mellitus. Recent. Progress. Med. 2010;101:274–276. [PubMed] [Google Scholar]
- 84.Indumathi D., Sujithra K., Srinivasan S., Vinothkumar V. Ameliorating effect of betanin, a natural chromoalkaloid by modulating hepatic carbohydrate metabolizing enzyme activities and glycogen content in streptozotocin-nicotinamide induced experimental rats. 2017;88:1069–1079. doi: 10.1016/j.biopha.2017.01.146. [DOI] [PubMed] [Google Scholar]
- 85.Lin K., Liou T., Hsiao L., Hwu C. Clinical and biochemical indicators of homeostasis model assessment-estimated insulin resistance in postmenopausal women. J. Chin. Med. Assoc. 2011;74:442–447. doi: 10.1016/j.jcma.2011.08.014. [DOI] [PubMed] [Google Scholar]
- 86.Swaminathan S., Elanthendral T., Edward K.D., Abirami M.J. Diagnostic usefulness of HOMA-β and HOMA-IR in Diabetes mellitus – a review. Int. J. Pharm. Res. Allied Sci. 2019;8(1):17. [Google Scholar]
- 87.Robey R.B., Hay N. Mitochondrial hexokinases, novel mediators of the anti-apoptotic effects of growth factors and Akt. Oncogene. 2006;25(34):4683–4696. doi: 10.1038/sj.onc.1209595. [DOI] [PubMed] [Google Scholar]
- 88.Ciscato F., Ferrone L., Masgras I., Laquatra C., Rasola A. Hexokinase 2 in cancer: a prima donna playing multiple characters. Int. J. Mol. Sci. 2021;22:4716. doi: 10.3390/ijms22094716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Heikkinen S., Pietila M., Halmekyto M., Suppola S., Pirinen E., Deeb S.S., Janne J., Laakso M. Hexokinase II-deficient mice: prenatal death of homozygotes without disturbances in glucose tolerance in heterozygotes. J. Biol. Chem. 1999;274(32):22517–22523. doi: 10.1074/jbc.274.32.22517. [DOI] [PubMed] [Google Scholar]
- 90.Barnett A. DPP-4 inhibitors and their potential role in the management of type 2 diabetes. Int. J. Clin. Pract. 2006;60(11):1454–1470. doi: 10.1111/j.1742-1241.2006.01178.x. [DOI] [PubMed] [Google Scholar]
- 91.Holst J.J. The physiology of glucagon-like peptide 1. Physiol. Rev. 2007;87(4):1409–1439. doi: 10.1152/physrev.00034.2006. [DOI] [PubMed] [Google Scholar]
- 92.Cho H. Protein tyrosine phosphatase 1B (PTP1B) and obesity. Vitam. Horm. 2013;91:405–424. doi: 10.1016/B978-0-12-407766-9.00017-1. [DOI] [PubMed] [Google Scholar]
- 93.Klemchuk P.P. Antioxidants. Ullmann'S. Encycl. Ind. Chem. 2000 doi: 10.1002/14356007.a03_091. ISBN 3527306730. [DOI] [Google Scholar]
- 94.Somogyi A., et al. Antioxidant measurements. Physiol. Meas. 2007;28:R41–R55. doi: 10.1088/0967-3334/28/4/R01. [DOI] [PubMed] [Google Scholar]
- 95.Weseler A.R., Bast A. Oxidative stress and vascular function: implications for pharmacologic treatments. Curr. Hypertens. Rep. 2010;12(3):154–161. doi: 10.1007/s11906-010-0103-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Asmat U., Abad K., Ismail K. Diabetes mellitus and oxidative stress –a concise review. Saudi Pharm. J. 2016;24:547–553. doi: 10.1016/j.jsps.2015.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Pham-Huy L.A., He H., Pham-Huy C. Free radicals, antioxidants in disease and health. IJBS. 2008;4(2):89–96. [PMC free article] [PubMed] [Google Scholar]
- 98.Maritim A.C., Sanders R.A., Watkins J.B. Diabetes, oxidative stress, and antioxidants: a review. J. Biochem. Mol. Toxicol. 2003;17(1):24–38. doi: 10.1002/jbt.10058. [DOI] [PubMed] [Google Scholar]
- 99.Giugliano D., Ceriello A., Paolisso G. Diabetes mellitus, hypertension, and cardiovascular disease: which role for oxidative stress? Metabolism. 1995;44(3):363–368. doi: 10.1016/0026-0495(95)90167-1. [DOI] [PubMed] [Google Scholar]
- 100.Furman B.L. Streptozotocin-induced diabetic models in mice and rats. Curr. Protoc. 2021;1 doi: 10.1002/cpz1.78. [DOI] [PubMed] [Google Scholar]
- 101.Bhatti J.S., Sehrawat A., Mishra J., Sidhu I.S., Navik U., Khullar N., Kumar S., Bhatti G.K., Reddy P.H. 2022. Oxidative stress in the pathophysiology of type 2 diabetes and related complications: Current therapeutics strategies and future perspectives. Free Radic. Biol. Med. 2022;184:114–134. doi: 10.1016/j.freeradbiomed.2022.03.019. [DOI] [PubMed] [Google Scholar]
- 102.Fatima M.T., Bhat A.A., Nisar S., Fakhro K.A., Akil A.S.A.S. The role of dietary antioxidants in type 2 diabetes and neurodegenerative disorders: An assessment of the benefit profile. Heliyon. 2023;9(1) doi: 10.1016/j.heliyon.2022.e12698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Morgantini C., Natali A., Boldrini B., Imaizumi S., Navab M., Fogelman A.M., Ferrannini E., Reddy S.T. Anti-inflammatory and antioxidant properties of HDLs are impaired in type 2 diabetes. Diabetes. 2011;60:2617–2623. doi: 10.2337/db11-0378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ghasemi A., Jeddi S. Streptozotocin as a tool for induction of rat models of diabetes: a practical guide. Excli. J. 2023;22:274–294. doi: 10.17179/excli2022-5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.