Abstract
Human granulocytic ehrlichiosis (HGE), a tick-borne zoonosis, is caused by an obligatory intragranulocytic bacterium, the HGE agent, a strain of Anaplasma phagocytophila. The equine model of HGE is considered valuable in understanding pathogenic and immune mechanisms of HGE. In the present study, cytokine mRNA expression by peripheral blood leukocytes (PBLs) in horses was examined during the course of infection by intravenous inoculation of A. phagocytophila or by allowing feeding by infected ticks. The p44 genes encoding the major outer membrane protein P44s of A. phagocytophila were detected by PCR in PBLs of all four horses from 4 to 20 days postexposure. During the 20-day infection period, interleukin-1β (IL-1β) and tumor necrosis factor alpha (TNF-α) mRNA expression was upregulated in PBLs of all four horses, and IL-8 mRNA expression was upregulated in three horses. Gamma interferon, IL-10, and IL-12 p35 mRNAs were weakly expressed in only one horse each. IL-2, IL-4, IL-6, and IL-12 p40 mRNA expression , however, could not be detected in the PBLs of any of the four horses. These results suggest that IL-1β, TNF-α, and IL-8 generation during A. phagocytophila infection has a primary role in HGE pathogenesis and immunomodulation.
Human granulocytic ehrlichiosis (HGE) is characterized by fever, chills, headache, myalgia, and laboratory findings including leukopenia, anemia, and thrombocytopenia, as well as elevated liver enzyme activities (3, 8). Delayed treatment, misdiagnosis, and/or the presence of immunosuppression may lead to a severe or fatal outcome (13, 21). HGE has been increasingly recognized in the United States (27) and various parts of Europe (20, 23, 30). HGE is caused by a strain of Anaplasma phagocytophila (the HGE agent), a gram-negative obligatory intragranulocytic bacterium. The white-footed mouse (Peromyscus leucopus) is considered to be a major wild-animal reservoir of A. phagocytophila in the northeastern United States, and Ixodes spp. ticks, the vector of Borrelia burgdorferi, the agent of Lyme disease, are considered to be the vector (12, 14, 29, 34). Strains of A. phagocytophila have also been known to cause tick-borne fever in ruminants in Europe and to cause equine ehrlichiosis in the United States (it has hence formerly been called Ehrlichia phagocytophila and Ehrlichia equi).
Pathogenesis and cellular immune responses of HGE are not well defined. The presence of low levels of A. phagocytophila in the blood of patients in the acute stage and autopsied patients' tissues (3, 21) and the nature of clinical signs and laboratory findings suggest the involvement of proinflammatory cytokines and chemokines in the pathogenesis. In vitro, we found that A. phagocytophila strain HZ, isolated from an acute-stage HGE patient, induced rapid and strong proinflammatory cytokine (interleukin-1β [IL-1β], tumor necrosis factor α [TNF-α], and IL-6) mRNA expression by human peripheral blood leukocytes (PBLs) and monocytes within 2 h and protein secretion within 24 h (16, 17). However, only IL-1β is upregulated in neutrophils, and the mRNA of these three cytokines is not upregulated in HL-60 cells. Within 2 h of incubation with A. phagocytophila, IL-8, IL-10, gamma interferon (IFN-γ), IL-2, and transforming growth factor β mRNAs are not consistently upregulated in human PBLs, suggesting that for these cytokines, gene expression either is not induced or is induced at later time points in vitro. Akkoyunlu et al. (2) reported IL-8 production by retinoic acid-treated HL-60 cells (human promyelocytic leukemia cell line) after 24 h (negative at 12 h for both mRNA and protein) and by human neutrophils 7 h postincubation with A. phagocytophila in vitro. That report also indicated that no IL-1α, IL-1β, or TNF-α is detected in the culture supernatant of retinoic acid-treated and nontreated HL-60 cells at 6 days after the addition of A. phagocytophila. Klein et al. reported that in infected dimethyl sulfoxide-treated HL-60 cells or human bone marrow cells, IL-8 and other chemokine levels, but not that of IL-1, IL-6, or TNF-α in the culture medium, were significantly elevated at 48 h postinfection (18).
IL-8 levels are significantly increased in the blood of HGE patients (2). Dumler et al. reported that IFN-γ and IL-10 levels are elevated in acute-phase (median, 4 days after onset) sera compared with convalescent (median, 31 days after onset) sera from HGE patients or normal controls whereas serum IL-1β, TNF-α, and IL-4 levels are not elevated (9). Using C3H mice, which do not show clinical signs and spontaneously clear the infection by 15 days after intraperitoneal inoculation of A. phagocytophila, splenic IL-12 and IFN-γ mRNA and serum IFN-γ levels are significantly elevated starting at 2 days postinfection, although the clearance of A. phagocytophila is not delayed in IFN-γ−/− mice (1).
Considering apparently conflicting data in vitro using cell lines, bone marrow cells, or human PBLs which were assayed at varied time points of incubation or in vivo using a transient mouse infection model or patients' sera, an equine model of HGE may be useful for clarifying the importance of these cytokines in HGE. Several reports have suggested that the horse serves as a more useful infection and disease model of HGE than the mouse, since A. phagocytophila isolated from patients induces clinical signs in horses similar to those of HGE and equine ehrlichiosis (7, 24, 25, 31, 32). However, cytokine responses in horses infected with A. phagocytophila have not been described so far. In the present study, we investigated cytokine mRNA expression by PBLs in horses following transmission of A. phagocytophila by intravenous inoculation or tick bite.
MATERIALS AND METHODS
A. phagocytophila.
HZ strain A. phagocytophila, isolated from an HGE patient (33), was propagated in HL-60 cells as previously described (15). The percentage of infected cells was determined by Diff-Quik (Baxter Scientific Products, Obetz, Ohio) staining as previously described (33).
Horse infection.
Four horses purchased from Ohio horse farms were kept in vector-proof stalls in the Equine Isolation Unit (College of Veterinary Medicine, The Ohio State University). All horses were mixed breed. Horse 1 was a 2-year-old male (380-kg body weight [BW]), horse 2 was a 5-year-old male (200-kg BW), horse 3 was a 5-year-old male (180-kg BW), and horse 4 was a 3-year-old female (170-kg BW). Prior to A. phagocytophila infection, these animals were confirmed as seronegative and PCR negative for A. phagocytophila. Horses 1 and 2 were intravenously (i.v.) inoculated with A. phagocytophila of high passage (HP; i.e., more than 50 passages in HL-60 cells) and low passage (LP; i.e., fewer than 10 passages), respectively, at 107 infected HL-60 cells (approximately 80% infected cells) in 5 ml of RPMI 1640 medium. A total of 100 and a total of 47 infected Ixodes scapularis adults were allowed to feed on horses 3 and 4, respectively. As nymphs, these ticks were acquisition fed on experimentally infected DBA/2 mice (37). Uninfected nymphs were kindly provided by S. R. Telford (Harvard School of Public Health, Boston, Mass.). All horses were euthanized at 24 days postinoculation (PI) or postattachment (PA). The use of horses for this study has been approved by the Ohio State University Institutional Animal Care and Use Committee.
Clinical evaluation, hematology, PBL preparation, morula examination, and indirect fluorescent antibody (IFA) testing.
Fever, depression, anorexia, diarrhea, leukopenia, laminitis, and other clinical signs were monitored daily for 20 days. For complete blood cell counts, blood samples were taken from the jugular veins of horses prior to injection or tick attachment (day 0) and every 4 days until 20 days PI or PA. To prepare the PBL fraction, the blood samples, which were collected in acid citrate dextrose anticoagulant tubes, were centrifuged at 500 × g for 5 min. Erythrocytes in the pellet were lysed in sterile 0.83% NH4Cl solution for 3 min at room temperature. Cells were washed twice by centrifugation (500 × g for 5 min) in phosphate-buffered saline (137 mM NaCl, 10 mM Na2HPO4, 2.7 mM KCl, 1.8 mM KH2PO4 [pH 7.2]). Viabilities of PBLs were >98%, as assessed by the trypan blue dye exclusion test. Intracytoplasmic microcolonies (morulae) of A. phagocytophila were examined in PBLs by using Diff-Quik staining and IFA using monoclonal antibody (MAb) 5C11 (15). The IFA test was performed as previously described (33). The serum antibody titer was expressed as the reciprocal of the highest dilution of serum that showed a positive reaction.
Reverse transcription (RT)-PCR for cytokine genes.
Total RNA was extracted from equine PBLs (107) by TRIzol reagent (GIBCO-BRL) and resuspended in 90 μl of diethyl pyrocarbonate-treated sterile water. As a positive control, PBLs (107) of uninfected healthy horses were stimulated with either concanavalin A (ConA; Sigma, St. Louis, Mo.) (10 μg/ml) or Escherichia coli lipopolysaccharide (LPS) (10 μg/ml) in RPMI 1640 medium at 37°C for 4 h. Total RNA (2 μg) was reverse transcribed in a 30-μl reaction mixture containing 1× reaction buffer (50 mM Tris-HCl [pH 8.3], 75 mM KCl, 3 mM MgCl2), 0.5 mM each deoxynucleoside triphosphate (dNTP), 1 U of RNase inhibitor (GIBCO-BRL), 1.5 μM oligo(dT) primer, and 10 U of Moloney murine leukemia virus reverse transcriptase (GIBCO-BRL) at 42°C for 1 h. The reaction was terminated by heating at 94°C for 5 min, and the cDNA was used in the PCR. The cDNA (2 μl) was amplified in a 50-μl reaction mixture containing 1× PCR buffer (10 mM Tris-HCl [pH 8.3] and 50 mM KCl), 1.5 mM MgCl2, 0.2 mM each dNTP, and 0.4 μM (each) 3′ and 5′ primers (0.5 μM each primer for equine IL-8 mRNA). The primers used for equine IL-1β, IL-2, IL-4, IL-6, IL-10, IL-12 p35/40, TNF-α, IFN-γ, and β-actin mRNA detection were as previously described (10, 11). Primer sequences for equine IL-8 mRNA expression, designed based on the equine cDNA sequence (GenBank accession number AF062377), were kindly provided by S. Giguere (Department of Large Animal Clinical Sciences, College of Veterinary Medicine, University of Florida). The sequences were as follows: sense, 5′-GACTTCCAAGCTGGCTGTTGC-3′, and antisense, 5′-GTCCTCTTTAGAAACGCCTGC-3′. To reduce nonspecific priming, all PCRs were performed by the hot-start method: Taq DNA polymerase (2 U/reaction; GIBCO-BRL) was added after incubation of the mixture at 94°C for 5 min. PCR was 25 cycles (27 cycles for equine IL-8), consisting of denaturation at 94°C for 45 s, annealing at 60°C (62°C for IL-8) for 45 s, and extension at 72°C for 2 min. The final extension was for 7 min. These conditions were within the linear range for PCR, as determined previously (16). PCR products (10 μl each) were electrophoresed in 1.5% agarose gel containing ethidium bromide (final concentration, 0.5 μg/ml) at 95 V for 1 h and photographed under UV illumination with a gel video system (Gel Print 2000i; Biophotonics Corporation, Ann Arbor, Mich.). DNA size markers (HaeIII fragments of φX174 replicative-form [RF] DNA; GIBCO-BRL) providing bands from 1,353 to 72 bp were run in parallel.
PCR of p44 genes of A. phagocytophila.
To detect A. phagocytophila DNA in equine PBLs, nested PCR, based on the multigene family p44 genes encoding immunodominant outer membrane protein P44s (36), was performed. The two primer pairs p3726 (5′-GCTAAGGAATTAGCTTATGA-3′)-p4257 (5′-AGAAGATCATAACAAGCATTG-3′) and p3761 (5′-CTGCTCKGCCAARACCTC-3′)-p4183 (5′-CAATAGTYTTAGCTAGTAACC-3′) (K = T+G; R = A+G; Y = C+T [mixed bases]) (22) that are located in conserved regions of p44 genes (Q. Lin, N. Zhi, H.-Y. Kim, H. Horowitz, G. Wormser, and Y. Rikihisa, 101st Gen. Meet. Am. Soc. Microbiol., abstr. D-204, p. 319, 2001) were used. The first and second PCR product sizes were 531 and 422 bp, respectively. The first PCR was performed with 3 min of denaturation at 94°C followed by 27 cycles consisting of 1 min each of denaturation at 94°C, annealing at 55°C, and extension at 72°C. In the first cycle, each 50-μl PCR mixture contained 2 μg of RNA, 5 μl of 10× reaction buffer, 0.2 mM each dNTP, 1.5 mM MgCl2, 1.25 U of Taq polymerase, and 25 pmol of each primer of the primer pair p3726-p4257. The second PCR was performed the same as the first PCR, except that the annealing temperature was 52°C, 1 μl of the first PCR product was used as template, and 10 pmol of each primer of the primer pair p3761-p4183 was used.
RESULTS
Clinical signs and hematology.
Horse 1, which had been inoculated i.v. with HP A. phagocytophila, developed fever (body temperature > 38.9°C; normal range, 37.5 to 38.6°C [19]) during days 5 to 8 and 15 to 18 PI and thrombocytopenia (<1.1 × 1011 platelets/liter; normal value for total equine platelets, [2.2 ± 0.93] × 1011/liter [6]) during days 4 to 8 PI. Horse 2, inoculated i.v. with LP A. phagocytophila, developed fever only at day 8 and thrombocytopenia (1.1 × 1011 platelets/liter) and slight neutropenia (1.5 × 109 leukocytes/liter; normal value, [4.4 ± 2.0] × 109/liter [6]) on day 16. Horses 3 and 4 did not show any significant clinical signs. Total red blood cell counts (5.9 × 1012 to 13.9 × 1012 cells/liter; normal value, [7.5 ± 2.5] × 1012/liter [6]) of all four horses were within the normal range during the 20 days PI. However, at 8 days PI, variable sizes (anisocytosis) and abnormal shapes (poikilocytosis) were detected in less than 1% of the red blood cells in all 4 horses. Leukocyte levels (5.1 × 109 to 16.7 × 109/liter; normal value, [7.6 ± 3.0] × 109/liter [6]) and differential cell count levels (1.0 × 109 to 7.9 × 109 lymphocytes/liter [normal value, (3.0 ± 1.9) × 109/liter] and 0.1 × 109 to 0.8 × 109 monocytes/liter [normal value, (0.25 ± 0.25) × 109/liter] [6]) were within normal ranges in all four horses. The reactive lymphocytes (less than 1%) were observed for horse 1 (days 8, 12, 16, and 20), for horse 2 (day 16), for horse 3 (days 16 and 20), and for horse 4 (every day examined). Slight eosinophilia was observed during days 12 to 16 in horse 3 (0.4 × 109 to 0.5 × 109 eosinophils/liter; normal value, [0.15 ± 0.15] × 109/liter [6]).
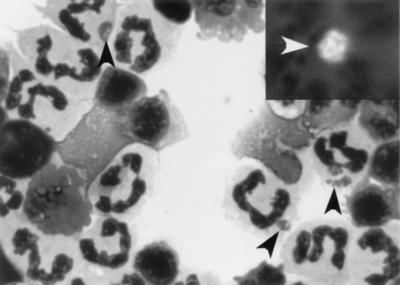
In horses 1 and 2, which were inoculated i.v., examination of PBLs by using Diff-Quik staining or IFA staining with MAb 5C11 revealed the presence of A. phagocytophila in neutrophils during days 4 to 12 PI (horse 1, 1.0% of neutrophils infected on day 4, 4.9% infected on day 8, and 2.2% infected on day 12; horse 2, 1% of neutrophils infected on day 4 and 3% infected during days 8 to 16) (Fig. 1). Morulae were not detected in the granulocytes of horses 3 and 4 throughout 20 days.
FIG. 1.
Microcolonies (morulae) of A. phagocytophila (black arrowheads) found in peripheral blood neutrophils from HP A. phagocytophila-infected horse 1 by using Diff-Quik staining and IFA with MAb 5C11 (insert, white arrowhead). Magnification, ×625.
Detection of p44 genes of A. phagocytophila.
The presence of immunodominant major outer membrane protein P44s has been demonstrated in all strains of A. phagocytophila so far examined (22, 36). Since P44 proteins are encoded by a multigene family, we have developed a highly sensitive p44-based nested-PCR method for HGE diagnosis (Lin et al., abstr. 101st Gen. Meet. Am. Soc. Microbiol., 2001). β-Actin DNA served as a control for the amount of horse PBL DNA across the samples. In all four horses, p44 genes were detected during days 4 to 20 PI (Fig. 2), indicating that ehrlichemia was established in all four horses. Although the nested PCR used was not quantitative, lack of progressive increase in band intensities following the day of infection in all four horses suggests an immune mechanism to contain the infection.
FIG. 2.
p44 gene detection of A. phagocytophila in the PBLs. The levels of A. phagocytophila in PBLs in horses infected by i.v. inoculation and/or by attachment of infected ticks were examined by PCR using p44 gene-specific primers. Input DNA was normalized by β-actin PCR. The PCR products were resolved on agarose gels containing ethidium bromide. M, DNA size markers (HaeIII fragments of φX174 RF DNA); N, no template; P, positive-control DNA from cell-cultured A. phagocytophila HZ.
IFA titers.
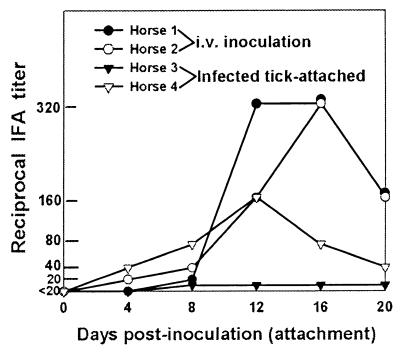
In the two horses inoculated i.v. with HP and LP A. phagocytophila, respectively, similar IFA immunoglobulin G (IgG) titers were observed (Fig. 3). In horses 1 and 2, seroconversions (>1:20) were detected from day 8 and day 4 PI, respectively. The maximum IgG titers of the 2 horses were 1:320 on days 12 and 16 PI, respectively. The IgG titers in horse 3, which had been infected by attaching infected ticks, were less than 1:20 throughout 20 days, and horse 4 showed a peak IgG titer of 1:160 on day 12 PA (Fig. 3).
FIG. 3.
IFA titers, showing development of IgG antibodies against A. phagocytophila in the four horses infected by intravenous inoculation of HP or LP A. phagocytophila or by attachment of HP A. phagocytophila-infected ticks.
Equine cytokine mRNA expression.
Expression of several equine mRNAs (IL-1β, IL-2, IL-4, IL-6, IL-8, IL-10, IL-12 p35/40, TNF-α, IFN-γ, and β-actin) by PBLs in the four horses infected with A. phagocytophila by i.v. inoculation or by attaching infected ticks was measured following the time course of infection by RT-PCR at a linear range of 25 or 27 PCR cycles. Contamination of RNA preparations with the genomic DNA was negligible, because PCR products from “minus-reverse transcriptase” negative controls were not detected in all specimens. Constitutively expressed β-actin mRNA served as a control for the amount of input RNA across the samples. As positive controls, ConA- and/or E. coli LPS-induced IL-1β, TNF-α, IL-6, IFN-γ, IL-8, IL-2, IL-4, IL-12 p35, IL-12 p40, and IL-10 mRNAs were clearly detectable in uninfected equine PBLs, using the primers and our RT-PCR conditions (Fig. 4). For horse 1 only, the results for all 10 cytokine and β-actin mRNAs examined are shown, and for the remaining horses, the results for only the cytokines whose mRNAs were expressed and IL-8 and β-actin mRNAs are shown (Fig. 4). Regardless of method of inoculation (i.e., intravenous inoculation or tick transmission), expression of IL-1β and TNF-α mRNAs was induced in all four horses by A. phagocytophila infection (Fig. 4).
FIG. 4.
Induction of cytokine mRNAs in PBLs from four horses infected with A. phagocytophila. Total RNA was extracted from equine PBLs and subjected to RT-PCR. The amount of cDNAs was normalized against β-actin mRNA in corresponding samples. M, DNA size markers (HaeIII fragments of φX174 RF DNA); N, no template. Uninfected horse PBLs were stimulated with ConA (C) or E. coli LPS (L) for 4 h in vitro. The results shown are representative of more than three assays.
IL-8 mRNA expression was detected in horses 1, 2, and 4 but not in horse 3. IFN-γ mRNA expression was weakly induced only in horse 2. A weak IL-10 mRNA expression level was also detected in horse 1. In horse 4, IL-12 p35 mRNA was weakly detected (Fig. 4). Expression of IL-2, IL-4, IL-6, and IL-12 p40 mRNAs was not detectable in the PBLs of any infected horses.
DISCUSSION
In our equine model of HGE, only IL-1β and TNF-α mRNAs were consistently upregulated in all four horses, regardless of A. phagocytophila cell culture passage number or route of infection. This suggests that A. phagocytophila-induced mild fever, neutropenia, and thrombocytopenia are mediated in horses through the induction of these two major proinflammatory cytokines, which are known to cause these changes (28). In our previous study, A. phagocytophila or rP44 induced IL-1β, TNF-α, and IL-6 mRNAs within 2 h PI and induced protein within 24 h PI in human PBLs in vitro in a dose-dependent manner (16, 17). However, IL-6 mRNA expression was not detectable in our equine model. The lack of IL-6 mRNA induction by PBLs in infected horses may be due to low numbers of A. phagocytophila in the blood of horses, since IL-6 mRNA induction in vitro requires 10-fold higher levels of A. phagocytophila than TNF-α or IL-1β mRNA induction does (16). The lack of IL-6 mRNA induction may be also related to the relatively mild nature of clinical signs of our horses. Coinfection of C3H mice with B. burgdorferi and A. phagocytophila was reported to increase the levels of A. phagocytophila and elevate IL-6 levels in the sera at 2 weeks postinfection beyond those of infection with B. burgdorferi alone (35). One report observed that in sera of acute-phase patients, the levels of IL-1β, TNF-α, and IL-6 are not elevated (9). Since, unlike those of experimentally infected animals, it is difficult to preserve these rapid-turnover cytokines in the specimens of human patients (5), it is premature to dismiss the role these cytokines play in HGE pathogenesis.
Acute-phase HGE patient sera contain significantly higher levels of IFN-γ than convalescent-phase sera (9). A. phagocytophila can transiently establish infection in C3H mice without any clinical signs, and these mice develop significant IFN-γ levels in serum during days 2 to 8 postintraperitoneal inoculation with A. phagocytophila-containing blood from infected SCID mice (1). Although in IFN-γ−/− mice, initial infection levels with A. phagocytophila are greater than in control mice, the speed of clearance of A. phagocytophila does not differ (1). In our study, IFN-γ mRNA expression was seen only in one horse. This finding may be related to the lack of spontaneous clearance of A. phagocytophila from the blood of all horses at even 20 days PI or PA. The role of IFN-γ in clearance of A. phagocytophila remains to be studied.
In the present study, IL-8 mRNA expression was upregulated in 3 horses; however, horse 3 had the weakest cytokine response overall and did not show IL-8 mRNA expression. Recently, Akkoyunlu et al. (2) proposed that IL-8 generated by neutrophils in response to A. phagocytophila facilitates the infection. Klein et al. reported that in dimethyl sulfoxide-treated infected HL-60 cells or human bone marrow cells, IL-8 and other chemokine levels, but not that of IL-1, IL-6, or TNF-α in the culture medium, were significantly elevated at 48 h postinfection (18). Since findings, including those of our present horse study, indicate that IL-8 mRNA is not upregulated at early stages of infection in vitro and in vivo, IL-8 does not seem to be critical for the initial establishment of infection.
Overall clinical signs for our horses, which were inoculated with a strain from an HGE patient in the state of New York, were milder than those reported with studies using the BDS or Webster strain from Wisconsin (4, 25, 31) but rather similar to those using inoculation with a New York strain as reported by Chang et al. (7). In the study of Pusterla et al. (32), at day 16 after i.v. inoculation and at day 18 after inoculation by tick attachment, A. phagocytophila Webster and BDS strains became undetectable by real-time quantitative PCR assays, based on the presence of the 16S rRNA gene in the blood of horses. However, A. phagocytophila HZ strain p44 genes were detected at day 20 of infection in four horses in the present study, suggesting that A. phagocytophila establishes subclinical persistent infection in horses, as suggested by Chang et al. (7). A pioneering phylogenic study, comparing different A. phagocytophila strains from patients and animals in the United States and Europe by using the ank gene as a marker, showed that Wisconsin strains are closer to Ehrlichia equi (A. phagocytophila, horse isolate, 99.18 to 99.24% amino acid identity) than New York strains (97.90 to 97.92% amino acid identity) (26). Thus, it is possible that Wisconsin strains are more pathogenic to horses but cleared more rapidly than New York strains.
Disease severity in horses inoculated i.v. with A. phagocytophila strain Webster was reported to be dependent on A. phagocytophila passage numbers in culture (31). This finding appears to be among those of studies that are not evident unless larger number of animals are tested, since we did not observe clear differences in clinical manifestations between horses infected with HP and LP A. phagocytophila HZ strains. Using a real-time quantitative PCR assay, Pusterla et al. reported 55-fold-lower initial ehrlichial load in PBLs from tick-infected horses than in those from horses inoculated i.v. (32). In the same study, however, higher ehrlichial levels in the blood of horses infected by tick attachment were observed at 7 days PI. According to the findings based on the use of PCR with p44 genes, our study appears to support their observation with respect to the lower rate of increase of ehrlichial growth with tick transmission than with intravenous inoculation. Pusterla et al. (32) did not see significant difference in antibody titer at day 30 PI between intravenous and tick transmission. In the present study, antibody responses were lower by tick attachment than by intravenous inoculation. More studies are needed to learn whether tick inoculation modulates host immune response to facilitate the infection.
Acknowledgments
This research was supported by grants AI30010 and AI40934 from the National Institutes of Health.
We thank Roger W. Stich and Debra Grove at the Ohio State University for technical assistance with tick attachment and S. R. Telford at Harvard School of Public Health, Boston, Mass., for uninfected nymphs. We thank Quan Lin at the Ohio State University for helping in extraction of DNA from blood samples.
REFERENCES
- 1.Akkoyunlu, M., and E. Fikrig. 2000. Gamma interferon dominates the murine cytokine response to the agent of human granulocytic ehrlichiosis and helps to control the degree of early rickettsemia. Infect. Immun. 68:1827-1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akkoyunlu, M., S. E. Malawista, J. Anguita, and E. Fikrig. 2001. Exploitation of interleukin-8-induced neutrophil chemotaxis by the agent of human granulocytic ehrlichiosis. Infect. Immun. 69:5577-5588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bakken, J. S., J. Krueth, C. Wilson-Nordskog, R. L. Tilden, K. Asanovich, and J. S. Dumler. 1996. Clinical and laboratory characteristics of human granulocytic ehrlichiosis. JAMA 275:199-205. [PubMed] [Google Scholar]
- 4.Barlough, J. E., J. E. Madigan, E. Derock, J. S. Dumber, and J. S. Bakken. 1995. Protection against Ehrlichia equi is conferred by prior infection with the human granulocytic ehrlichia (HGE agent). J. Clin. Microbiol. 33:3333-3334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beutler, B. A., I. W. Milsark, and A. Cerami. 1985. Cachectin/tumor necrosis factor: production, distribution, and metabolic fate in vivo. J. Immunol. 135:3972-3977. [PubMed] [Google Scholar]
- 6.Beutler, E., M. A. Lichtman, B. S. Coller, and T. J. Kipps. 1995. Williams hematology: examination of the blood, p. 8-15; comparative hematology, p. 77-85. McGraw-Hill, Inc., New York, N.Y.
- 7.Chang, Y.-F., V. Novosel, E. Dubovi, S. J. Wong, F. K. Chu, C. F. Chang, F. Del Piero, S. Shin, and D. H. Lein. 1998. Experimental infection of the human granulocytic ehrlichiosis agent in horses. Vet. Parasitol. 78:137-145. [DOI] [PubMed] [Google Scholar]
- 8.Chen, S.-M., J. S. Dumler, J. S. Bakken, and D. H. Walker. 1994. Identification of a granulocytotropic Ehrlichia species as the etiologic agent of human disease. J. Clin. Microbiol. 32:589-595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dumler, J. S., E. R. Trigiani, J. S. Bakken, M. E. Aguero-Rosenfeld, and G. P. Wormser. 2000. Serum cytokine responses during acute human granulocytic ehrlichiosis. Clin. Diagn. Lab. Immunol. 7:6-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Franchini, M., U. Gilli, M. K. Akens, R. V. Fellenberg, and V. Bracher. 1998. The role of neutrophil chemotactic cytokines in the pathogenesis of equine chronic obstructive pulmonary disease (COPD). Vet. Immunol. Immunopathol. 66:52-65. [DOI] [PubMed] [Google Scholar]
- 11.Giguere, S., and J. F. Prescott. 1999. Quantitation of equine cytokine mRNA expression by reverse transcription-competitive polymerase chain reaction. Vet. Immunol. Immunopathol. 67:1-15. [DOI] [PubMed] [Google Scholar]
- 12.Hodzic, E., D. Fish, C. M. Maretzki, A. M. De Silva, S. Feng, and S. W. Barthold. 1998. Acquisition and transmission of the agent of human granulocytic ehrlichiosis by Ixodes scapularis ticks. J. Clin. Microbiol. 36:3574-3578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jahangir, A., C. Kolbert, W. Edwards, P. Mitchell, J. S. Dumler, and D. H. Persing. 1998. Fatal pancarditis associated with human granulocytic ehrlichiosis in a 44-year-old man. Clin. Infect. Dis. 27:1424-1427. [DOI] [PubMed] [Google Scholar]
- 14.Katavolos, P., P. M. Armstrong, J. E. Dawson, and S. R. Telford III. 1998. Duration of tick attachment required for transmission of granulocytic ehrlichiosis. J. Infect. Dis. 177:1422-1425. [DOI] [PubMed] [Google Scholar]
- 15.Kim, H.-Y., and Y. Rikihisa. 1998. Characterization of monoclonal antibodies to the 44-kilodalton major outer membrane protein of the human granulocytic ehrlichiosis agent. J. Clin. Microbiol. 36:3278-3284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim, H.-Y., and Y. Rikihisa. 2000. Expression of interleukin-1β, tumor necrosis factor-α, and interleukin-6 in human peripheral blood leukocytes exposed to human granulocytic ehrlichiosis agent or recombinant major surface protein P44. Infect. Immun. 68:3394-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim, H.-Y., and Y. Rikihisa. 2002. Roles of p38 mitogen-activated protein kinase, NF-κB, and protein kinase C in proinflammatory cytokine mRNA expression by human peripheral blood leukocytes in response to Anaplasma phagocytophila. Infect. Immun. 70:4132-4141. [DOI] [PMC free article] [PubMed]
- 18.Klein, M. B., S. Hu, C. C. Chao, and J. L. Goodman. 2000. The agent of human granulocytic ehrlichiosis induces the production of myelosuppressing chemokines without induction of proinflammatory cytokines. J. Infect. Dis. 182:200-205. [DOI] [PubMed] [Google Scholar]
- 19.Kobluk, C. N., T. R. Ames, and R. J. Geor. 1995. The horse disease and clinical management: equine gastrointestinal surgery, p. 329-361. W. B. Saunders Company, Philadelphia, Pa.
- 20.Lebech, A. M., K. Hansen, P. Pancholi, L. M. Sloan, J. M. Magera, and D. H. Persing. 1998. Immunoserologic evidence of human granulocytic ehrlichiosis in Danish patients with Lyme neuroborreliosis. Scand. J. Infect. Dis. 30:173-176. [DOI] [PubMed] [Google Scholar]
- 21.Lepidi, H., J. E. Bunnell, M. E. Martin, J. E. Madigan, S. Stuen, and J. S. Dumler. 2000. Comparative pathology and immunohistology associated with clinical illness after Ehrlichia phagocytophila-group infections. Am. J. Trop. Med. Hyg. 62:29-37. [DOI] [PubMed] [Google Scholar]
- 22.Lin, Q., N. Zhi, N. Ohashi, H. W. Horowitz, M. E. Aguero-Rosenfeld, J. Raffalli, G. P. Wormser, and Y. Rikihisa. 2002. Analysis of sequences and loci of p44 homologs expressed by Anaplasma phagocytophila in acutely infected patients. J. Clin. Microbiol. 40:2981-2988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lotric-Furlan, S., T. Avsic-Zupanc, M. Petrovec, W. L. Nicholson, J. W. Sumner, J. E. Childs, and F. Strle. 2001. Clinical and serological follow-up of patients with human granulocytic ehrlichiosis in Slovenia. Clin. Diagn. Lab. Immunol. 8:899-903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Madigan, J. E., J. E. Barlough, J. S. Dumler, N. S. Schankman, and E. DeRock. 1996. Equine granulocytic ehrlichiosis in Connecticut caused by an agent resembling the human granulocytotropic ehrlichia. J. Clin. Microbiol. 34:434-435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Madigan, J. E., P. J. Richter, R. B. Kimsey, J. E. Barlough, J. S. Bakken, and J. S. Dumler. 1995. Transmission and passage in horses of the agent of human granulocytic ehrlichiosis. J. Infect. Dis. 172:1141-1144. [DOI] [PubMed] [Google Scholar]
- 26.Massung, R. F., J. H. Owens, D. Ross, K. D. Reed, M. Petrovec, A. Bjoersdorff, R. T. Coughlin, G. A. Beltz, and C. Murphy. 2000. Sequence analysis of the ank gene of granulocytic ehrlichiae. J. Clin. Microbiol. 38:2917-2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McQuiston, J. H., C. D. Paddock, R. C. Holman, and J. E. Childs. 1999. The human ehrlichioses in the United States. Emerg. Infect. Dis. 5:635-642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morris, D. D. 1991. Endotoxemia in horses: a review of cellular and humoral mediators involved in its pathogenesis. J. Vet. Intern. Med. 5:167-181. [DOI] [PubMed] [Google Scholar]
- 29.Pancholi, P., C. P. Kolbert, P. D. Mitchell, K. D. Reed, Jr., J. S. Dumler, J. S. Bakken, S. R. Telford III, and D. H. Persing. 1995. Ixodes dammini as a potential vector of human granulocytic ehrlichiosis. J. Infect. Dis. 172:1007-1012. [DOI] [PubMed] [Google Scholar]
- 30.Petrovec, M., S. Lotric-Furlan, T. Avsic-Zupanc, F. Strle, P. Brouqui, V. Roux, and J. S. Dumler. 1997. Human disease in Europe caused by a granulocytic Ehrlichia species. J. Clin. Microbiol. 35:1556-1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pusterla, N., J. E. Madigan, K. M. Asanovich, J. S. Chae, E. Derock, C. M. Leutenegger, J. B. Pusterla, H. Lutz, and J. S. Dumler. 2000. Experimental inoculation with human granulocytic ehrlichia agent derived from high- and low-passage cell culture in horses. J. Clin. Microbiol. 38:1276-1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pusterla, N., C. M. Leutenegger, J.-S. Chae, H. Lutz, R. B. Kimsey, J. S. Dumler, and J. E. Madigan. 1999. Quantitative evaluation of ehrlichial burden in horses after experimental transmission of human granulocytic ehrlichia agent by intravenous inoculation with infected leukocytes and by infected ticks. J. Clin. Microbiol. 37:4042-4044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rikihisa, Y., N. Zhi, G. P. Wormser, B. Wen, H. W. Horowitz, and K. E. Hechemy. 1997. Ultrastructural and antigenic characterization of a granulocytic ehrlichiosis agent directly isolated and stably cultivated from a patient in New York State. J. Infect. Dis. 175:210-213. [DOI] [PubMed] [Google Scholar]
- 34.Telford, S. R., III, J. E. Dawson, P. Katavolos, C. K. Warner, C. P. Kolbert, and D. H. Persing. 1996. Perpetuation of the agent of human granulocytic ehrlichiosis in a deer tick-rodent cycle. Proc. Natl. Acad. Sci. USA 93:6209-6214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thomas, V., J. Anguita, S. W. Barthold, and E. Fikrig. 2001. Coinfection with Borrelia burgdorferi and the agent of human granulocytic ehrlichiosis alters murine immune responses, pathogen burden, and severity of Lyme arthritis. Infect. Immun. 69:3359-3371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhi, N., N. Ohashi, and Y. Rikihisa. 1999. Multiple p44 genes encoding major outer membrane proteins are expressed in the human granulocytic ehrlichiosis agent. J. Biol. Chem. 274:17828-17836. [DOI] [PubMed] [Google Scholar]
- 37.Zhi, N., N. Ohashi, T. Tajima, J. Mott, R. W. Stich, D. Grover, S. R. Telford III, Q. Lin, and Y. Rikihisa. 2002. Transcript heterogeneity of p44 multigene family in human granulocytic ehrlichiosis agent transmitted by ticks. Infect. Immun. 70:1175-1184. [DOI] [PMC free article] [PubMed] [Google Scholar]