Abstract
Nitric oxide (NO) affects many physiological systems by activating cGMP signaling cascades through soluble guanylate cyclase (sGC). In the accepted model, NO binds to the sGC heme, activating the enzyme. Here, we report that in the presence of physiological concentrations of ATP and GTP, NO dissociation from the sGC heme is ≈160 times slower than the rate of enzyme deactivation in vitro. Deactivated sGC still has NO bound to the heme, and full activation requires additional NO. We propose an activation model where, in the presence of both ATP and GTP, tonic NO forms a stable heme complex with low sGC activity; acute production of NO transiently and fully activates this NO-bound sGC.
Keywords: heme, nucleotide regulation
Nitric oxide (NO) mediates blood vessel relaxation, complex aspects of myocardial function, perfusion and function of all major organs, synaptic plasticity in the brain, platelet aggregation, skin function, and numerous other physiological processes, by targeting and activating soluble guanylate cyclase (sGC) (reviewed in refs. 1–5). Dysregulation of NO signaling, then, contributes to many types of disease state, from erectile dysfunction and heart disease, to neurodegeneration, stroke, hypertension, and gastrointestinal disease, to name a few (reviewed in refs. 6–8).
In vivo and ex vivo tissue studies of NO have revealed two fundamentally distinct and paradoxical signaling modes: tonic and acute. Tonic NO describes the continual low-level production of NO that elicits a long-lasting low-level cGMP signal (9). Under resting conditions such as normotension, inhibition of nitric oxide synthase (NOS) or sGC results in vasoconstriction; therefore, tonic NO-elicited cGMP production maintains homeostatic vascular tone. Relaxation of smooth muscle requires an acute burst of NO synthesis, typically triggered by acetylcholine, and cGMP levels rise rapidly (10). These data, which describe two separate effects of NO, cannot be explained by the established binary model for NO regulation of sGC: that NO activates sGC solely by binding to its heme cofactor.
The N-terminal ≈180 aa of the β1 subunit of sGC form an evolutionarily conserved protoporphyrin-IX heme domain with spectral properties similar to the holoenzyme (11–13). NO binding to the sGC heme is diffusion-limited; however, oxygen does not bind to sGC, and carbon monoxide (CO) binding is at least 106-fold weaker than NO. Thus, the sGC heme environment is a specific NO sensor. The C-terminal domains of each subunit are homologous to the catalytic domains of adenylate cyclase and fold together to form the active site of the enzyme (14). In the existing activation model (Fig. 1, black scheme), NO binds to the heme of sGC (15), forming a six-coordinate intermediate (16–18). The rate of conversion of this intermediate to the final five-coordinate ferrous-nitrosyl species depends on the concentration of NO; thus, nonheme NO accelerates rupture of the proximal histidine–iron bond. Breaking of this bond is thought to result in a conformational change in the catalytic domain of the enzyme, accelerating the basal rate of conversion of GTP to cGMP several hundred fold (17, 19, 20).
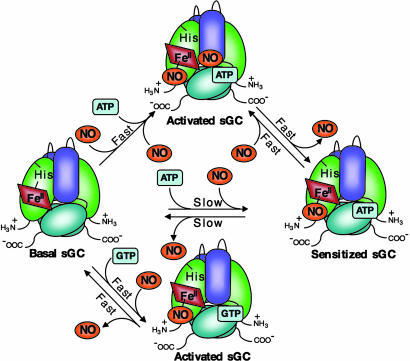
Fig. 1.
Models for NO activation of sGC. In the scheme depicted in black, NO binds rapidly to the basally active five-coordinate ferrous heme, forming a six-coordinate ferrous-nitrosyl intermediate. At a rate that depends on NO concentration, the final five-coordinate complex is activated several hundred-fold. In the scheme depicted in red, the modulation of the formation and dissociation of the sGC heme-NO complex is shown, as well as the activation state of ferrous-nitrosyl sGC, by ATP, GTP, and NO.
Although the phenomena of tonic and acute effects of NO/cGMP are widely acknowledged, the in vivo data are clearly inconsistent with the in vitro binary model for sGC activation by NO. In vivo data suggest that NO is both a long-lasting partial agonist and a transient full agonist of sGC, whereas the in vitro binary model suggests that NO simply switches the enzyme between inactive and fully active states. The sGC heme-NO complex is extremely stable, inconsistent with a rapid deactivation profile in vivo. Recent data also suggest that full activation of sGC by NO at the heme requires high levels of the cyclase reaction products [cGMP and/or pyrophosphate (PPi)] before NO binding (21). Furthermore, ATP at physiological concentrations inhibits sGC activity by as much as 50% (22, 23). Clearly, NO and nucleotides affect sGC function in ways not previously appreciated. We have studied the activation and deactivation of sGC in the presence of physiological concentrations of ATP (≈1 mM) and GTP (≈200 μM) (24), and have uncovered two distinct modes of NO activation. One activation state is low and stable and is due to NO binding to the heme. An acute transient activation state of the enzyme depends on nonheme NO. Together, these two states support a model that explains, under physiologically relevant nucleotide levels, how NO activates sGC to effect the tonic and acute NO signaling modes observed in vivo.
Materials and Methods
Expression and Purification of Recombinant His-Tagged sGC. Briefly, a C-terminal His-tag was added to the α1 subunit of rat lung sGC. The Bac-to-Bac baculovirus expression system (Invitrogen) was used to generate recombinant α and β baculoviruses according to the manufacturer's protocol. Sf9 cells (American Type Culture Collection) were cultured in Ex-Cell 420 insect serum-free medium (JRH Biosciences) supplemented with 10% FCS (HyClone) and 1% antibiotic-antimycotic (Invitrogen). For protein expression, 1-liter cultures were coinfected with H6α1 and β1 baculoviruses and harvested after 3 days in an orbital shaker at 28°C. sGC was purified to homogeneity by using Ni affinity chromatography and anion exchange chromatography. Purified sGC (exhibiting an A278/A431 < 1.1) was concentrated to 5–10 μM in a Vivaspin 6 50k filter (VWR Scientific), flash-frozen, and stored in liquid N2. Protein purity was assessed by SDS/PAGE and was routinely >95%. Protein concentrations were calculated from the A431 by using an extinction coefficient of 148,000 M–1·cm–1 (25).
Preparation of Heme Protein NO Traps. Horse heart myoglobin (Mb) was dissolved in 50 mM Hepes (pH 7.4) and 50 mM NaCl, reduced with sodium dithionite (Na2S2O4) in an anaerobic chamber (Coy Laboratory Products), desalted on a PD-10 column (Amersham Pharmacia), and exposed to air to form oxymyoglobin (MbO2). The β2(1–217) (residues 1–217 of the sGC β2 isoform) H-NOX domain construct was expressed in Escherichia coli and purified by anion-exchange and gel-filtration chromatography. Plasmid encoding the Mycobacterium bovis truncated hemoglobin (HbN) was a gift from Michel Guertin (Laval University, Quebec, Canada). HbN was expressed and purified as described in ref. 26.
Dissociation of NO from the sGC Heme. The dissociation of NO from the sGC heme at 10°C was measured by using the CO/Na2S2O4 trapping method described in ref. 27. A solution of Na2S2O4 in 50 mM Hepes (pH 7.4) and 50 mM NaCl was prepared in an anaerobic chamber and saturated with CO (Matheson) by bubbling for 10 min. Disodium 1-(hydroxy-NNO-azoxy)-l-proline (PROLI/NO) stocks were prepared in 10 mM NaOH and kept on ice. sGC-NO was prepared by the addition of a small excess of PROLI/NO in the absence or presence of nucleotide as indicated, in 50 mM Hepes (pH 7.4), 50 mM NaCl, 20 mM MgCl2, and 2 mM DTT at 25°C. sGC-NO (100 μl at 1.6 μM) was placed in an anaerobic cuvette, and the headspace was replaced with argon. The cuvette and trap solutions were equilibrated at 10°C for 1 min, and the reaction was initiated by the addition of 300 μl of CO/Na2S2O4 solution, using a Hamilton gas-tight syringe. The final reaction concentrations were 30 mM Na2S2O4, 400 nM sGC-NO, 5 mM MgCl2, 1mM ATP, and 200 μM GTP or guanosine 5′-[α,β-methylene]triphosphate (GMPCPP). Any remaining excess NO was immediately destroyed by the vast excess of Na2S2O4. The time from addition of trap to initiation of data collection was ≈15 s. Electronic absorption spectra were collected with a Cary 3E spectrophotometer at 15 nm/s. A buffer baseline was subtracted for each spectrum, and spectra were corrected for baseline drift by normalization to the average absorbance from 448 to 450 nm. Difference spectra were obtained by subtraction of the time 0 spectrum for the sample containing no nucleotide from all subsequent spectra. ΔA423 values were extracted from the difference spectra and plotted versus time for each experiment.
Deactivation of sGC-NO by the NO Trap β2(1–217). Deactivation reactions were carried out at 22°C as follows. A small excess of PROLI/NO was added to sGC in 50 mM Hepes (pH 7.4), 50 mM NaCl, 5 mM MgCl2, and 2 mM DTT (with or without 715 μMATP and/or 37 μM GMPCPP) in 140 μl. Deactivation was initiated by the addition of 20 μl of β2(1–217) in 50 mM Hepes (pH 7.4), 50 mM NaCl, 2 mM DTT, and 15 mM GTP to the reaction mixture. The final concentrations were 48.3 μM β2(1–217), 230 nM sGC-NO, 4.4 mM MgCl2, 1.9 mM GTP, and, where indicated, 625 μM ATP and 32 μM GMPCPP. Aliquots (20 μl) were withdrawn at the indicated time points and quenched by addition to 480 μl of 125 mM Zn(CH3CO2)2, to which was then added 500 μl of 125 mM Na2CO3. The cGMP formed was quantified with a cGMP enzyme immunoassay (EIA) kit (EIA Format B, Biomol), per the manufacturer's instructions. The decline in enzyme activity (as a percentage of initial activity) was obtained by fitting the accumulation of cGMP to Eq. 1 as described in ref. 28:
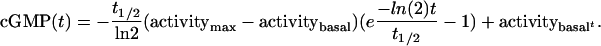 |
[1] |
Data were replotted according to Eq. 2 describing a decline in enzyme activity:
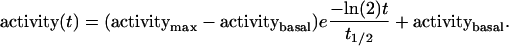 |
[2] |
Effect of Nucleotides on the Activity of Ferrous-Nitrosyl sGC. To make ferrous-nitrosyl sGC, aliquots of diethylammonium (Z)-1-(N,N-diethylamino)diazen-1-ium-1,2-diolate (DEA/NO) were added to sGC in the absence or presence of 30 μM GMPCPP and/or 1 mM ATP, and the reaction was monitored by electronic absorbance spectroscopy. Addition of DEA/NO was continued until only a small shoulder at 431 nm remained, at which point the protein was assayed. In some experiments, PROLI/NO [t1/2 = 1.8 s at 37°C (29)] was used; no difference was observed when using this compound. Full conversion to 399 nm was not attained to ensure that no excess NO existed in solution. Using the extinction coefficients of sGC and ferrous-nitrosyl sGC, we generated simulated spectra of mixed populations and overlaid them on observed spectra for each addition of NO to sGC (Fig. 6, which is published as supporting information on the PNAS web site). This allowed us to calculate the percentage of ferrous-nitrosyl sGC in each mixture. Assays in the spectrophotometer were initiated by addition of GTP, mixing, and initiation of data collection. Spectra were acquired every minute throughout all assays, which contained 400 nM sGC, 6 mM MgCl2, and 3 mM GTP. After 5–6 min, the reactions were quenched by the withdrawal of 100 μl from the cuvette and addition to 400 μl of 125 mM Zn(CH3CO2)2, to which was then added 500 μl of 125 mM Na2CO3. The cGMP formed was quantified by EIA.
Activation of Ferrous-Nitrosyl sGC by Excess NO. Assays were conducted in the spectrophotometer at 22°C as follows. sGC (400 nM) in 50 mM Hepes, 2 mM DTT, and 5 mM MgCl2 (pH 7.4) was premixed in a cuvette with or without 30 μM GMPCPP and increasing concentrations of ATP (0 μM, 375 μM, 750 μM, 1.5 mM, or 3.3 mM). Stoichiometric PROLI/NO was added to the sGC/nucleotide mix for 1 min, and then 2-min assays were initiated by the addition of GTP to 1.5 mM. Spectra were acquired every 30 s throughout the course of each assay. For each assay, the final concentration of ferrous-nitrosyl sGC was determined by comparison to calculated spectral mixtures of sGC and ferrous-nitrosyl sGC as above. Experiments were conducted in triplicate, and cGMP accumulation was measured by EIA. For experiments with excess NO, assays were performed as above, except that the sGC concentration was 67 nM, and 1.5-ml Eppendorf tubes were used.
Effect of Nucleotides on NO Binding to the sGC Heme. sGC (400 nM) with or without ATP and/or GMPCPP in 50 mM Hepes (pH 7.4), 2 mM DTT, and 5 mM MgCl2 was placed in a cuvette at 22°C. The same substoichiometric amount of PROLI/NO was added to each sGC/nucleotide mix. Electronic absorption spectra were acquired after 1 min, corrected for baseline drift, and overlaid for comparison of ferrous-nitrosyl sGC formation, as determined by absorbance increase at 399 nm and decrease at 431 nm. To examine the rates of Fe–His bond cleavage in the absence and presence of nucleotides, sGC (400 nM) in 50 mM Hepes (pH 7.4), 2 mM DTT, and 5 mM MgCl2 was premixed at 4°C with 0 μMor200 μMGTP and 0 μM, 500 μM, or 3 mM ATP. PROLI/NO (2 μM) was added, and spectra were collected every 12 s.
Effect of YC-1 on the Activation of sGC by NO. Ferrous-nitrosyl sGC was prepared by the addition of DEA/NO to obtain mostly ferrous-nitrosyl sGC, as described above. YC-1 stocks were made up in DMSO. In all assays, the final concentration of DMSO was 1%, which does not significantly affect enzyme activity. Assays were carried out in duplicate at 22°C as follows. A 50-μl aliquot of enzyme (sGC, ferrous-nitrosyl sGC, or ferrous-nitrosyl sGC plus excess NO) with or without YC-1 was added to 50 μl of 50 mM Hepes (pH 7.4), 50 mM NaCl, 5 mM MgCl2, and 1.6 mM GTP. Final concentrations were 200 nM sGC, 1.5 mM MgCl2, 800 μM GTP, and 100 μM YC-1, in a reaction volume of 100 μl. Assays were quenched, and cGMP was quantified by EIA. Final ferrous-nitrosyl sGC concentrations were determined by comparison to calculated spectral mixtures of sGC and ferrous-nitrosyl sGC as before. The effect of YC-1 on the deactivation of sGC activated by excess NO was also investigated. sGC with or without YC-1 in 50 mM Hepes (pH 7.4) and 50 mM NaCl was activated with DEA/NO (4 μM) in 90 μl. An aliquot (10 μl) of an NO trap solution, containing HbN as the NO trap, MgCl2, and GTP, was added to the sGC-NO solution. The final assay concentrations were 67 nM sGC, 5 μM HbN, 5 mM MgCl2, 3 mM GTP, and 100 μM YC-1. Assays were quenched, and cGMP was quantified by EIA.
Miscellaneous. The NO donors DEA/NO and PROLI/NO were from Cayman Chemical. No difference was observed for any experiment when NO gas was used instead of a NO donor. The noncyclizable GTP analogue GMPCPP was from Jena Bioscience. All other chemicals were from Sigma unless otherwise stated.
Results and Discussion
We first analyzed rates of NO dissociation from the sGC heme. sGC-NO was produced by addition of a small excess of the NO donor PROLI/NO to sGC in the presence of GTP, ATP, or both nucleotides; Mg2+ was always present. To remove excess NO, prevent rebinding, and chemically destroy dissociated NO, a CO/dithionite trap was used (27, 30). In the absence of nucleotide, or in the presence of ATP, the NO-heme off-rate is extremely slow (0.00031 ± 0.00002 s–1; t1/2 = 37 min). In the presence of the substrate GTP, the NO-heme off-rate is rapid (0.18 ± 0.01 s–1; t1/2 = 3.9 s); however, when both ATP and GTP are present, the NO-heme off-rate is again extremely slow (0.00031 ± 0.00002 s–1; t1/2 = 37 min) (Fig. 2A). The same rates are observed when a noncyclizable analog of GTP (GMPCPP) is used (data not shown). Thus, GTP, in a turnover-independent fashion, accelerates the NO off-rate from the sGC heme by ≈500-fold, and ATP blocks this GTP effect. Intriguingly, the GTP effect occurs only if it is present with sGC before NO addition, or if it is added to sGC in the presence of excess NO. GTP has no effect on the NO-heme off-rate if it is added simultaneously with the NO trap (data not shown). The rapid dissociation of NO in the presence of GTP would be consistent with the rapid deactivation rates observed in vivo. However, physiological concentrations of ATP block the ability of GTP to accelerate the dissociation of NO from the sGC heme. This finding suggests that in the presence of ATP and GTP, deactivation of sGC should be extremely slow.
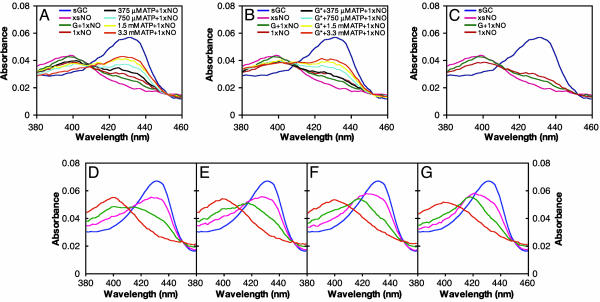
Fig. 2.
The rate of dissociation of NO from the sGC heme does not correlate with the rate of enzyme deactivation. (A) NO-heme dissociation rate at 10°C in the absence or presence of nucleotides. sGC was premixed with 200 μM GTP and/or 1 mM ATP, and excess NO was added via PROLI/NO.ACO/dithionite trap was used to destroy excess and dissociated NO. The increase in absorbance at 423 nm, because of formation of the heme-CO complex, is plotted versus time. Data from duplicate runs were averaged and fit to a single exponential to obtain rate constants. (B) Deactivation of sGC by an NO trap at 22°C in the absence or presence of nucleotides. sGC was premixed with GMPCPP and/or ATP, or no nucleotide, and excess NO was added via PROLI/NO. Deactivation was initiated by simultaneous addition of a NO trap [β2(1–217), which removes excess and dissociated NO] and MgGTP. Final concentrations were 48.3 μM β2(1–217), 230 nM sGC-NO, 4.4 mM MgCl2, and 1.9 mM GTP (with or without 625 μM ATP and/or 32 μM GMPCPP). Aliquots were withdrawn at the indicated time points, and the cGMP content of each was measured. Data were fit to an equation describing the accumulation of cGMP (Eq. 1). (C) The decline in enzyme activity as the derivative (Eq. 2) of the accumulation of cGMP shown in B. Data are shown as a percentage of initial activity and are representative of two experiments performed in duplicate. (D) Activity of ferrous-nitrosyl sGC formed in the absence or presence of nucleotides. sGC (400 nM) was premixed with 30 μM GMPCPP (G*) and/or 1 mM ATP or no nucleotide. Approximately 1 eq of NO was added, and assays were initiated by addition of GTP. Spectra were recorded during each assay. Concentrations of ferrous-nitrosyl sGC were calculated (Fig. 6) for each reaction, and the data show specific activity of the ferrous-nitrosyl species. Basal activity and activity in the presence of excess NO are shown for comparison. (E) Activation of ferrous-nitrosyl sGC by excess NO. ATP (from 375 μM to 3.3 mM) was premixed with 30 μM GMPCPP (G*) and sGC. The activity of the ferrous-nitrosyl species in the absence (light bars) or presence (dark bars) of excess NO were measured. (F) Activity from E as a percentage of maximum activity.
The current binary model states that deactivation of sGC correlates with NO dissociation from the heme (Fig. 1, black scheme). Thus, we predicted that, because the NO-heme off-rate is slow in the presence of ATP and GTP, the deactivation of sGC would be correspondingly slow. Deactivation studies of sGC under similar conditions as for NO dissociation were carried out. sGC was premixed with GMPCPP,‡‡ ATP, or both, and excess NO. Deactivation assays were initiated by the addition of substrate and a NO trap, which acts by removing all excess and dissociated NO from solution as the assay progresses. In the presence of GMPCPP, deactivation was rapid (t1/2 = ≈7 s), in good agreement with previously reported values [t1/2 = ≈4 s (28)]. Surprisingly, the deactivation of sGC is extremely rapid in every situation, regardless of nucleotide or NO trap (Fig. 2 B and C; and see Fig. 7, which is published as supporting information on the PNAS web site). ATP does not slow deactivation of sGC despite the slow NO-heme off-rate we observe when ATP is present. The rapid deactivation of sGC observed in the presence of ATP and GTP, although consistent with in vivo observations of sGC deactivation, is inconsistent with the paradigm that NO binding solely to the heme results in full activation. In the presence of ATP and GTP, it is the removal of nonheme NO that results in the rapid deactivation of sGC.
The ≈160-fold difference in the rates of NO dissociation and sGC deactivation as described above suggests that NO remains bound to deactivated sGC. In fact, we were able to isolate deactivated sGC and show that the heme remained largely NO-bound (see Supporting Text and Fig. 8, which are published as supporting information on the PNAS web site). This finding is consistent with a recent report in which a low-activity species of sGC with NO bound to the heme was described. In this report, when excess NO was removed from sGC, the activity of the resulting ferrous-nitrosyl enzyme was 10–20% of full activity (21). Together, these observations indicate that the formation of ferrous-nitrosyl sGC is not always sufficient for full enzyme activation. Indeed, when we deactivate sGC in the presence of ATP and GTP, a ferrous-nitrosyl species with low-activity results. This is contrary to the current model, which maintains that ferrous-nitrosyl sGC is the activated form of sGC, and that loss of NO from the heme is the mechanism by which deactivation occurs.
Binding of NO to the sGC heme is not sufficient for full activation; some other factor must be required for full activation. In the report by Russwurm and Koesling (21), the authors show that high concentrations of substrate GTP (5 mM) or the cyclase reaction products cGMP (1 mM) or pyrophosphate (PPi) (600 μM), when present with sGC before the addition of NO, result in fully active enzyme without excess NO. They concluded that it is these products, not GTP, that are responsible for enabling full activation of sGC by NO. However, the concentration of cGMP used in their experiments is orders of magnitude higher than estimated physiological concentrations of cGMP [0.1–10 μM (31)]. In light of our data above indicating that the effect of GTP on sGC activation by NO is turnover-independent, we confined the experiments reported here to more physiological concentrations of GTP when investigating the ability of nucleotides to regulate NO activation of sGC.
To determine the activity of the ferrous-nitrosyl species of sGC formed in the absence of excess NO and in the presence of ATP and GTP, a concentration of NO substoichiometric with the sGC heme was used to form the ferrous-nitrosyl complex. For each experiment, the concentration of ferrous-nitrosyl sGC was calculated (Fig. 6), and the activity due to that species was determined. When sGC is premixed with GMPCPP, formation of the ferrous-nitrosyl sGC species leads to full activity. If GMPCPP is added after the formation of the ferrous-nitrosyl sGC species, activity is low (data not shown). When ATP is present with GMPCPP, ATP essentially blocks GMPCPP from affecting formation of fully active ferrous-nitrosyl enzyme (Fig. 2D). Thus, in the presence of ATP and GTP, binding of NO to the sGC heme does not fully activate the enzyme, implying that excess NO is required to fully activate the ferrous-nitrosyl sGC.
We next investigated the effect of excess NO on the low-activity ferrous-nitrosyl sGC species formed in the presence of ATP and GTP. sGC was premixed with a fixed concentration of GMPCPP and increasing concentrations of ATP. Activities were then determined in the presence of substoichiometric or excess NO. As before, the concentration of ferrous-nitrosyl sGC was calculated, and the activity due to that species was determined. With substoichiometric NO, when no nucleotide is premixed with sGC, the activity of the ferrous-nitrosyl enzyme is low (Fig. 2 E and F). Premixing GMPCPP with sGC leads to a ferrous-nitrosyl species with full activity. As the concentration of ATP increases, the ability of GMPCPP to effect formation of the fully active ferrous-nitrosyl species is progressively blocked, resulting in an increasing proportion of low-activity ferrous-nitrosyl sGC. Full activation of this low-activity species is only possible by the addition of excess NO.
The data presented thus far suggest a previously uncharacterized model for activation and deactivation of sGC (Fig. 3). Under physiological ATP and GTP concentrations, low concentrations of NO (tonic NO) bind to the sGC heme, resulting in a low-activity stable species. This ferrous-nitrosyl species is sensitized to further activation by additional NO. High concentrations of NO (acute NO) rapidly and fully activate sGC. As soon as NO concentrations drop, the enzyme deactivates, returning to its sensitized ferrous-nitrosyl state. When cellular ATP levels are low, any ferrous-nitrosyl sGC formed in the presence of GTP will be fully active. It is currently unknown where ATP and GTP bind to exert their effect on sGC activity. A recent modeling and mutational study suggests that both ATP and GTP bind to a pseudosubstrate site in the catalytic domains (22); however, it remains a possibility that ATP and/or GTP might bind elsewhere on the enzyme.
Fig. 3.
A previously uncharacterized model for sGC activation and deactivation. When ATP and GTP are present (the most likely physiological scenario), the ferrous-nitrosyl species is extremely stable at low NO concentrations and has low activity. Higher NO concentrations fully activate sGC, albeit transiently. When ATP levels drop (such as under conditions of cellular stress), GTP and NO at the heme are sufficient for full enzyme activity, which is also transient. Although ATP and GTP are depicted binding to the same or overlapping sites, it remains possible that the nucleotides bind to separate sites on the enzyme.
While studying the effect of nucleotides on ferrous-nitrosyl sGC, we noticed that ATP inhibits NO binding to the sGC heme, whereas GTP increases binding. We observed that when a fixed substoichiometric concentration of NO is added to sGC (λmax 431 nm) in the presence of increasing concentrations of ATP, less formation of the ferrous-nitrosyl species (λmax at 399 nm) occurs (Fig. 4A). These observations were unaffected by the presence of a fixed concentration of GMPCPP (Fig. 4B). Furthermore, GMPCPP alone increases ferrous-nitrosyl formation compared with the nucleotide-free sample (Fig. 4C). To observe the effect of nucleotide on heme–NO binding over time, sGC was mixed with a fixed amount of GTP and increasing concentrations of ATP. NO was then added, and binding was monitored at 4°C. In the absence of nucleotide, as described in Fig. 1 (black scheme), binding of NO to the heme proceeds through a six-coordinate intermediate, with a λmax at 420 nm (18). In the presence of GTP, the six-coordinate intermediate is barely evident (Fig. 4D). This finding indicates that conversion of the six-coordinate intermediate to the five-coordinate complex is accelerated by GTP. Similar data have been reported (21). However, when ATP is included with GTP, a peak at 420 nm appears. This peak is indicative of the six-coordinate intermediate and becomes more prominent as the concentration of ATP is increased (Figs. 4 E–G). Thus, acceleration of the formation of five-coordinate ferrous-nitrosyl sGC by GTP is inhibited by ATP.
Fig. 4.
ATP inhibits the formation of the sGC heme-NO complex. sGC (400 nM) was premixed with no nucleotide, 30 μM GMPCPP, or 30 μM GMPCPP and 0 μM, 375 μM, 750 μM, 1.5 mM, or 3.3 mM ATP at 22°C. The same amount of PROLI/NO was added to all samples, which were then analyzed by absorption spectroscopy. Final spectra are shown. The spectra of ferrous sGC and sGC with excess NO (xsNO) are shown for comparison. (A) ATP prevents formation of ferrous-nitrosyl sGC, whereas GMPCPP (G*) increases formation. (B) The experiment shown in A was repeated with GMPCPP (G*) and ATP together. ATP prevents GMPCPP from increasing ferrous-nitrosyl formation. (C) GMPCPP (G*) accelerates formation of ferrous-nitrosyl sGC. (D) The effect of nucleotides on the binding of NO to the sGC heme at 4°C. sGC (400 nM) was premixed with 200 μM GTP, 200 μM GTP and 500 μM ATP (E), 200 μM GTP and 3 mM ATP (F), or 3 mM ATP (G). Excess PROLI/NO was added, and spectra were collected every 12 s to monitor NO-heme binding. The t = 0 spectrum is blue, followed by pink, green, and orange for t = 12, 24, and 36 s, respectively. Spectra acquired in the absence of nucleotide were the same as those acquired in the presence of 3 mM ATP (data not shown). In the presence of increasing amounts of ATP, the six-coordinate intermediate (λmax at 420 nm) is visible. Little intermediate is observed with GTP alone; ATP blocks GTP from accelerating the formation of the final five-coordinate NO-heme complex.
The ability of ATP to slow formation of the final heme-NO complex and the acceleration of this conversion by GTP correlates with the effect of each nucleotide on NO dissociation rates. Importantly, ATP blocks any acceleration by GTP of the formation or dissociation of the five-coordinate NO-heme complex. These observations lead us to propose a biochemical scheme relating NO binding dynamics at the sGC heme with enzyme activity (Fig. 1, red scheme). In the presence of GTP, formation and dissociation of the final heme-NO complex are rapid, and the activity of the corresponding ferrous-nitrosyl species is high. However, in the presence of ATP and GTP, heme-NO complex formation and dissociation are slow, and the corresponding ferrous-nitrosyl species has a low activity. This low-activity ferrous-nitrosyl species requires nonheme NO to be fully activated. Physiologically, the most likely scenario is one in which both ATP and GTP are present with sGC; in this scenario, NO will bind to sGC to form stable low-activity ferrous-nitrosyl complexes sensitized to full activation by subsequent acute NO signals.
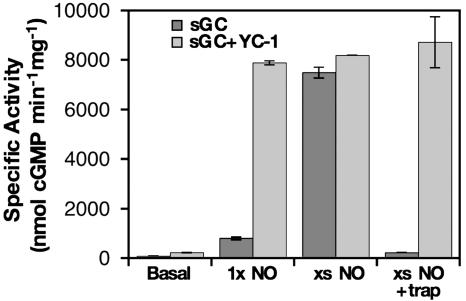
To date, physiological and therapeutic NO signaling studies have been designed and interpreted based on the binary model of NO activation of sGC. Clearly, in the presence of ATP and GTP, NO regulates not one, but two activation states of sGC. At low NO concentrations, such as the tonic NO production in tissues, the sGC heme forms a stable complex with NO. This ferrous-nitrosyl species has a persistent, low activity when ATP and GTP levels are normal. Acute concentrations of NO (typically in the low to mid nanomolar) fully activate ferrous-nitrosyl sGC, and deactivation of fully activated sGC is rapid. Although direct observation of the ligation state of sGC is not possible in vivo, YC-1, an exogenous activator of sGC (32), might act as a reporter for the presence of the low-activity ferrous-nitrosyl species. Previous reports have shown that the effects of YC-1 on basal and NO-stimulated activities of sGC are minimal, but that it synergistically activates low-activity ferrous-CO sGC (33, 34). Because ferrous-nitrosyl sGC has low activity in the presence of ATP and GTP (vide supra), the effect of YC-1 on the activity of this species was determined. We found that YC-1 fully activates the low-activity ferrous-nitrosyl enzyme (Fig. 5). Furthermore, when YC-1 and excess NO are mixed with sGC, rapid deactivation with a NO trap does not occur, as reported (28). Given that YC-1 does not affect the rate of dissociation of NO from the sGC heme (S.P.L.C. and M.A.M., unpublished work), we conclude that it is a stable synergistic activator of low-activity ferrous-nitrosyl sGC.
Fig. 5.
YC-1 fully activates low-activity ferrous-nitrosyl sGC. Shown are the activities of basal sGC, ferrous-nitrosyl sGC, and ferrous-nitrosyl sGC with excess NO (200 nM sGC in each experiment) with or without 100 μM YC-1 at 22°C. Also shown is the effect of YC-1 on the deactivation of sGC (67 nM) activated by excess NO. YC-1 was always added to sGC before PROLI/NO.
Many physiological studies with YC-1 describe it as a NO-independent activator of sGC, or point to its effects as proof that CO is coactivating sGC. For instance, YC-1 dilates blood vessels without exogenous NO or acetylcholine; this YC-1 effect in tissues is reversed extremely slowly (35). Other studies found that, after an acute NO signal in cerebellar cells, sGC is deactivated almost immediately to a low-activity “desensitized” state (36, 37). Subsequent addition of YC-1 fully reactivates this sGC species. Our data suggest that the use of YC-1 in vivo stimulates cGMP production by synergizing with the low-activity ferrous-nitrosyl sGC that is likely present in tissues. This finding may explain why YC-1 can have such a potent, long-lived effect on resting tissue or deactivated sGC: low-activity sGC with a stable ferrous-nitrosyl complex will be stably and fully activated by YC-1. We proposed that YC-1 synergizes with a low-activity NO-bound species of sGC to mimic activation by high concentrations of nonheme NO.
The data presented in this article suggest that tonic and acute NO signal transduction in vivo is due to the two NO activation states we observe in vitro. The stable low-activity state of sGC and the transient high-activity state, both of which are regulated by NO, appear to be essential properties of NO/cGMP signaling. A low-activity NO-bound species of sGC was recently observed, but because it did not form in the presence of substrate, it was discounted as physiologically irrelevant (21). The data we report here show that ATP mediates formation of this low-activity NO-bound species; furthermore, we show that NO dissociates from this species ≈500 times more slowly than the NO-bound species formed in the presence of GTP alone. It has been proposed that the ability of ATP to directly affect sGC activity provides the cell with a mechanism for linking NO/cGMP signaling with changes in cellular metabolism (23). Here, we have extended the understanding of how ATP affects NO/cGMP signaling, providing insight into the mechanism of direct ATP regulation of sGC activity in the context of physiological nucleotide levels. Furthermore, the studies we describe here on NO-heme association and dissociation, and sGC activation and deactivation, provide a model consistent with in vivo observations of the amplitude and duration of tonic and acute sGC activity. Perhaps the most striking example of tonic and acute NO/cGMP signaling working in concert is in hippocampal long-term potentiation (LTP) (38). Tonic NO is required before and after the acute NO signal to achieve LTP. As a behavioral correlate to LTP, blocking the hippocampal NO/cGMP pathway in rats immediately after an inhibitory avoidance learning task produces amnesia (39, 40). Furthermore, when YC-1 is administered before or after a learning task, it enhances learning and memory (41, 42). Because we show here that NO can affect sGC activity in two fundamentally distinct ways, we believe that NO signaling studies in tissues, and the development of therapeutics, must consider this previously uncharacterized NO duality.
Supplementary Material
Acknowledgments
We thank the members of the Marletta laboratory for discussions and critical reading of the manuscript. This work was supported by the Laboratory Directed Research and Development Fund of Lawrence Berkeley National Laboratory.
Author contributions: S.P.L.C., J.A.W., and M.A.M. designed research; S.P.L.C. and J.A.W. performed research; S.P.L.C., J.A.W., and M.A.M. analyzed data; and S.P.L.C., J.A.W., and M.A.M. wrote the paper.
Abbreviations: β2(1–217), residues 1–217 of the soluble guanylate cyclase β2 isoform; DEA/NO, diethylammonium (Z)-1-(N,N-diethylamino)diazen-1-ium-1,2-diolate; EIA, enzyme immunoassay; GMPCPP, guanosine 5′-[α,β-methylene]triphosphate; NO, nitric oxide; PROLI/NO, disodium 1-(hydroxy-NNO-azoxy)-l-proline; sGC, soluble guanylate cyclase.
Footnotes
For activity studies, we used the GTP analogue GMPCPP, which is not turned over by sGC, for premixing instead of GTP. Premixing with low concentrations of GMPCPP produces the same effect as GTP in spectral studies and does not interfere with enzyme activity.
References
- 1.Cals-Grierson, M. M. & Ormerod, A. D. (2004) Nitric Oxide 10, 179–193. [DOI] [PubMed] [Google Scholar]
- 2.Denninger, J. W. & Marletta, M. A. (1999) Biochim. Biophys. Acta 1411, 334–350. [DOI] [PubMed] [Google Scholar]
- 3.Hare, J. M. & Colucci, W. S. (1995) Prog. Cardiovasc. Dis. 38, 155–166. [DOI] [PubMed] [Google Scholar]
- 4.Shah, A. M. & MacCarthy, P. A. (2000) Pharmacol. Ther. 86, 49–86. [DOI] [PubMed] [Google Scholar]
- 5.Toda, N. & Okamura, T. (2003) Pharmacol. Rev. 55, 271–324. [DOI] [PubMed] [Google Scholar]
- 6.Bredt, D. S. (1999) Free Radical Res. 31, 577–596. [DOI] [PubMed] [Google Scholar]
- 7.Dawson, V. L. & Dawson, T. M. (1998) Prog. Brain Res. 118, 215–229. [DOI] [PubMed] [Google Scholar]
- 8.Takahashi, T. (2003) J. Gastroenterol. 38, 421–430. [DOI] [PubMed] [Google Scholar]
- 9.Pohl, U. & de Wit, C. (1996) Pflügers Arch. 432, R107–R110. [PubMed] [Google Scholar]
- 10.Buvinic, S. & Huidobro-Toro, J. P. (2001) Eur. J. Pharmacol. 424, 221–227. [DOI] [PubMed] [Google Scholar]
- 11.Iyer, L. M., Anantharaman, V. & Aravind, L. (2003) BMC Genomics 4, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karow, D. S., Pan, D., Tran, R., Pellicena, P., Presley, A., Mathies, R. A. & Marletta, M. A. (2004) Biochemistry 43, 10203–10211. [DOI] [PubMed] [Google Scholar]
- 13.Pellicena, P., Karow, D. S., Boon, E. M., Marletta, M. A. & Kuriyan, J. (2004) Proc. Natl. Acad. Sci. USA 101, 12854–12859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Winger, J. A. & Marletta, M. A. (2005) Biochemistry 44, 4083–4090. [DOI] [PubMed] [Google Scholar]
- 15.Ignarro, L. J., Degnan, J. N., Baricos, W. H., Kadowitz, P. J. & Wolin, M. S. (1982) Biochim. Biophys. Acta 718, 49–59. [DOI] [PubMed] [Google Scholar]
- 16.Makino, R., Matsuda, H., Obayashi, E., Shiro, Y., Iizuka, T. & Hori, H. (1999) J. Biol. Chem. 274, 7714–7723. [DOI] [PubMed] [Google Scholar]
- 17.Stone, J. R. & Marletta, M. A. (1996) Biochemistry 35, 1093–1099. [DOI] [PubMed] [Google Scholar]
- 18.Zhao, Y., Brandish, P. E., Ballou, D. P. & Marletta, M. A. (1999) Proc. Natl. Acad. Sci. USA 96, 14753–14758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wedel, B., Humbert, P., Harteneck, C., Foerster, J., Malkewitz, J., Bohme, E., Schultz, G. & Koesling, D. (1994) Proc. Natl. Acad. Sci. USA 91, 2592–2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao, Y. & Marletta, M. A. (1997) Biochemistry 36, 15959–15964. [DOI] [PubMed] [Google Scholar]
- 21.Russwurm, M. & Koesling, D. (2004) EMBO J. 23, 4443–4450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chang, F. J., Lemme, S., Sun, Q., Sunahara, R. K. & Beuve, A. (2005) J. Biol. Chem. 280, 11513–11519. [DOI] [PubMed] [Google Scholar]
- 23.Ruiz-Stewart, I., Tiyyagura, S. R., Lin, J. E., Kazerounian, S., Pitari, G. M., Schulz, S., Martin, E., Murad, F. & Waldman, S. A. (2004) Proc. Natl. Acad. Sci. USA 101, 37–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Traut, T. W. (1994) Mol. Cell. Biochem. 140, 1–22. [DOI] [PubMed] [Google Scholar]
- 25.Brandish, P. E., Buechler, W. & Marletta, M. A. (1998) Biochemistry 37, 16898–16907. [DOI] [PubMed] [Google Scholar]
- 26.Couture, M., Yeh, S. R., Wittenberg, B. A., Wittenberg, J. B., Ouellet, Y., Rousseau, D. L. & Guertin, M. (1999) Proc. Natl. Acad. Sci. USA 96, 11223–11228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kharitonov, V. G., Sharma, V. S., Magde, D. & Koesling, D. (1997) Biochemistry 36, 6814–6818. [DOI] [PubMed] [Google Scholar]
- 28.Russwurm, M., Mergia, E., Mullershausen, F. & Koesling, D. (2002) J. Biol. Chem. 277, 24883–24888. [DOI] [PubMed] [Google Scholar]
- 29.Saavedra, J. E., Southan, G. J., Davies, K. M., Lundell, A., Markou, C., Hanson, S. R., Adrie, C., Hurford, W. E., Zapol, W. M. & Keefer, L. K. (1996) J. Med. Chem. 39, 4361–4365. [DOI] [PubMed] [Google Scholar]
- 30.Moore, E. G. & Gibson, Q. H. (1976) J. Biol. Chem. 251, 2788–2794. [PubMed] [Google Scholar]
- 31.Trivedi, B. & Kramer, R. H. (1998) Neuron 21, 895–906. [DOI] [PubMed] [Google Scholar]
- 32.Wu, C. C., Ko, F. N., Kuo, S. C., Lee, F. Y. & Teng, C. M. (1995) Br. J. Pharmacol. 116, 1973–1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Friebe, A., Schultz, G. & Koesling, D. (1996) EMBO J. 15, 6863–6868. [PMC free article] [PubMed] [Google Scholar]
- 34.Stone, J. R. & Marletta, M. A. (1998) Chem. Biol. 5, 255–261. [DOI] [PubMed] [Google Scholar]
- 35.Galle, J., Zabel, U., Hubner, U., Hatzelmann, A., Wagner, B., Wanner, C. & Schmidt, H. H. (1999) Br. J. Pharmacol. 127, 195–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bellamy, T. C. & Garthwaite, J. (2001) J. Biol. Chem. 276, 4287–4292. [DOI] [PubMed] [Google Scholar]
- 37.Bellamy, T. C., Wood, J., Goodwin, D. A. & Garthwaite, J. (2000) Proc. Natl. Acad. Sci. USA 97, 2928–2933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bon, C. L. & Garthwaite, J. (2003) J. Neurosci. 23, 1941–1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bernabeu, R., de Stein, M. L., Fin, C., Izquierdo, I. & Medina, J. H. (1995) NeuroReport 6, 1498–1500. [DOI] [PubMed] [Google Scholar]
- 40.Bernabeu, R., Schroder, N., Quevedo, J., Cammarota, M., Izquierdo, I. & Medina, J. H. (1997) NeuroReport 8, 2221–2224. [DOI] [PubMed] [Google Scholar]
- 41.Chien, W. L., Liang, K. C., Teng, C. M., Kuo, S. C., Lee, F. Y. & Fu, W. M. (2005) Eur. J. Neurosci. 21, 1679–1688. [DOI] [PubMed] [Google Scholar]
- 42.van Staveren, W. C., Markerink-van Ittersum, M., Steinbusch, H. W., Behrends, S. & de Vente, J. (2005) Brain Res. 1036, 77–89. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.