Abstract
Cancer remains one of the deadliest diseases globally, significantly impacting patients' quality of life. Addressing the rising incidence of cancer deaths necessitates innovative approaches such as nanomedicine and artificial intelligence (AI). The convergence of nanomedicine and AI represents a transformative frontier in cancer healthcare, promising unprecedented advancements in diagnosis, treatment, and patient management. This narrative review explores the distinct applications of nanomedicine and AI in oncology, alongside their synergistic potential. Nanomedicine leverages nanoparticles for targeted drug delivery, enhancing therapeutic efficacy while minimizing adverse effects. Concurrently, AI algorithms facilitate early cancer detection, personalized treatment planning, and predictive analytics, thereby optimizing clinical outcomes. Emerging integrations of these technologies could transform cancer care by facilitating precise, personalized, and adaptive treatment strategies. This review synthesizes current research, highlights innovative individual applications, and discusses the emerging integrations of nanomedicine and AI in oncology. The goal is to provide a comprehensive understanding of how these cutting-edge technologies can collaboratively improve cancer diagnosis, treatment, and patient prognosis.
Graphical Abstract
Keywords: Cancer, Nanomedicine, Artificial intelligence, Nanoparticles, Drug delivery, Digital healthcare, Oncology, AI in healthcare
Introduction
Cancer remains a major global cause of death, profoundly affecting patients' quality of life [1]. The increasing global burden of cancer necessitates innovative approaches for improved diagnosis, prevention, and treatment [2]. Recent advancements in nanomedicine present promising solutions through the development of novel therapeutic platforms. Nanoparticles, such as albumin nanoparticles [3], liposomes [4], and polymeric micelles [5], have demonstrated considerable promise because of their unique characteristics, including small diameter, effective binding, targeting capabilities, enhanced bioavailability, and reduced toxicity [6].
Approximately 19.3 million new cancer cases and nearly 10 million cancer-related deaths were reported globally in 2020. With over 200 types of malignancies identified, cancer is forecasted to become the leading cause of death by 2030 [7, 8]. Data from the World Health Organization (WHO) and the International Agency for Research on Cancer (IARC) predict a 77% increase in global cancer cases by 2050, from an estimated 20 million in 2022 to over 35 million. This escalation is expected to result in a doubling of cancer-related deaths by 2050, with an estimated 18.5 million deaths compared to 9.7 million in 2022.
Africa is expected to experience the most substantial percentage increase in cancer cases by 2050, with a projected rise of almost 140%, from 1.2 million cases in 2022 to 2.8 million. Asia, which had the highest number of cancer cases globally at over 9.8 million in 2022, is projected to see a 77% increase, totaling 17.4 million cases by 2050 [9, 10].
The incorporation of AI into cancer diagnosis and treatment marks a significant breakthrough in combating the disease. AI technologies can dramatically enhance how cancer is detected and treated by increasing diagnostic accuracy and speed, tailoring treatment plans, and forecasting patient outcomes [11]. By examining vast datasets, AI can uncover trends beyond human capabilities, enabling earlier detection and more personalized, effective therapy options [12].
In the field of nanomedicine, AI is essential for optimizing the design and delivery of nanoparticle-based therapies [13]. Machine learning algorithms enable researchers to predict nanoparticle interactions with biological systems, enhance targeting capabilities, and reduce potential side effects [14]. AI-driven models can accelerate the development of new nanomedicines by identifying promising compounds and formulations more efficiently than traditional methods [15].
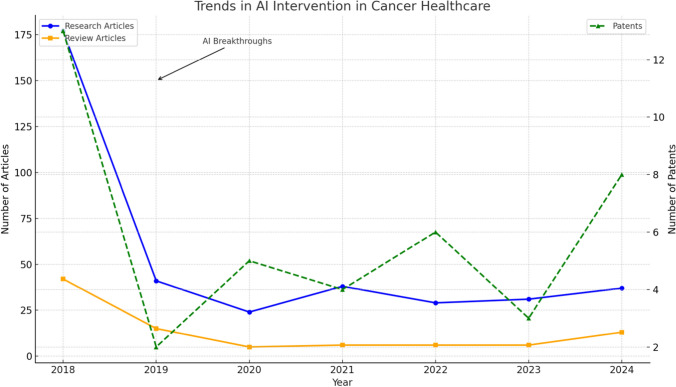
Figure 1 illustrates the annual number of research articles, review articles, and patents related to AI applications in cancer healthcare, based on PubMed data. The left y-axis represents the number of research and review articles, while the right y-axis indicates the number of patents. Key milestones, including significant AI breakthroughs (2019), a surge in FDA approvals (2021), and AI integration milestones (2024), are highlighted. The observed trends suggest increasing research interest and technological advancements in the field.
Fig. 1.
Trends in AI intervention in cancer healthcare (PubMed) from 2018 to 2024
Research articles show a notable increase in 2018, possibly reflecting the early adoption of AI technologies in oncology. However, a sharp decline in subsequent years may indicate initial challenges in clinical translation and regulatory approval.
In contrast, review articles remained relatively stable, suggesting a continuous effort to synthesize knowledge and evaluate AI applications. Patents, representing technological innovations, showed a gradual increase with a notable peak in 2024, reflecting enhanced AI integration in healthcare systems. Significant milestones, such as AI breakthroughs in 2019 and a surge in FDA approvals in 2021, accelerated patent filings and research activity.
Overall, the figure highlights the growing role of AI in cancer healthcare, driven by scientific advancements, regulatory support, and emerging innovations. This trend suggests a promising future for AI-powered oncology solutions, with potential improvements in early diagnosis, personalized treatment, and patient outcomes.
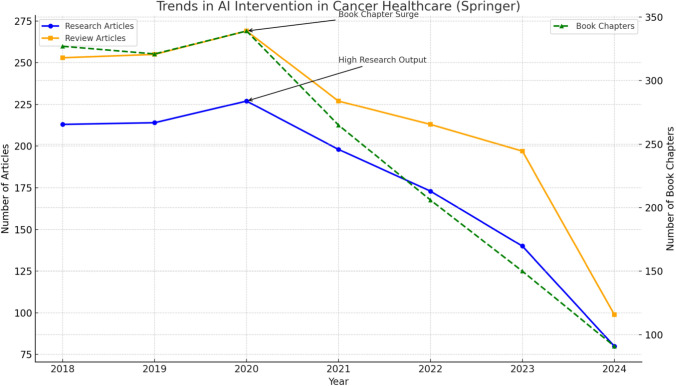
Figure 2 shows the annual number of research articles, review articles, and book chapters published on AI applications in cancer healthcare, based on Springer data. The left y-axis represents the number of research and review articles, while the right y-axis shows the number of book chapters. Significant events like the surge in research output (2020) and a peak in book chapter publications (2020) are highlighted. The decline in recent years may suggest saturation or shifts toward practical implementation.
Fig. 2.
Trends in AI intervention in cancer healthcare (Springer) from 2018 to 2024
Research articles and review articles saw a gradual increase from 2018 to 2020, reflecting the growing interest in AI-driven oncology research. Notably, a surge in research articles in 2020 may correspond to increased funding and technological advancements.
Book chapters peaked in 2020, possibly due to the need for comprehensive knowledge dissemination and academic exploration. However, post-2021, there was a significant decline across all categories. This reduction may reflect a transition from exploratory research to clinical applications and real-world implementation.
Overall, the figure highlights the dynamic nature of AI research in cancer healthcare, with early enthusiasm leading to significant publications and subsequent focus on application and validation.
To sum up, the integration of artificial intelligence with nanomedicine holds considerable promise for transforming cancer care. AI facilitates early detection, personalizes treatment plans, and advances the development of new nanomedicines, which are critical for managing the growing global cancer burden and improving patient outcomes. This review addresses different nanoparticles used in oncology, evaluates potential nano-oncology products, and explores AI’s role in clinical applications and digital health. It also highlights recent progress in nano-enabled drug delivery systems, aiming to provide a detailed overview of how the fusion of nanomedicine and AI is enhancing cancer therapy.
Nanotechnology in cancer
Nanotechnology in oncology, or nano-oncology, involves using nanoscale medicinal agents as anticancer treatments [16]. This emerging approach has shown promising outcomes in cancer detection [17], treatment [18], and prevention [19] over the past two decades, making it a cutting-edge approach in oncology. Now, let's delve into the various applications of nanoparticles in oncology, starting with liposomes.
Liposomes
Liposomes, derived from natural or synthetic phospholipids such as cholesterol, are among the most commercially viable nano-therapeutic platforms [20]. Capable of encapsulating both water-soluble and fat-soluble drugs, they enhance the solubility and stability of therapeutic agents. Their biocompatibility and biodegradability make them perfect for directed therapy, reducing the systemic toxicity associated with traditional chemotherapy [21]. Additionally, liposomes can be engineered with targeting molecules for accurate delivery of cytotoxic agents to tumor sites [22].
The benefits of liposomes include improved pharmacokinetic profiles of encapsulated drugs, longer circulation times, and passive targeting of tumors and inflammatory sites through the enhanced permeability and retention (EPR) effect. They also enhance drug solubility and offer a slow, sustained release, reducing the systemic toxicity typically associated with free drugs. Fulton et al. reviewed the applications of liposomes in overcoming pharmacokinetic limitations and enhancing the therapeutic efficacy of chemotherapeutic drugs [23]. However, despite their efficacy in treating various cancers, liposomes still present potential toxicity and lack precise targeting and disposition. These issues have hindered their clinical translation, indicating a need for more focused research to resolve these challenges [24].
In a notable breakthrough, Cho et al. developed an innovative tumor-targeting therapy by integrating an acid-responsive smart membrane with conjugated platycodin D2 (PCD2) and liposomes. This combination demonstrated effective tumor targeting in colorectal cancer xenografts. PCD2 not only induced potent antitumor effects through apoptotic cell death but also contributed to the stabilization of the liposome membrane [25]. This research underscores the potential of liposomal systems as a promising therapy for colorectal cancer, providing targeted delivery and addressing the limitations of current treatment approaches.
Extracellular vesicles
Extracellular vesicles (EVs) offer remarkable potential for drug delivery because of their unique biological composition and efficient cellular absorption. Their surfaces can be modified to target tumor areas specifically [26], making them particularly appealing for treating brain cancer, given their natural ability to cross the blood–brain barrier [27]. Additionally, EVs can transport a range of bioactive molecules useful for early cancer detection and ongoing disease assessment [28]. Their natural origin and cell communication capabilities make them excellent candidates for personalized medicine and immunotherapy [29].
In a comprehensive review, Terstappen et al. explored the developments and challenges of blood–brain barrier (BBB)-crossing strategies for delivering chemotherapeutic agents. They focused on non-invasive approaches, such as nanoparticles and EVs potential in treating CNS disorders, including brain cancer [30].
Similarly, Palazzolo et al. summarized the biomedical applications of EVs, emphasizing their potential in advanced cancer diagnostics through liquid biopsies and as innovative carriers for drug delivery. They discussed the diagnostic efficiency of EVs in several observational clinical studies, including those on pancreatic cancer or pancreatic ductal adenocarcinoma (NCT03032913), lung metastasis (NCT03108677), and oropharyngeal squamous cell carcinoma (NCT02147418) [31]. Further, Ms. Jayaseelan VP explored commercial EV biomarkers including ExoDx® Prostate and ExoDx® Lung, which are employed to identify prostate and lung cancer markers from biological samples [32]. Building on this, tumor extracellular vesicles (TEVs) have emerged as promising natural nanoparticles rich in tumor antigens, which are increasingly explored for vaccine development. Recently, Han et al. repurposed TEVs to create a novel class of personalized cancer vaccines. Their study demonstrated that these modified TEVs show significant promise in suppressing tumor progression, presenting new treatment possibilities for recurrent cancers [33].
Nanoemulsions
Nanoemulsions are nanoscale emulsions used to deliver medicines to tumor sites. They can be formulated with generally regarded as safe (GRAS) oils, which helps reduce negative side effects [34, 35]. Their small droplet size enhances the solubility and efficacy of hydrophobic agents, leading to improved outcomes and reduced dosages [36]. Furthermore, nanoemulsions may be tailored to precisely target specific tumor cells, ensuring accurate administration of therapeutic agents [37].
A critical obstacle in cancer chemotherapy is the presence of multi-drug-resistant (MDR) tumors, which significantly contribute to cancer-related mortality. To address this issue, various anticancer drugs encapsulated in nanoemulsions have been investigated for their potential to overcome MDR. Ganta et al. devised an innovative theranostic nanomedicine specifically designed to bypass MDR processes, utilizing a clinically relevant GRAS-grade nanoemulsion. This formulation was tested for efficacy in ovarian cancer cell lines, and its tumor accumulation was monitored using magnetic resonance imaging (MRI). The study demonstrated that the nanoemulsion delivered docetaxel through receptor-mediated uptake, resulting in increased cytotoxicity and the ability to overcome taxane resistance, outperforming docetaxel alone [38].
Similarly, Kaur et al. explored the efficacy of a Boswellia oil-based nanoemulsion combining paclitaxel and erucin to combat drug-resistant cancers. Their optimized formulation achieved markedly greater cytotoxic effect against breast cancer cells compared to the separate administration of paclitaxel or erucin alone. This finding underscores the potential of nanoemulsions to overcome MDR in cancers and to precisely target tumor sites [39].
Dendrimers
Dendrimers are artificial nanocarriers characterized by their tree- or branch-like structure composed of radially symmetric subunits. Their highly branched, well-defined architecture allows for excellent surface functionalization and targeting possibilities [40, 41]. E4 This unique structural design improves their capacity for specific binding to cancer cells, thereby enhancing the targeted release of chemotherapeutics to tumor sites and reducing damage to normal tissues [42]. Additionally, dendrimers can be loaded with contrast agents, which enhances imaging techniques for the early detection and monitoring of tumors [43].
Dendrimers show particular promise for brain tumor therapy due to their ability to transport xenobiotics across the BBB and effectively target brain tumors. These nanocarriers are being developed for innovative therapeutic applications, including sustained drug release, immunotherapy, and various anticancer treatments. For instance, polyamidoamine (PAMAM), polypropylenimine, poly-L-lysine, and functionalized dendrimers have significantly advanced the diagnosis and treatment of brain tumors. In this context, Kaurav et al. provided a comprehensive review of these dendrimers, exploring current applications, future prospects, and advancements in dendrimer types, synthesis methods, and mechanisms of action [44].
Similarly, Kharwade et al. examined the structure, characteristics and commercial production of PAMAM dendrimers. Their work also focused on the commercially available PAMAM dendrimers, such as Superfect® and Polyfect®, emphasizing their targeted drug delivery applications in pharmaceuticals as well as other biomedical uses [45]. However, the large-scale production of high-generation, defect-free PAMAM dendrimers is hindered by the complexities of the purification process and the precise management of energy parameters.
Inorganic nanoparticles
Inorganic nanoparticles, including gold and silver nanoparticles, carbon nanotubes, carbon quantum dots, metallic oxide fragments, and mesoporous silica nanoparticles, are extensively employed in medical applications due to their precise targeting capabilities, advantageous pharmacokinetic properties, and diagnostic functions. Their inherent stability, ease of functionalization, and distinctive optical and magnetic features make them exceptionally valuable in this field [46].
For example, gold nanoparticles are particularly well-suited for imaging and diagnostic applications, including photothermal therapy [47]. Similarly, mesoporous silica nanoparticles can be engineered to encapsulate and release drugs in response to specific stimuli, thereby improving targeted drug delivery and reducing systemic toxicity [48].
In a notable advancement, Huang et al. engineered tumor-targeted lipid-dendrimer-calcium-phosphate nanoparticles to deliver siRNA targeting PD-L1 and IL-2-encoding plasmid DNA to hepatocellular carcinoma (HCC). The study highlights the potential of a dual delivery strategy for siRNA and plasmid DNA to reprogram the immune resistant tumor microenvironment and advance cancer immunological therapy [49].
Additionally, Pugazhendhi et al. provided a comprehensive review on the role of inorganic nanoparticles in pharmacotherapy, exploring strategies for developing effective drug delivery systems and comparing the benefits and limitations of traditional chemotherapy versus nanotherapy for improving cancer treatment [50].
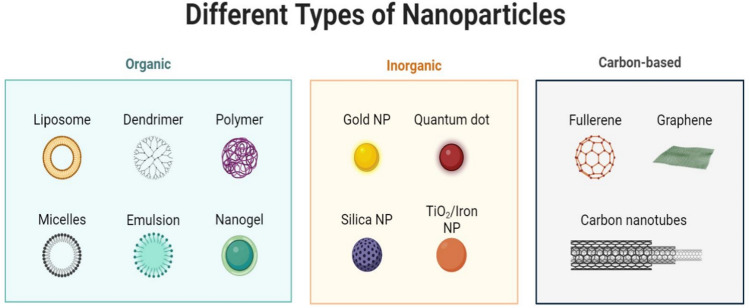
Figure 3 illustrates various types of nanoparticles, displaying their unique structures and properties. This figure highlights the potential applications of nanoparticles in nanomedicine, particularly in cancer diagnosis and therapy. It underscores the versatility and effectiveness of organic and inorganic nanoparticles in enhancing the precision and efficacy of cancer treatments.
Fig. 3.
Different types of nanoparticles
Solid lipid nanoparticles (SLNs)
Solid lipid nanoparticles (SLNs) utilize solid lipids instead of liquid lipids in a colloidal drug delivery system, offering versatility for multiple administration routes, such as intravenous, oral, local, and topical [51]. SLNs offer controlled drug release, which minimizes dosing frequency and reduces systemic toxicity [52]. Additionally, they can be engineered for targeted drug delivery to specific cancer cells, thereby enhancing drug delivery precision [53].
One notable challenge with cisplatin, a widely used chemotherapeutic drug, is its intrinsic and acquired resistance, along with significant side effects. Raut et al. demonstrated that SLN encapsulation effectively addresses these issues by improving cisplatin’s efficacy and reducing its toxicity [54]. Supporting this, Aldawsari et al. found that cisplatin-SLN formulations greatly reduced cell multiplication in human breast cancer and exhibited potent cytotoxic effects compared to free cisplatin, while showing minimal effects on normal cells [55]. These findings highlight the potential of cisplatin-loaded SLNs to overcome administration limitations and enhance treatment outcomes in breast cancer patients.
Expanding on the broader applications of SLNs, German-Cortés et al. provided a comprehensive overview of SLNs, covering their composition, production methods, and administration routes. Their review also explores recent advancements in utilizing SLNs for cancer treatment [56].
Carbon nanotubes
Carbon nanotubes (CNTs) are highly effective in targeting cancer cells by absorbing near-infrared (NIR) radiation, which causes them to heat up and kill the cells [57]. These nanotubes are also proficient at delivering various medicinal substances into living cells and can safely penetrate biofilms [58]. Additionally, when functionalized with imaging agents, CNTs enhance contrast in imaging techniques such as MRI and CT [59]. Their ability to convert NIR radiation into heat offers a non-penetrative method for the thermal ablation of tumors [60].
In drug delivery, bromocriptine, used for cancers linked with excessive prolactin production, shows enhanced target specificity and drug penetration when conjugated with nanosystems, significantly inducing apoptosis in lung cancer cells [61]. Kamazani et al. designed a novel nanomedicine based on multi-walled carbon nanotubes (MWCNTs) conjugated with bromocriptine. Their study indicated that this targeted nano-drug delivery system might be useful for treating lung cancer due to its improved performance based on neurotransmitter pathways [62].
Muzi et al. explored the benefits of drug encapsulation inside the inner cavities of CNTs, which protects drugs from premature inactivation. By loading a hydrophobic platinum (IV) complex into functionalized multi-walled carbon nanotubes (MWCNTs), they achieved effective induction of cell death in HeLa human cancer cells. Larger diameter CNTs were more cytotoxic, but smaller diameter nanotubes provided prolonged drug release, improving anticancer effectiveness. CNTs also enhanced drug accumulation within cells relative to free drug treatments [63].
Naief et al. provided a comprehensive review of CNT applications in cancer treatment, particularly in photothermal and photodynamic therapies. Their review underscores the diverse use of CNTs in these therapies and emphasizes the need for defining appropriate procedures for their therapeutic use. It also highlights the necessity for further research in human in vivo and in vitro applications [64].
Polymeric nanoparticles (PNPs)
In cancer treatment, polymeric nanoparticles are gaining prominence for their ability to improve patient care through the precise, controlled release of therapeutic agents. These nanoparticles can maintain optimal drug concentrations at tumor sites, enhancing treatment efficacy [65]. They are well suited for a range of anticancer therapies due to their biocompatibility and capability to encapsulate both hydrophilic and hydrophobic drugs [66]. Furthermore, by functionalizing polymeric nanoparticles with targeting ligands, precise drug delivery to cancer cells can be achieved [67].
Combining polymeric nanoparticles with other materials can further enhance their therapeutic potential. For instance, they can be synthesized by incorporating inorganic components like carbon nanotubes, polymers, silica, metal oxide nanoparticles, and graphene with organic compounds such as lipids, proteins, and phospholipids. This hybrid approach offers significant advantages over traditional drug formulations. Ferreira Soares et al. reviewed the development of polymer-hybrid nanoparticles for biomedical applications, highlighting cutting-edge synthesis techniques and their uses in both in vitro and in vivo contexts [68]. Their work offers valuable insights into the latest methodologies and practical applications of these nanoparticles for improving therapeutic strategies.
One prominent example of a multifunctional polymer used in nanoparticle synthesis is Poly Lactic-co-Glycolic Acid (PLGA). This biodegradable copolymer, consisting of lactic and glycolic acids, is celebrated for its capacity to enhance therapeutic efficacy. With U.S. FDA approval for long-acting release formulations, PLGA is extensively used to encapsulate anticancer drugs due to its excellent bioavailability, and ability to lower systemic toxicity. Clinical formulations such as Decapeptyl, Suprecur MP, and Lupron Depot showcase its successful application in cancer therapy. Makadia and Siegel explored different fabrication methods for PLGA-based formulations and drug delivery systems, and examined the factors affecting their degradation and drug release profiles [69]. Their exploration of these advancements opens new avenues for improving cancer treatment methodologies.
Quantum dots
Quantum dots (QDs) are semiconductor nanoparticles with sizes ranging from two to ten nanometers, distinguished by their exceptional optical and electronic properties that are crucial for advancing cancer diagnosis and treatment. QDs enable highly sensitive imaging techniques, facilitating early detection and monitoring of tumor growth [70]. In addition, QDs can serve as carriers for the precise delivery of therapeutics to cancer site, significantly minimizing the impact on surrounding healthy tissues [71]. In photodynamic therapy, QDs generate reactive oxygen species upon light activation, selectively killing cancer cells [72].
Following the US FDA’s approval of the first human clinical trial for quantum-dot technology in 2011, there has been continued advancement in the field. Recently, the FDA has also approved a clinical trial for Cornell dots (C-dots) in the treatment of melanoma. In their thorough review, Namdev Dhas et al. discussed the synthesis, surface modification, and theranostic applications of QDs in cancer treatment, reflecting the rising importance of QDs in oncology [73].
Carbon quantum dots (CQDs), a subset of QDs, are emerging as significant nanomaterials within the carbon family. In their research, Snehasis Mishra et al. developed highly fluorescent carbon quantum dots (CQDs) from orange juice via a one-step hydrothermal method and subsequently fabricated CQD/Ag heterostructures. These heterostructures exhibited notable anticancer effects against human colorectal carcinoma (HCT 116) cells by inducing ROS-dependent mitochondrial-mediated apoptosis and showed potential as bioimaging agents [74].
Following a similar strategy, Wang et al. developed copper-doped carbon quantum dots (Cu-CQDs) using a one-step hydrothermal synthesis method. Their findings indicated that Cu-CQDs not only demonstrated impressive biosafety but also possessed significant anticancer properties, effectively disrupting malignant nature and maintaining oxidative homeostasis in breast cancer cells [75]. Overall, these nanoparticle-based strategies represent the forefront of precision oncology, offering targeted, efficient, and safer alternatives to conventional cancer therapies.
AI technologies in cancer therapy
AI and its associated technologies have the potential to revolutionize cancer treatment by optimizing the administration of nanomedicines to achieve and maintain optimal drug concentrations in both the bloodstream and targeted areas [76]. The application of AI and machine learning (ML) in automated diagnostic systems is addressing emerging challenges in early disease detection, especially for cancer. As ML technology advances, neural network algorithms are developing to solve problems in ways similar to human cognition. Deep learning (DL), a branch of ML, replicates human data processing capabilities to recognize patterns, interpret spoken language, discover novel drugs, advance personalized medicine, refine diagnostic tests, and assist in clinical decision-making [77].
AI's primary focus in combating the rising global cancer mortality rate is leveraging biological computation. AI can be categorized into three types: Narrow AI, General AI, and Superintelligent AI. Narrow AI, also known as Weak AI, is designed to enable computers to understand and analyze complex biological processes that are often challenging for humans to grasp. This technology has led to the development of systems capable of detecting biological changes with human-like intelligence by analyzing real-time data from large populations for precise clinical insights. For example, Narrow AI powers voice-activated assistants like Google Assistant, Apple’s Siri, Amazon’s Alexa, and Microsoft’s Cortana, which process user inputs, query search engines, and deliver relevant results [78].
General AI, with its potential to outperform humans, could revolutionize fields like medicine by scanning global research, formulating solutions, and simulating outcomes to cure diseases like cancer. General AI combines human-like consciousness with vast data access, enabling continuous, focused problem-solving without rest. However, the risks include uncontrolled upgrades and misuse by rogue states or unethical corporations. Such misuse could lead to privacy breaches, hacking, and unethical manipulation, prioritizing profit over public good [79].
Super AI surpasses human intelligence in computation and decision-making, revolutionizing healthcare by analyzing vast medical data for superior diagnostics, treatment plans, and patient monitoring. Its predictive power shifts healthcare from reactive to proactive, identifying health risks and disease outbreaks early. For instance, it can predict carcinogenesis from patient data trends, enabling effective precautions and resource use.
Super AI enhances patient protection through real-time monitoring of medical devices, detecting adverse events like drug toxicity or surgical complications early, and alerting providers for timely intervention. Challenges include data privacy, ethical concerns, and potential errors, but robust regulations and fail-safe mechanisms can mitigate these risks, ensuring reliable ASI performance [80].
Major multinational tech giant Nvidia announced plans in November 2020 to build an AI supercomputer for healthcare studies and drug delivery. AI in healthcare aims to identify therapeutic targets, uncover intricate protein interactions, and solve complex biological problems. AI also offers sophisticated medical imaging techniques that improve the accuracy of cancer diagnosis by recognizing abnormal cellular changes [81].
The article by Dlamini et al. introduces an "AI-based precision oncology approach," which targets individual cells and serves as a foundation for tools designed to overcome the limitations of next-generation sequencing (NGS) [82]. AI in medicine can help map therapeutic targets, interpret complex protein interactions, and address challenging physiological questions. For example, the National Health Service (NHS) in Scotland is conducting a clinical trial using the DL NHS 111 algorithm to assist individuals with minor medical issues at home via phone. Similarly, AI chatbots are being utilized by patients for symptom identification and to receive recommendations for further actions in community and primary care settings. Babylon Health and Ada are leading examples of online healthcare platforms that use AI technology to deliver digital health services aimed at improving clinical outcomes. These platforms employ sophisticated algorithms to offer preliminary medical advice, suggest next steps for diagnosis or treatment, and support early disease detection [83, 84].
Expanding on the role of AI and ML in cancer care, the creation of a Clinical Linked Data Graph (LDG) exemplifies a major advancement in the integration of diverse biomedical datasets to drive research and improve patient care. By employing semantic web technology, the LDG connects a wide array of bioinformatics resources, thereby facilitating large-scale AI and ML processes for discovering new cancer treatments and enhancing diagnostic capabilities. This approach reflects a broader trend in cancer research where sophisticated data analysis techniques, similar to those used in autonomous vehicles for navigation and decision-making, are harnessed to address complex challenges in cancer treatment and diagnosis. For instance, AI-driven models used in the LDG can predict patient responses to treatments or identify new drug targets, much like how autonomous vehicles use AI to navigate and make real-time decisions [85].
Through this narrative review article, we aim to highlight the latest AI-developed methods and approaches that advance sophisticated cancer detection and treatment, driven by developments in machine learning tools.
Cancer nanomedicine
Cancer nanomedicine leverages nanotechnology to identify, monitor, and manage cancer by combining medicinal agents with nanoscale technology. These advancements can be integrated into biological machinery, nanobiosensors, and medical equipment, significantly improving therapeutic outcomes in complex and fatal diseases by maintaining targeted treatment dosages [86].
The U.S. Food and Drug Administration (FDA) has approved several nanomedicine formulations for cancer treatment [87]. Notable examples include Doxil [88], Myocet™ [89], and DaunoXome [90]. Additionally, nanoparticle formulations like Abraxane [91], approved for metastatic breast cancer, advanced non-small cell lung cancer, and metastatic pancreatic cancer, have also received FDA approval. Other FDA-approved formulations include injectable liposomal irinotecan (Onivyde™) [92] for metastatic pancreatic cancer, and a combination of cytarabine and daunorubicin (Vyxeos) [93] for treating acute myeloid leukemia.
Furthermore, several other nanomedicine formulations have been approved, showcasing the diversity in composition, mechanisms of action, and therapeutic targets. Hensify (NBTXR3) [94], composed of hafnium oxide nanoparticles, is used in radiotherapy for locally advanced soft tissue sarcoma (STS). Pazenir [95], an albumin-bound nanoparticle formulation of paclitaxel, is used to treat metastatic cancers of the breast, pancreas, and non-small cell lung cancer. Nanotherm [96], composed of iron oxide nanoparticles, is used in magnetic field-induced thermal ablation for the treatment of brain tumors, prostate cancer, and pancreatic cancer. Marqibo [97], a liposomal vincristine, is indicated for acute lymphoblastic leukemia, while DepoCyt [98], a liposomal cytarabine, is used in neoplastic meningitis. Mepact [99], another liposome-based drug, contains mifamurtide MTP-PE for osteosarcoma. Genexol-PM [100], a PEG-PLA polymeric micelle containing paclitaxel, is used for breast, lung, and ovarian cancer. Oncaspar [101], a polymer protein conjugate carrying pegaspargase/L-asparaginase, is used in acute lymphoblastic leukemia.
In addition to these approved formulations, many are also undergoing various phases of clinical trials. These multimodal drug delivery systems represent significant progress in oncology by improving drug delivery, reducing toxicity, and enhancing therapeutic efficacy. Table 1 lists these multimodal drug delivery systems approved for different types of cancer. This table highlights the advancements in nanomedicine that have been approved for cancer treatment, showcasing the diversity in composition, mechanisms of action, and therapeutic targets. These nanomedicines improve drug delivery, reduce toxicity, and enhance therapeutic efficacy, representing significant progress in oncology.
Table 1.
USFDA approved nanomedicine in cancer treatment
| S. No | Drug | Type of nano drug delivery system | Applications | References |
|---|---|---|---|---|
| 1 | Doxil | Injectable Liposomal | AIDS related kaposi’s sarcoma and cancer of ovaries | [86] |
| 2 | Myocet ™ | Injectable Liposomal | Metastatic Breast cancer | [89] |
| 3 | DaunoXome | Injectable Liposomal | Advanced HIV associated kaposi’s sarcoma | [90] |
| 4 | Abraxane | Nanoparticle |
Metastatic Breast cancer Metastatic Pancreatic cancer Advanced non-small cell lung cancer |
[91] |
| 5 | Irinotecan (Onibyde™) | Injectable Liposomal | Metastatic Pancreatic cancer | [92] |
| 6 | Vyxeous (Cytarabine &Daunorubicin) | Injectable Liposomal | Acute Myeloid leukemia | [93] |
| 7 | Hensify (NBTXR3) | Intratumoural Injectable Nanoparticle | Locally Advanced Soft Tissue Sarcoma | [94] |
| 8 | Pazenir | Injectable Nanoparticle-Bound Albumin | Metastatic Breast Cancer, Metastatic Adenocarcinoma of the Pancreas, and Non-Small Cell Lung Cancer | [95] |
| 9 | Nanotherm | Injectable Nanoparticle | Glioblastoma, Prostate, and Pancreatic cancer | [96] |
| 10 | Marqibo | Injectable Liposomal | Acute Lymphoblastic Leukemia | [97] |
| 11 | DepoCyt | Injectable Liposomal | Neoplastic meningitis | [98] |
| 12 | Mepact | Injectable Liposomal | Osteosarcoma | [99] |
| 13 | Genexol-PM |
Injectable PEG-PLA Polymeric Micelle |
Breast, Lung, and Ovarian cancer | [100] |
| 14 | Oncaspar | Injectable Pegylated Protein Drug Conjugate | Acute Lymphoblastic Leukemia | [101] |
Ligand-coated liposomes and nanoparticles can dramatically enhance drug delivery to cancer sites. For instance, a blood clotting factor XIII analog combined with doxorubicin-loaded liposomes resulted in a 40-fold increase in drug accumulation at tumor sites [102]. Recent advancements have focused on nanoparticles capable of carrying both therapeutic and diagnostic agents. These nanomedicines are engineered to address the limitations of conventional cancer treatments. A prime example is theranostic liposomes loaded with docetaxel and QDs, coated with RGD-TPGS (arginine-glycine-aspartic acid peptide with D-α-tocopheryl polyethylene glycol 1000 succinate). These liposomes have shown significantly improved efficacy over traditional treatments, as indicated by a notable reduction in reactive oxygen species production without inducing edema or brain injury [103].
Clinical applications of AI in cancer
AI models in healthcare operate through distinct mechanisms to analyze data and generate predictions. ML algorithms, including supervised, unsupervised, and reinforcement learning, work by identifying patterns in medical data. In supervised learning, labeled datasets train algorithms like SVMs or RF to detect anomalies such as tumors in medical images [104]. Unsupervised learning identifies hidden patterns to classify cancer subtypes, while reinforcement learning optimizes treatment plans by learning from past patient outcomes [105]. NNs mimic the human brain using interconnected nodes arranged in layers. Input data passes through these layers, undergoing transformations using activation functions like rectified linear unit (ReLU) or Sigmoid, while back propagation and gradient descent algorithms minimize errors by adjusting weights [106]. DL, a subset of neural networks, uses multiple hidden layers for complex tasks like tumor segmentation and genomic analysis. CNNs are particularly effective for medical image interpretation, extracting spatial features, while RNNs and long short-term memory (LSTM) networks analyze sequential patient data [107]. Transformer models like bidirectional encoder representations from transformers (BERT) and generative pre-trained transformer (GPT) apply self-attention mechanisms to interpret clinical text and research data. Additionally, Explainable AI (XAI) ensures transparency by providing insights into how AI models arrive at their decisions. Techniques like SHAP (SHapley Additive exPlanations) and LIME (Local Interpretable Model-agnostic Explanations) attribute importance to input features, while visualization tools generate heatmaps to highlight regions of interest in medical images [108–110]. Through these mechanisms, AI enhances cancer diagnosis, predicts treatment responses, and personalizes patient care, making it an indispensable tool in modern healthcare.
Personalized therapy—AI
The potential of AI is evident in various medical specialties, from diagnostic imaging and early disease detection to the identification of subtle abnormalities. By simulating human cognitive abilities, AI enables physicians to provide optimal and personalized treatments to cancer patients [111].
One notable example is IBM Watson for Oncology, which compiles data from medical records, textbooks, journals, and expert oncologists to analyze and recommend possible therapies. This aids oncologists in identifying the most effective treatment options.
Other platforms making significant strides in AI-driven healthcare include Google's DeepMind, Microsoft's Hannover project, PathAI, Tempus Labs, Microsoft Azure AI for Healthcare, PathAI’s Tumor Detection Algorithms, Varian’s ARIA Oncology Information System, ClinicalTrials.gov search tools, and BenevolentAI. These platforms, summarized in Table 2, are advancing AI applications in personalized cancer therapy by leveraging data analysis and machine learning to enhance treatment precision and efficacy.
Table 2.
Software used in personalized therapy for cancer patients
| AI Application | Technology | Use | References |
|---|---|---|---|
| Genomic Data Analysis | IBM Watson |
Helps in cancer patients personalized therapy Analyzes genetic mutations for targeted therapies |
[112, 113] |
| Radiotherapy planning in head and neck cancer | Google’s Deepmind | Analyses the data collected from various sources and offers treatment options based on it | [114, 115] |
| Personalized drug combinations | Microsoft’s Project Hanover | Provide best treatment options by using data and expertise in oncology | [116, 117] |
| Biomarker Discovery | PathAI | Identifies cancer biomarkers from pathology slides | [118] |
| Drug Response Prediction | Tempus Labs | Predicts effective therapies using clinical and molecular data | [119] |
| Clinical Decision Support | Microsoft Azure AI for Healthcare | Supports oncologists with personalized treatment recommendations | [120] |
| Tumor Segmentation & Planning | PathAI’s Tumor Detection Algorithms | Segments tumors from images for personalized treatment planning | [121] |
| Precision Radiation Therapy | Varian’s ARIA Oncology Information System | Optimizes radiation therapy plans based on patient data | [122] |
| Clinical Trial Matching | ClinicalTrials.gov Search Tools | AI-driven tools match patients with suitable clinical trials | [123] |
| Personalized Immunotherapy | BenevolentAI | Develops new immunotherapies based on genetic and molecular data | [124] |
Nanomedicine for cancer—AI technologies
AI can significantly enhance cancer therapy by optimizing drug combinations to maximize efficacy and minimize side effects, particularly for multifunctional nanopharmaceuticals. Researchers have leveraged AI to determine the optimal combinations of cytotoxic medications [125]. In a notable study, Russo et al. explored AI's capacity to predict outcomes of chemotherapy, both as a monotherapy and in conjunction with targeted treatments, for metastatic colorectal cancer patients. Their research showed encouraging results in predicting both therapeutic efficacy and adverse effects [126].
Innovative AI techniques have been used to optimize the combinations of nanodiamond with key anticancer drugs such as doxorubicin, mitoxantrone, bleomycin, and conventional paclitaxel, leading to more effective treatment strategies for breast cancer cells [127]. These AI-optimized drug-nanomedicine combinations outperformed randomly chosen ones [128]. Additionally, Weiss et al. employed a simplified feedback system control based on a stochastic search algorithm to refine drug combinations for for targeting the renal cancer cell line 786-O. Their findings revealed that combinations of axitinib, erlotinib, dasatinib, and AZD4547 were effective in reducing cancer cell viability while minimizing side effects on non-malignant cells [129, 130].
CURATE.AI, an AI-driven platform, has been used to refine treatment regimens for metabolic disorders [131], tuberculosis [132], and metastatic castration-resistant prostate cancer [133] through patient-specific drug dosing adjustments. Tan et al. assessed the feasibility of CURATE.AI for guiding chemotherapy dosing in solid tumors with the PRECISE CURATE.AI pilot clinical trial. The study underscored CURATE.AI’s potential to address big data challenges in personalized medicine, providing preliminary efficacy and safety data for upcoming precision oncology trials [134].
Application of AI in nanomedicine for cancer healthcare
Artificial Intelligence (AI) has emerged as a transformative force in nanomedicine, significantly advancing cancer diagnosis, treatment, and monitoring. By leveraging vast datasets, AI algorithms enhance the design of nanocarriers, predict cancer behavior, and facilitate personalized therapies. The convergence of AI and nanotechnology paves the way for more precise and effective cancer management.
-
i.
AI in nanocarrier design and drug delivery optimization
i
AI algorithms, including machine learning (ML) and deep learning (DL), are instrumental in the rational design of nanocarriers. These models predict the optimal size, shape, surface charge, and functionalization of nanoparticles to maximize therapeutic efficacy while minimizing toxicity. Additionally, AI can optimize drug-loading capacities and release kinetics, tailoring nanoparticles for specific tumor microenvironments [135].
-
ii.
Predictive modeling for cancer targeting
AI-powered predictive models simulate nanoparticle interactions within the body, forecasting biodistribution, tumor accumulation, and potential off-target effects. In silico modeling aids in selecting appropriate biomarkers and ligands for nanoparticle surface modifications, resulting in precise drug delivery systems that improve therapeutic outcomes and minimize adverse effects [136, 137].
-
iii.
Personalized treatment strategies
AI algorithms analyze genomic, proteomic, and clinical data to generate personalized treatment plans. By predicting individual patient responses to nanomedicine-based therapies, AI enables the selection of the most suitable nanoparticle formulations and drug combinations. This personalized approach is particularly advantageous for managing heterogeneous tumors and reducing resistance [138].
-
iv.
Real-time monitoring and adaptive therapy
AI-enhanced imaging techniques, when paired with nanoparticle contrast agents, offer real-time insights into tumor progression and treatment response. AI algorithms analyze imaging data to adapt treatment strategies dynamically, ensuring timely interventions and maximizing therapeutic efficacy. Additionally, wearable biosensors and nanodevices, integrated with AI, provide continuous patient monitoring for early detection of complications [139].
-
v.
Drug discovery and development
AI accelerates drug discovery by analyzing chemical libraries and predicting interactions between nanoparticles and biological systems. Machine learning models identify optimal drug-nanocarrier combinations, streamlining formulation development and reducing the time and cost of bringing new therapies to market [140].
-
vi.
Emerging integrations of AI and nanomedicine
The convergence of AI with nanotechnology is driving innovative integrations that further enhance cancer healthcare:
a) Theranostic nanoplatforms: AI-powered theranostic platforms combine diagnostic and therapeutic functions within a single nanostructure. These platforms can detect cancer biomarkers, deliver targeted therapy, and monitor treatment responses in real-time, offering a comprehensive personalized management solution [141].
b) AI-driven nano-robotics: AI-controlled nano-robots demonstrate promise in precise drug delivery and tumor targeting. These nano-robots autonomously navigate through the bloodstream, identify cancer cells, and release therapeutics in a controlled manner, minimizing damage to healthy tissues [142].
c) Multi-omics data integration: AI algorithms integrate genomic, transcriptomic, and proteomic data with nanomedicine approaches to uncover novel biomarkers and predict therapeutic responses. This integration enhances patient stratification and informs the development of personalized nano-therapeutics [143].
d) Quantum computing for nanomedicine: Quantum computing, combined with AI, enables rapid simulation of complex biological environments. This enhances nanoparticle modeling and expedites the development of next-generation nanomedicines [144].
As research progresses, the synergistic combination of AI and nanomedicine is expected to further enhance cancer care. Future advancements may lead to sophisticated AI-driven solutions for early detection, personalized treatment, and improved patient outcomes, ultimately making cancer a more manageable disease.
AI in medicine
AI empowers computers and robots to emulate human behaviors, support healthcare diagnosis, and perform surgical procedures. AI applications in medicine include drug development, generating medical statistical datasets, and analyzing diseases like cancer [145]. AI-powered surgical robots [146] and nanorobots [147] offer targeted drug delivery, enhancing precision and efficacy.
ML and deep learning assist in clinical diagnostics and treatment decisions [148]. For instance, AI is used in surgical robots for procedures such as heart valve repair [149], gynecology [150], and prostatectomy [151]. Future cancer treatments may utilize unsupervised learning and reinforcement learning to identify hidden patterns and optimize strategies. Additionally, AI plays a significant role in computational biology, genetics, and molecular medicine for identifying medicinal targets and managing protein interactions [152].
The review by Vijayakumar and Shetty on robotic surgery in oncology highlights its advantages, barriers, and the Indian scenario [153]. For example, Yang et al. detailed a case in which the 4th generation da Vinci Robotic Surgical System (Xi) was employed to perform a uniportal right upper lobectomy, highlighting the advanced capabilities of robotic-assisted surgery. The operation lasted 90 min, and the patient had a smooth recovery, being discharged three days post-surgery [154].
Robotic surgery for rectal cancer overcomes the technical challenges associated with traditional laparoscopic methods, leading to improved radical operation outcomes. A notable development in this field is the introduction of Verb Surgical, a collaboration between Google and Johnson & Johnson, which is advancing robotic-guided procedures. Liu et al. provided a comprehensive overview of the current status of robotic technology in rectal cancer treatment, detailing advancements such as robotic total mesorectal excision, lateral lymph node dissection, and the integration of AI in surgical processes [155].
In drug formulation, computational methods can optimize methotrexate nanosuspension by analyzing drug molecule interactions and conditions leading to aggregation. Techniques such as coarse-grained simulations and chemical calculations are employed to understand drug-dendrimer interactions and to evaluate drug encapsulation efficiency. Software like LAMMPS and GROMACS 4 can assess the impact of surface chemistry on nanoparticle internalization into cells. Mehta et al. offered an in-depth review of computational modeling tools utilized in drug formulation design, emphasizing their applications and potential to advance personalized medicine and enhance therapeutic outcomes [156].
Challenges in AI-assisted robotic surgery
AI-assisted robotic surgery has significantly improved precision and patient outcomes in cancer care, but its implementation presents several challenges that must be addressed for broader adoption and safer clinical use. The substantial costs of acquiring, maintaining, and operating AI-driven surgical systems limit their accessibility, particularly in resource-limited settings, preventing equitable access to advanced surgical care. Surgeons and healthcare teams require specialized training to operate these systems effectively. The steep learning curve and the need for continuous education can delay adoption and temporarily increase the risk of errors [157, 158]. Additionally, AI systems are vulnerable to technical malfunctions and software issues, posing risks during critical procedures. Surgeons must retain conventional surgical skills to manage emergencies when technology fails [159]. Another major limitation is the lack of haptic feedback, which reduces a surgeon’s ability to detect subtle tissue abnormalities, potentially leading to unintended tissue damage [160]. Data privacy and security also remain significant concerns, as AI-powered systems collect and analyze large volumes of sensitive patient data. Ensuring robust cybersecurity measures and maintaining compliance with regulations like Health Insurance Portability and Accountability Act (HIPAA) and General Data Protection Regulation (GDPR) are essential to protect patient information [161]. Furthermore, AI algorithms may reflect biases present in their training data, leading to disparities in surgical outcomes [162]. Ethical concerns regarding accountability in cases of adverse events further complicate the integration of AI in surgical practice [163]. The transparency and explainability of AI systems are also limited, with many models operating as “black boxes.” Implementing explainable AI (XAI) methods can enhance clinician trust by providing clearer insights into AI-driven recommendations [164]. Legal and regulatory frameworks must also be established to define accountability for AI-related errors and ensure the responsible use of AI in surgical procedures [165]. Lastly, the successful integration of AI-assisted robotic systems with existing healthcare infrastructure, including electronic health records (EHRs), is essential. Addressing interoperability challenges will streamline workflows and support informed decision-making. By overcoming these challenges through technological advancements, transparent algorithms, continuous training, and clear regulations, AI-assisted robotic surgery can fulfill its potential in enhancing cancer care [166].
AI in medical imaging
AI-driven bioinformatic algorithms and computational models significantly impact Medical Imaging Technology (MIT), aiding in the detection of abnormal cell division and physiological changes [167]. By enhancing diagnostic accuracy in fields such as neuroradiography, MRI, and radiology, AI extends its applications to record-keeping, data extraction, image categorization, and analysis [168, 169].
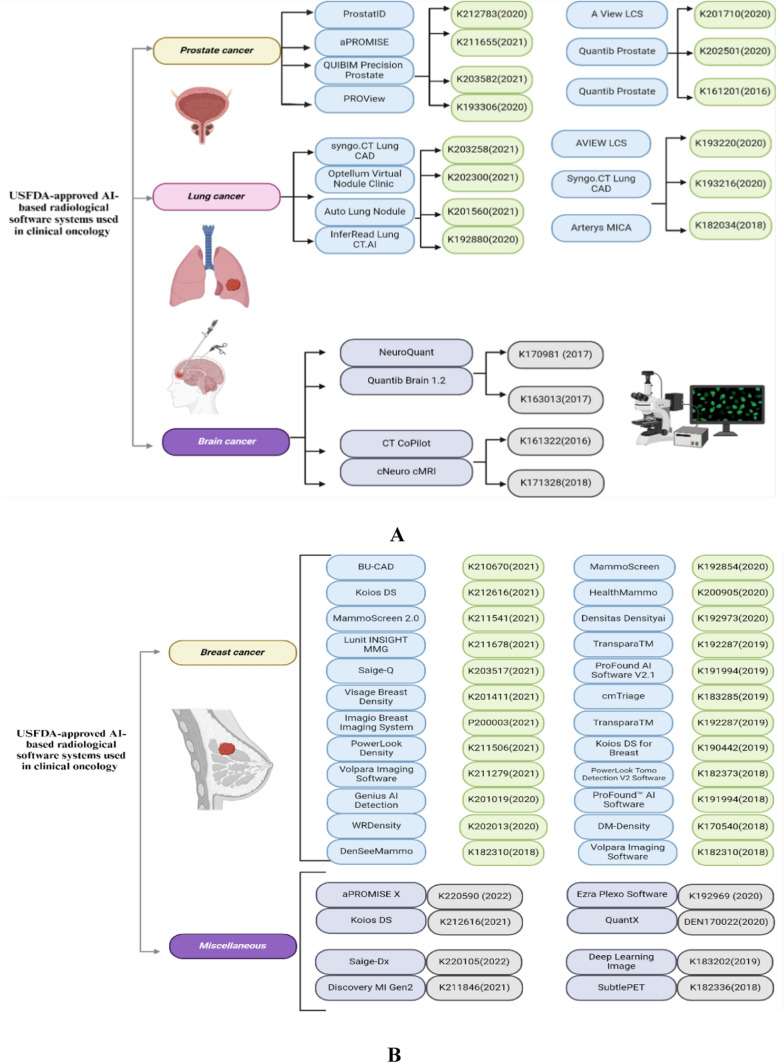
In their extensive review, Belge Bilgin et al. outlined a range of USFDA-approved AI-powered radiological software systems for clinical oncology, covering diagnostic tools for various cancers, including those of the breast, prostate, lung, and brain [170]. These advancements are summarized in Fig. 4A and 4B, displaying the software with their respective USFDA approval numbers and approval years.
Fig. 4.
A USFDA-approved AI-based radiological software systems used in prostate, lung and brain cancers (Biorender Agreement No. OX272ZMRSN). B USFDA-approved AI-based radiological software systems used breast and Miscellaneous cancers (Biorender Agreement No. RH272ZRN58)
Integrating AI into medical imaging processes allows for more precise and efficient diagnoses by automating the detection and interpretation of complex imaging data. This technology is adept at revealing subtle abnormalities that might escape human detection, thus promoting earlier disease diagnosis and better treatment outcomes. For instance, Ng et al. explored the use of Mia version 2.0 from Kheiron Medical Technologies for mammography, demonstrating that AI-assisted double reading was at least as effective as human double reading and, in some cases, even showed superior performance [171].
Radiologists must develop advanced digital skills to integrate and utilize computerized data in medical imaging effectively. As AI continues to evolve, adapting to new technologies and workflows becomes crucial. Mastery of AI-driven tools will enable radiologists to leverage these advancements to improve patient outcomes.
In summary, AI in medical imaging offers substantial benefits by enhancing diagnostic accuracy and efficiency. It supports various aspects of biomedical imaging, from data management to detailed image analysis, requiring radiologists to stay adept with digital technologies to maximize these benefits.
Big data in precision oncology—AI
The introduction of next-generation sequencing (NGS) technology in 2005 has dramatically improved the management of large-scale sequencing data and has become a vital component in the development of targeted therapies [172]. NGS applications in whole-genome, whole-exome, and RNA sequencing help identify altered genes and abnormal cellular pathways, which are crucial in cancer studies and diagnosis [173]. The integration of artificial intelligence (AI) with NGS platforms further enhances our ability to decode complex genetic patterns, facilitating precision oncology by providing insights into specific genetic mutations and their impacts on cancer progression [174]. For instance, Abraham et al. developed MI GPSai, an advanced AI molecular classifier that integrates DNA and RNA sequencing data to accurately predict tumor types across a diverse range of diagnostic categories. By analyzing 77,044 cases from the Caris Molecular Database, which includes samples from 2008 to 2020, they demonstrated the classifier’s effectiveness in identifying various cancers, including those with unknown primary origins [175]. This innovative approach highlights the potential of AI to enhance diagnostic precision in oncology, offering new avenues for the identification and classification of tumors that are often challenging to diagnose.
Furthermore, AI-driven analysis of big data from NGS can uncover novel biomarkers for early detection and prognosis, improving the chances of successful treatment outcomes. Harnessing the power of large-scale genetic data processing is changing the landscape of cancer treatment, moving us toward more precise and patient-centered therapies. Table 3 presents examples of AI-driven discovery of novel biomarkers in cancer.
Table 3.
AI-driven discovery and analysis of novel biomarkers in cancer
| Biomarker | Cancer type | Therapy/Significance | AI used | References |
|---|---|---|---|---|
| BRCA1/2 Mutations | Breast and Ovarian Cancer | Guides the use of PARP inhibitors | Deep Learning Algorithms | [176] |
| Microsatellite Instability-High (MSI-H) / Deficient Mismatch Repair (dMMR) | Colorectal and Various Cancers | Predicts responsiveness to immune checkpoint inhibitors | Machine Learning Models | [177] |
| Tumor Mutational Burden (TMB) | Various Cancers | Indicates likelihood of positive response to immune checkpoint inhibitors | Neural Networks | [178] |
| EGFR Mutations | Lung Cancer | Guides the use of tyrosine kinase inhibitors | AI-Powered NGS Platforms | [179] |
| ALK and ROS1 Rearrangements | Lung Cancer | Targets for ALK inhibitors and ROS1 inhibitors | AI-Based Genomic Analysis Tools | [180] |
| BRAF V600E Mutation | Melanoma and Colorectal Cancer | Target for BRAF inhibitors | Deep Learning Genomic Analysis | [181] |
| PIK3CA Mutations | Hormone Receptor-Positive Breast Cancer | Target for PI3K inhibitors | Machine Learning Algorithms | [182] |
| IDH1/2 Mutations | Gliomas | Used for prognosis and targeted therapy with IDH inhibitors | AI-Enhanced Genetic Sequencing | [183] |
| TP53 Mutations | Breast, Ovarian, Lung Cancer | Important for prognosis and potential targets for novel therapies | AI-Based Mutation Detection Systems | [184] |
| Homologous Recombination Deficiency (HRD) | Ovarian and Breast Cancer | Sensitivity to PARP inhibitors | Deep Learning Algorithms | [185] |
In summary, the synergy between big data, NGS, and AI is paving the way for groundbreaking advancements in precision oncology, enhancing our ability to diagnose, treat, and understand cancer at a molecular level. This collaborative approach signifies a new era in oncology, where treatments are increasingly personalized and outcomes are significantly improved.
AI in digital pathology and drug discovery
ANNs and machine learning algorithms process information through interconnected layers of neurons, akin to the way human brain cells function. These layers include various types such as dense (fully connected), convolutional, pooling, and normalization layers, each designed to perform specific transformations on the data [186]. In the context of digital pathology, customized convolutional layers are particularly useful for processing high-resolution images of tissue samples. These layers can be tailored to identify and analyze features within the images that are indicative of disease, such as abnormal cell shapes, sizes, and patterns [187].
In drug discovery, AI and ML techniques analyze extensive datasets to pinpoint potential drug candidates [188] and predict their efficacy and safety [189]. ANNs excel at learning from existing data, identifying intricate patterns and relationships often overlooked by conventional methods. This can significantly accelerate the drug discovery process by identifying promising compounds earlier in the pipeline and predicting their interactions with biological targets. Table 4 highlights the role of AI in transforming digital pathology and drug discovery.
Table 4.
AI in digital pathology and drug discovery
| Application | Specific example | AI technology used | Impact/Benefits | References |
|---|---|---|---|---|
| Digital Pathology | Tumor Detection and Grading | Deep Learning (e.g., CNNs) | Enhanced accuracy and efficiency in diagnosing cancer | [190] |
| Digital Pathology | Detection of Prostate Cancer | Machine Learning (e.g., SVMs) | Improved detection rates and reduced diagnostic variability | [191] |
| Digital Pathology | Identifying Breast Cancer Subtypes | Neural Networks (e.g., RNNs) | Accurate classification of different cancer subtypes, aiding personalized treatment plans | [192] |
| Drug Discovery | Predicting Drug-Target Interactions | AI-Powered Bioinformatics (e.g., DTIs) | Accelerated identification of potential drug candidates | [193] |
| Drug Discovery | AI-Driven Compound Screening | Machine Learning Algorithms (e.g., Random Forest) | Efficient and high-throughput screening of chemical compounds for drug discovery | [194] |
| Drug Discovery | Personalized Medicine Development | Neural Networks (e.g., LSTM) | Tailored treatment plans based on individual genetic profiles | [195] |
| Drug Discovery | Predicting Drug Synergies | AI-Based Simulation Models (e.g., GANs) | Optimized drug combinations for better therapeutic outcomes | [196, 197] |
Cancer mechanisms by AI
AI technologies are revolutionizing our understanding of cancer mechanisms by enabling detailed analysis and insights into genetic, cellular, and molecular processes. In genetic mutation analysis, AI models like machine learning algorithms (e.g., Random Forest) are crucial in identifying driver mutations that contribute to cancer progression, providing essential data for targeted therapies. Deep learning algorithms such as CNNs enhance our understanding of altered cellular pathways, aiding in the development of targeted treatments [198, 199].
For instance, Warnat-Herresthal et al. demonstrated that ML-based transcriptomics could aid in diagnosing myeloid leukaemia [200]. Similarly, Ben Azzouz et al. utilized an ML approach with transcriptomics data to classify triple-negative breast cancer subtypes, addressing the challenge of heterogeneity in treatment [201]. Additionally, ML-based transcriptomics have been employed in developing prognostic biomarkers for prostate cancer [202], diagnosing colorectal cancer [203], and predicting immune responses [204].
Additionally, AI's role extends to detecting epigenetic changes, where neural networks like RNNs analyze DNA methylation patterns, uncovering how these modifications contribute to cancer. AI-powered image analysis, particularly using CNNs, is instrumental in examining the tumor microenvironment. This is crucial for understanding tumor-immune interactions and developing immunotherapies. For instance, Ye et al. proposed an AI-assisted scoring system called the Tumor Growth Pattern (TGP-I) score to evaluate the relationship between TGP and tumor-infiltrating lymphocytes at the invasive margin, and to predict its prognostic value for stratifying colorectal cancer patients [205]. Their findings demonstrated that the TGP-I score could independently predict prognosis, with higher scores correlating with poorer survival outcomes.
Moreover, AI-powered evolutionary models trace clonal evolution in tumors, offering insights into tumor diversity and progression. This is essential for understanding cancer dynamics and enhancing treatment strategies. These AI-driven advancements in cancer research exemplify the transformative potential of integrating advanced computational techniques into oncology, leading to more accurate, efficient, and personalized cancer treatments [206].
Furthermore, Fuzzy Cognitive Maps (FCMs), blending fuzzy logic with ANNs, aid in decision-making within complex systems characterized by high levels of uncertainty. Papageorgiou et al. developed two FCMs specifically for the intricate task of detecting bladder tumors, known for their complex categorization. They applied the Active Hebbian Learning (AHL) algorithm to train one FCM and the Nonlinear Hebbian Learning (NHL) algorithm to the other. Both algorithms achieved high precision in identifying malignant tumors than benign tumors [207–209].
These AI methodologies facilitate the development of comprehensive tumor atlases, allowing healthcare providers worldwide to share diagnostic and therapeutic data. This collective knowledge base enhances the ability to identify effective treatment protocols and track patient outcomes over time. Moreover, AI applications in oncology help automate the analysis of high-throughput data, reducing the likelihood of human error and increasing the reproducibility of results. Overall, integrating AI into understanding cancer mechanisms and optimizing treatment strategies represents a significant advancement in personalized medicine.
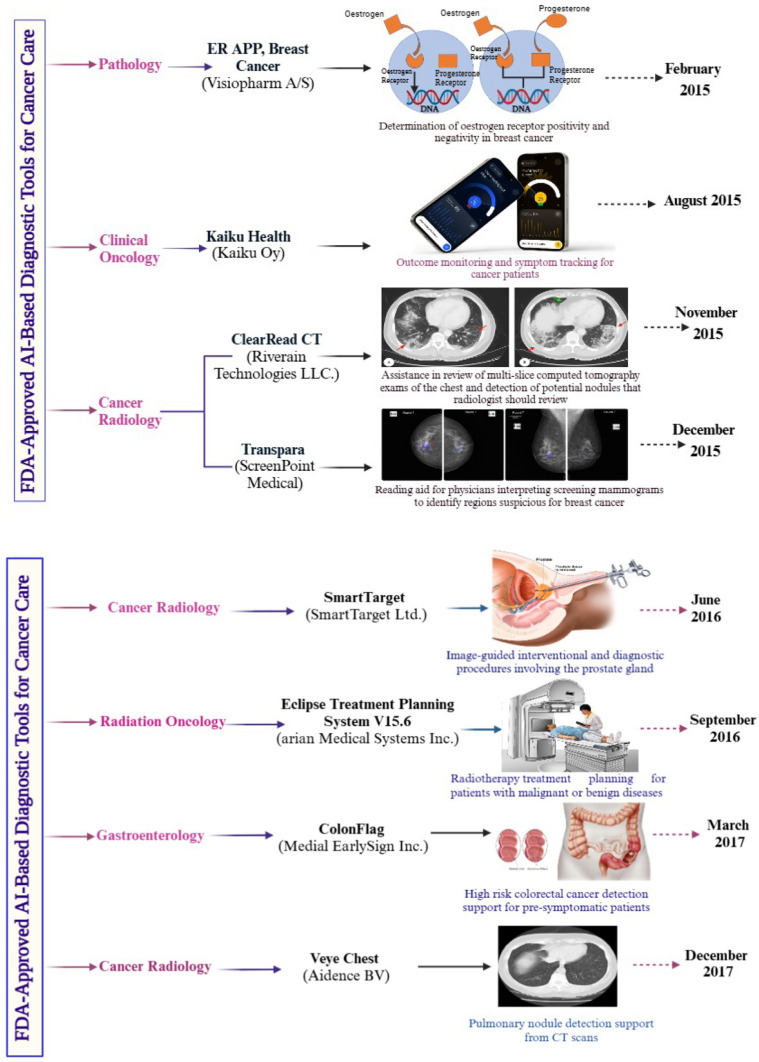
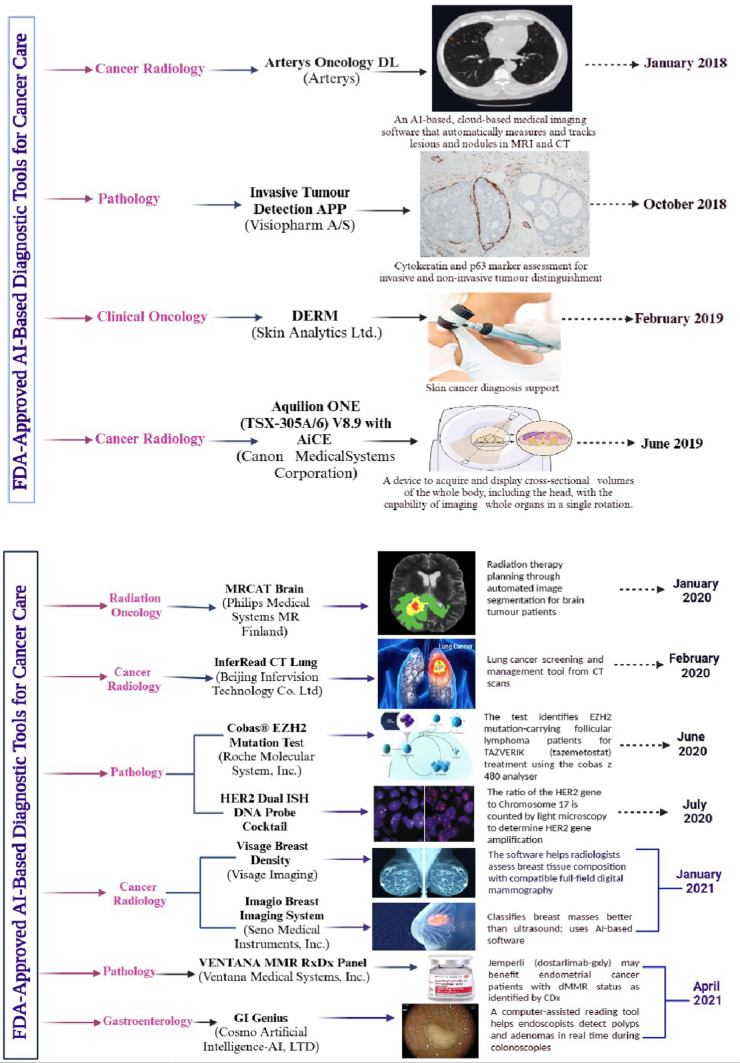
Figure 5 highlights FDA-approved AI-based diagnostic tools for cancer care as of 2021. The figure provides information on the approval month, device name, description, and specific applications across oncology specialties, including pathology, radiology, clinical oncology, radiation oncology, and gastroenterology. These AI tools exemplify advancements in diagnostic accuracy, early cancer detection, and personalized treatment planning.
Fig. 5.
FDA-approved AI-based diagnostic tools for cancer care: enhancing diagnosis and personalized treatment (Biorender Agreement Numbers. LL28329KSD, AQ282ZMGYI, GE28302QB7 & RE2830N3V5)
Figure 5 presents a curated selection of AI-driven diagnostic devices that received FDA approval for use in cancer care. Each device is listed with its corresponding approval date, name, a brief description, and its intended application within various oncology domains. AI algorithms in these tools are designed to analyze medical images, pathology slides, genomic data, and other clinical information to assist healthcare professionals in making accurate and timely diagnoses.
Notable applications include enhancing imaging interpretation in radiology, supporting pathologists in detecting malignancies, assisting oncologists in treatment decision-making, and improving cancer screening programs. For example, AI systems can identify suspicious lesions in mammograms, classify tumor subtypes in pathology slides, and predict therapeutic responses using patient data. By streamlining diagnostic workflows and reducing human error, these technologies contribute significantly to personalized cancer care.
This figure underscores the growing role of AI in transforming cancer diagnostics and management, paving the way for further innovations in oncology.
AI in surgery
AI applications in surgery are rapidly advancing, transforming the landscape of surgical procedures and improving patient outcomes. AI-assisted surgeries have demonstrated significant benefits, including a 30.6% reduction in the incidence of mastectomy, showcasing AI's capacity to improve precision and minimize the need for invasive procedures [210].
AI also plays a crucial role in image-guided biopsy procedures, where ML models analyze imaging data and pathology reports to accurately predict the malignancy of suspicious nodules. This reduces the necessity for unnecessary surgical resections, sparing patients from potential complications and optimizing the utilization of medical resources. Elia et al. conducted an analysis of radiomic features extracted from CT and PET-CT findings of 71 patients with Solitary Pulmonary Nodules (SPN) who had undergone initial pulmonary resection. Three distinct ML algorithms: functional tree, Rep Tree, and J48 were applied. Histological examination revealed malignancy in 64.8% of nodules, with the J48 model demonstrating the highest predictive value (AUC 0.9) [211].
Moreover, AI-powered robotic surgery systems, like the da Vinci Surgical System, have become increasingly prevalent. These systems offer surgeons enhanced dexterity, precision, and control, facilitating minimally invasive surgeries that lead to shorter recovery times and reduced risk of complications. AI algorithms continuously learn from surgical data to improve the performance of these robotic systems, adapting to the unique anatomy and needs of each patient [212].
The integration of AI in surgery extends beyond the operating room. Preoperative planning tools use AI to simulate different surgical scenarios, helping surgeons to anticipate potential challenges and plan the most effective approach. During surgery, AI can provide real-time feedback and assist with decision-making, ensuring that the procedure stays on track and any deviations are promptly addressed [213].
Postoperatively, AI algorithms monitor patient recovery, analyzing data from wearable devices and electronic records to detect early signs of adverse events. This continuous monitoring allows for timely interventions, improving post-operative treatment strategies and reducing hospital readmissions [214]. Figure 6 illustrates the anticipated uses of AI technologies in electronic medical care and oncology, highlighting the potential of AI to transform various aspects of healthcare delivery.
Fig. 6.
AI applications in digital health care and oncology. Source: [Compiled by the author Eashwar Sai Komarla Rajasekhar]. This figure presents various applications of artificial intelligence (AI) in digital health care and oncology, illustrating how AI technologies are transforming cancer diagnosis, treatment, and patient management
Ethical aspects of AI in therapy
Evaluating the potential risks and benefits of any technology is crucial, and AI in cancer nanotherapy is no exception. A significant challenge in deep learning is the interpretability issue, referring to the difficulty in explaining or understanding AI algorithms [215, 216]. Explainable AI allows humans to understand how algorithms function, including the values they encode [217]. Currently, less explainable algorithms tend to be more accurate, raising the question of whether accuracy and explainability must be mutually exclusive or if both can be achieved simultaneously.
Human clinicians are held accountable for their decisions, despite sometimes imperfect explanations, both legally and ethically. They can typically provide rationale for their actions and are obligated to do so when required. In contrast, healthcare AI systems may make personalized diagnostic, prognostic, and treatment decisions that are less transparent. This contrasts with traditional decision-support tools, which use clear formulas for general recommendations. Explainable AI in medicine is actively researched, and integrating AI into clinical practice will require robust quality assurance processes to ensure consistency, reviewability, and comprehension of results. Clear guidelines on explainability are crucial before implementation [218].
AI has the potential to significantly impact areas with radiologist shortages and enhance screening programs. By analyzing imaging data, AI can uncover new imaging biomarkers, potentially revolutionizing therapy and guiding further research. However, challenges remain regarding standardization, transparency, ethics, regulations, training, accreditation, and safety. For instance, the Armed Forces Medical Services, with its diverse units and roles, could integrate AI-enabled radiological services to support small, remote hospitals and alleviate the workload in larger facilities. Overcoming obstacles such as IT integration, funding, software and hardware updates, training, and cybersecurity is crucial for the successful adoption of AI [219].
Despite the significant progress, practical AI applications in nanooncology face many obstacles. AI cannot be a foolproof solution to all problems nor entirely replace human roles. It should complement the insights and understanding that humans provide. Maintaining human oversight and control is vital.
Another ethical concern is the potential for AI to make poor decisions and mishandle risks. Biases in data or flaws in models can lead to unintended consequences, necessitating careful oversight and regulation to ensure reliability and prevent harm. Transparency in AI decision-making and accountability for errors are crucial for maintaining trust and efficacy in AI-driven nanotherapies.
Furthermore, the potential for AI to replace human interaction in therapeutic contexts raises questions about the quality of cancer care. While AI can provide valuable support, the emotional and psychological aspects of human care are difficult to replicate. Maintaining holistic and empathetic cancer healthcare practices requires ensuring that AI complements, rather than replaces, human caregivers [220–222].
The ethical aspects of AI in therapy are complex, involving patient dependence, accountability, decision-making reliability, and the balance between AI and human interaction. It's crucial to address these ethical challenges to maximize the benefits of AI while minimizing risks in healthcare.
Case studies of AI in cancer treatment
IBM Watson for Oncology
IBM Watson for Oncology (WFO) uses AI to analyze extensive medical literature, clinical trial data, and patient records, offering evidence-based treatment recommendations to oncologists. In a large-scale study across hospitals in China, WFO demonstrated over 90% concordance with expert oncologists for breast, lung, and colorectal cancers, accelerating decision-making and expanding access to advanced cancer care in resource-limited settings.
Zhou et al. assessed the alignment between WFO’s recommendations and actual clinical decisions at a Chinese cancer center, aiming to compare treatment approaches between China and the U.S. The retrospective study analyzed data from 362 cancer patients treated between April and October 2017. WFO categorized its recommendations as "recommended," "for consideration," or "not recommended," with concordance defined when oncologists' decisions aligned with the first two categories.
The highest concordance was observed in ovarian cancer (96%), followed by lung and breast cancers, both slightly above 80%. Rectal cancer had a 74% concordance rate, while colon and cervical cancers each reached 64%. In contrast, gastric cancer showed a markedly low concordance of 12%, with 88% of cases treated based on physician discretion.
The study concluded that concordance rates varied across cancer types, with gastric cancer showing the lowest alignment. Differences in cancer incidence and pharmaceutical availability were identified as primary reasons for the discordance [223].
Tempus xT platform
Tempus leverages AI-driven genomic analysis to personalize cancer treatment by integrating molecular data with clinical information, providing actionable insights for oncologists. Through collaborations with institutions like the Mayo Clinic and other leading cancer centers, Tempus has identified biomarkers that guide targeted therapies, significantly improving outcomes in lung and pancreatic cancers [224].
In a study by Beaubier et al., 500 patient samples representing various tumor types were analyzed using the Tempus xT platform, which applies DNA-seq, RNA-seq, and immunological biomarkers. The integration of tumor and germline data enhanced mutation detection and reduced false positives, while RNA-seq further improved gene fusion detection and cancer type classification.
Using DNA-seq alone, 29.6% of patients were matched to precision therapies backed by strong evidence or well-powered studies. This proportion increased to 43.4% with the addition of RNA-seq and immunotherapy biomarker data. When clinical criteria were incorporated, 76.8% of patients were matched to at least one relevant clinical trial based on biomarker insights from the xT assay.
These findings emphasize the value of comprehensive molecular profiling. By combining tumor-normal and transcriptome sequencing, the Tempus platform offers a more effective approach than tumor-only DNA panel testing for identifying personalized therapies and clinical trial opportunities, ultimately enhancing patient care [225].
PathAI
PathAI applies AI algorithms to analyze pathology slides, assisting pathologists in diagnosing cancers with greater accuracy and efficiency. Collaborations with institutions like the Dana-Farber Cancer Institute have demonstrated reduced diagnostic errors and faster diagnoses for breast and prostate cancers. The AI system enhances cancer grading accuracy, directly influencing treatment decisions.
Novartis has also partnered with PathAI to leverage AI in pathology, aiming to uncover hidden patterns in cancer tissue slides. PathAI’s system was trained using 400 annotated pathology images from breast and lung cancer patients, enabling it to distinguish tumors from normal tissue and identify various cell types. By analyzing cell distributions and abundances, the AI can potentially predict patient responses to immunotherapy. Unlike the labor-intensive process faced by pathologists, AI provides rapid, large-scale analysis, accelerating drug development and improving cancer care [226].
In another collaboration, PathAI partnered with Aster Insights to accelerate oncology discoveries through curated multimodal datasets. By integrating PathAI’s PathExplore™ AI tumor microenvironment (TME) quantification panel with Aster Insights’ Avatar platform, the partnership enables comprehensive analysis of histopathology images alongside clinical and molecular data. With access to over 400,000 cancer patient samples from the Oncology Research Information Exchange Network® (ORIEN®), PathExplore uses AI to detect and classify millions of cells and tissue regions in hematoxylin and eosin (H&E) stained slides. This analysis provides critical insights into immune cell infiltration and complex tissue structures. Combined with genomic data from Aster’s platform, researchers can better understand cancer biology and predict patient responses to therapies. PathAI and Aster Insights offer these joint solutions to biopharma companies to accelerate drug discovery, identify novel biomarkers, and advance personalized cancer treatments [227].
Aidoc
Aidoc’s AI-powered imaging analysis platform aids radiologists in detecting cancerous lesions from CT and MRI scans. In hospitals across the United States, Aidoc has been deployed to identify lung nodules, brain metastases, and liver tumors. This real-time assistance has reduced diagnostic turnaround times and improved early detection rates, leading to more timely cancer interventions.
Wiklund and Medson conducted a retrospective single-center cross-sectional study to assess the impact of Aidoc Brief Case and Aidoc Medical algorithms on report turnaround time, time to treatment, and detection rate in patients with cancer-associated incidental pulmonary embolism (iPE). The study included adult cancer patients before (July 1, 2018, to June 30, 2019) and after (November 1, 2020, to April 30, 2021) the implementation of AI algorithms for iPE detection and triage. The results showed a significant increase in the reported iPE prevalence post-AI implementation (2.5% vs. 0.8%, P < 0.001). Additionally, both report turnaround time (median 0.66 h vs. 24.68 h, P < 0.001) and time to treatment (median 0.98 h vs. 28.05 h, P < 0.001) were markedly reduced. The study concluded that AI integration effectively enhanced iPE detection rates and expedited diagnosis and treatment in cancer patients [228].
OncoAI
OncoAI is an AI-powered predictive analytics platform that models cancer progression and treatment responses. By integrating patient-specific genomic data with real-world clinical outcomes, OncoAI provides oncologists with personalized predictions. Real-world implementations in academic medical centers have demonstrated improved survival rates and reduced treatment-related complications in cases of advanced colorectal and ovarian cancers.
In a study led by Sako et al., Onc.AI's deep learning radiomic biomarker was developed and validated using both real-world data (RWD) and clinical trial data to estimate patient responses to immune checkpoint inhibitor (ICI) therapies.
The study collected retrospective data from 1829 advanced non–small cell lung cancer (NSCLC) patients across 10 academic and community institutions in the United States and Europe. The data sets included a discovery set (Data Set A-Discovery, n = 1173) and an independent test set (Data Set B, n = 458). Onc.AI's radiomic pipeline employed a deep learning feature extractor and a survival model to generate the computed tomography (CT) response score (CTRS) using pretreatment routine CT or positron emission tomography (PET)-CT scans. An enhanced version of the CTRS (eCTRS) incorporated additional clinical factors such as age, sex, treatment line, and lesion annotations.
The results demonstrated the biomarker's ability to identify patients likely to benefit from ICI therapy. In the RWD Test Data Set B, the CTRS predicted a high probability of response with a progression-free survival (PFS) hazard ratio (HR) of 0.46 (95% CI 0.26 to 0.82) and an overall survival (OS) HR of 0.50 (95% CI 0.28 to 0.92) in the first-line ICI monotherapy cohort. Further validation using a clinical trial data set (Data Set C, n = 54) evaluating the PD-1 inhibitor sasanlimab showed an adjusted OS HR of 0.33 (95% CI 0.14 to 0.91), reinforcing the biomarker's predictive accuracy.
Onc.AI's innovative approach using deep learning radiomic biomarkers offers a transformative solution in personalized cancer care by providing actionable insights into treatment responses. This case exemplifies how AI-powered solutions can enhance clinical decision-making, optimize therapeutic strategies, and ultimately improve patient outcomes in oncology [229].
Regulatory hurdles and clinical translation challenges
The integration of nanomedicine and AI in cancer health care offers transformative potential. However, the clinical translation of these technologies is fraught with significant regulatory and practical challenges that must be addressed to ensure their successful adoption.
Regulatory challenges in nanomedicine
Nanomedicine products often exhibit complex behaviors due to their nanoscale properties, which can lead to unpredictable interactions within biological systems. Regulatory agencies, including the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA), require extensive preclinical and clinical data to evaluate the safety, efficacy, and quality of nanomedicine products. The lack of standardized characterization protocols and comprehensive understanding of nanomaterial toxicity further complicates the approval process.
Additionally, regulatory frameworks must accommodate the diverse nature of nanomedicine formulations, including nanoparticles, liposomes, and dendrimers. Establishing clear guidelines for Good Manufacturing Practices (GMP) and ensuring consistency in production are essential. Furthermore, the need for robust in vitro and in vivo models to predict human responses presents a major challenge in regulatory assessments [230].
Clinical translation challenges
Translating nanomedicine from bench to bedside involves navigating numerous obstacles. Clinical trials for nanomedicine therapies require careful consideration of dosage, biodistribution, and long-term effects. The heterogeneity of cancer types further complicates the design of effective clinical trials, necessitating the use of stratified patient populations and personalized treatment approaches.
Furthermore, manufacturing scalability and cost-effectiveness remain significant hurdles. Nanoparticle formulations often demand specialized equipment and rigorous quality control measures, driving up production costs. Ensuring the reproducibility of nanomedicine products across different batches is critical for regulatory approval and clinical adoption [231].
Regulatory and translation challenges in AI
In the realm of AI applications, regulatory challenges revolve around the transparency, reliability, and interpretability of algorithms. AI-driven diagnostic tools and predictive models require continuous validation using diverse datasets to minimize biases and ensure generalizability. Regulatory agencies are increasingly developing guidelines for AI systems, emphasizing explainability and real-world performance.
Moreover, the integration of AI into clinical workflows demands seamless interoperability with existing electronic health records (EHRs) and medical devices. Regulatory bodies are also concerned with data privacy and security, particularly given the sensitive nature of patient information used in AI model training and validation [232].
AI biases in clinical decision making and data security risk
Artificial Intelligence (AI) has emerged as a transformative force in cancer clinical decision-making, enhancing diagnostic accuracy, predicting patient outcomes, and recommending personalized treatments. However, biases and data security risks present significant challenges. AI biases often originate from non-representative training data, leading to inaccurate or discriminatory results. Marginalized populations are disproportionately affected, facing misdiagnoses and suboptimal treatment recommendations. Additionally, algorithmic bias may result from flawed model design, reinforcing existing disparities in healthcare [233].
In cancer care, biased AI systems can lead to delayed diagnoses and ineffective therapies, undermining patient trust. Promoting diversity in datasets, conducting algorithm audits, and applying fairness metrics are essential steps to mitigate bias. Collaboration among oncologists, data scientists, and ethicists can ensure transparent and equitable AI applications [234].
Data security is another critical concern in AI-driven oncology. Patient data, containing sensitive medical information, is vulnerable to cyberattacks and privacy breaches. Malicious manipulation of AI training data can compromise model integrity. Implementing robust cybersecurity measures, such as encryption, data anonymization, and access controls, is crucial to safeguard information. AI-powered monitoring systems can provide real-time threat detection and response [235].
Furthermore, adherence to regulations like the Health Insurance Portability and Accountability Act (HIPAA) and the General Data Protection Regulation (GDPR) is necessary for ethical data management. By addressing biases and strengthening data security, AI can fulfill its potential to provide more accurate, equitable, and secure cancer care, ultimately improving patient outcomes and advancing healthcare innovation [236].
Here are some examples to illustrate AI biases and data security risks in cancer clinical decision-making:
-
i.
AI biases in cancer diagnosis
In breast cancer screening, AI models trained predominantly on data from Caucasian populations have shown lower accuracy when diagnosing breast cancer in African American and Asian women. This can lead to misdiagnoses or delayed treatment, contributing to health disparities [237].
-
ii.
Bias in treatment recommendations
AI systems used for recommending cancer therapies may prioritize certain treatments based on data from clinical trials that underrepresent minority groups or low-income populations. For instance, a model may recommend immunotherapy that is less effective for certain genetic backgrounds [238].
-
iii.
Data manipulation and model corruption
Cyberattacks like data poisoning can manipulate training datasets used in cancer AI applications. Malicious actors can introduce biased or false data, leading to inaccurate diagnoses or harmful treatment suggestions [239].
-
iv.
Privacy breaches in oncology data
A cancer hospital using AI for patient management experienced a data breach exposing sensitive information. Without robust encryption and access control, attackers accessed patient medical histories, compromising both privacy and regulatory compliance [240].
-
v.
Algorithmic transparency issues
A proprietary AI tool used for predicting cancer recurrence refused to disclose its algorithmic decision-making process. This lack of transparency prevented oncologists from understanding potential biases and verifying treatment recommendations [241].
These examples emphasize the importance of ensuring diversity in datasets, maintaining algorithm transparency, and implementing stringent data security measures to mitigate biases and protect patient data in AI-driven cancer care.
Path forward
To overcome these challenges, a collaborative effort among researchers, clinicians, regulatory agencies, and industry stakeholders is essential. Developing standardized frameworks for nanomedicine characterization and AI model validation will facilitate smoother regulatory approval processes. Additionally, fostering adaptive regulatory pathways that allow iterative improvements based on real-world evidence can expedite the clinical translation of these technologies.
Establishing multidisciplinary regulatory advisory boards and promoting transparent dialogue between developers and regulators can further bridge the gap between innovation and implementation. Ultimately, addressing these regulatory and clinical translation challenges will be crucial to realizing the full potential of nanomedicine and AI in revolutionizing cancer health care.
Conclusion
Nanomedicine alone has not achieved the desired outcomes in improving treatment efficacy. Artificial neural networks and deep learning can significantly aid clinicians in cancer diagnosis and research by selecting the best nanomedicine combinations and maintaining appropriate drug levels in the blood or target site. These tools facilitate continuous updates and optimal decision-making. Importantly, the deployment of AI in clinics will not replace radiologists and other medical professionals. AI cannot operate entirely autonomously and will always require human engagement. However, if AI-enabled nanomedicine proves to be more effective, safer, and provides better treatment outcomes than traditional cancer nanomedicine therapy, it will likely gain widespread adoption.
Our review includes various nanoparticle types, showcasing the potential of AI and its clinical applications in cancer through nanomedicine. Reproducible computer-assisted drug development, with its increased specificity and lower costs, is a promising tool for future drug development. Additionally, big data analytics and machine learning tools can manage and evaluate large collections of complex health data, minimizing limitations and reducing false positives.
Acknowledgements
The authors are thankful to the Kampala International University and Meenakshi Faculty of Pharmacy, MAHER University for providing necessary facilities to carry out the research work.
Author contributions
PS, RKK, and SPNB contributed to the design of the study, data collection, and writing of the manuscript. SPNB, ESKR, ZE and AAKJ contributed to the study design, data collection, and manuscript revision. PS and RKK critically reviewed the manuscript. All authors approved the final manuscript.
Funding
The author(s) received no specific funding for this work.
Data availability
The datasets/information used for this study is available on within the manuscript.
Declarations
I hereby declare that this submission is entirely my own work, in my own words, and that all sources used in researching it are fully acknowledged and all quotations properly identified.
Ethics approval and consent to participate
Not applicable.
Consent for publication
All the authors have read and agreed to the final copy of the finding as contained in the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Nayak MG, George A, Vidyasagar MS, Mathew S, Nayak S, Nayak BS, et al. Quality of life among cancer patients. Indian J Palliat Care. 2017;23:445–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Prager GW, Braga S, Bystricky B, Qvortrup C, Criscitiello C, Esin E, et al. Global cancer control: responding to the growing burden, rising costs and inequalities in access. ESMO Open. 2018;3: e000285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen W, Zhou S, Ge L, Wu W, Jiang X. Translatable high drug loading drug delivery systems based on biocompatible polymer nanocarriers. Biomacromol. 2018;19:1732–45. [DOI] [PubMed] [Google Scholar]
- 4.Su S, Kang PM. Recent advances in nanocarrier-assisted therapeutics delivery systems. Pharmaceutics. 2020;12:837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou Q, Zhang L, Yang T, Wu H. Stimuli-responsive polymeric micelles for drug delivery and cancer therapy. Int J Nanomedicine. 2018;13:2921–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jain A, Bhattacharya S. Recent advances in nanomedicine preparative methods and their therapeutic potential for colorectal cancer: a critical review. Front Oncol. 2023;13:1211603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferlay J, Colombet M, Soerjomataram I, Parkin DM, Piñeros M, Znaor A, et al. Cancer statistics for the year 2020: an overview. Intl Journal of Cancer. 2021;149:778–89. [DOI] [PubMed] [Google Scholar]
- 8.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J Clin. 2021;71:209–49. [DOI] [PubMed] [Google Scholar]
- 9.Siegel RL, Giaquinto AN, Jemal A. Cancer statistics, 2024. CA Cancer J Clin. 2024;74:12–49. [DOI] [PubMed] [Google Scholar]
- 10.World Health Organization. Latest global cancer data: Cancer burden rises to 18.1 million new cases and 9.6 million cancer deaths in 2018. 2018.
- 11.Sufyan M, Shokat Z, Ashfaq UA. Artificial intelligence in cancer diagnosis and therapy: current status and future perspective. Comput Biol Med. 2023;165: 107356. [DOI] [PubMed] [Google Scholar]
- 12.Johnson KB, Wei W-Q, Weeraratne D, Frisse ME, Misulis K, Rhee K, et al. Precision medicine, AI, and the future of personalized health care. Clin Transl Sci. 2021;14:86–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vidhya KS, Sultana A, Naveen Kumar M, Rangareddy H. Artificial intelligence’s impact on drug discovery and development from bench to bedside. Cureus. 2023;15: e47486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Visan AI, Negut I. Integrating artificial intelligence for drug discovery in the context of revolutionizing drug delivery. Life (Basel). 2024;14:233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yousfan A, Al Rahwanji MJ, Hanano A, Al-Obaidi H. A comprehensive study on nanoparticle drug delivery to the brain: application of machine learning techniques. Mol Pharm. 2024;21:333–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jain KK. Nanooncology. In: The Handbook of Nanomedicine. New York, NY: Springer New York; 2017. p. 321–420.
- 17.Alrushaid N, Khan FA, Al-Suhaimi EA, Elaissari A. Nanotechnology in cancer diagnosis and treatment. Pharmaceutics. 2023;15:1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chehelgerdi M, Chehelgerdi M, Allela OQB, Pecho RDC, Jayasankar N, Rao DP, et al. Progressing nanotechnology to improve targeted cancer treatment: overcoming hurdles in its clinical implementation. Mol Cancer. 2023;22:169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zeineldin R, Syoufjy J. Cancer nanotechnology: opportunities for prevention, diagnosis, and therapy. In: Zeineldin R, editor. Cancer nanotechnology. New York: Springer, New York; 2017. p. 3–12. [DOI] [PubMed] [Google Scholar]
- 20.Pande S. Liposomes for drug delivery: review of vesicular composition, factors affecting drug release and drug loading in liposomes. Artif Cells, Nanomed Biotechnol. 2023;51:428–40. [DOI] [PubMed] [Google Scholar]
- 21.Nsairat H, Khater D, Sayed U, Odeh F, Al Bawab A, Alshaer W. Liposomes: structure, composition, types, and clinical applications. Heliyon. 2022;8: e09394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang S, Chen Y, Guo J, Huang Q. Liposomes for tumor targeted therapy: a review. Int J Mol Sci. 2023;24:2643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fulton MD, Najahi-Missaoui W. Liposomes in cancer therapy: how did we start and where are we now. Int J Mol Sci. 2023;24:6615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.England C, Ng C, Berkel V, Frieboes H. A review of pharmacological treatment options for lung cancer: emphasis on novel nanotherapeutics and associated toxicity. CDT. 2015;16:1057–87. [DOI] [PubMed] [Google Scholar]
- 25.Cho E, Mun S-J, Jeon M, Kim HK, Baek H, Ham YS, et al. Tumor-targeted liposomes with platycodin D2 promote apoptosis in colorectal cancer. Mater Today Bio. 2023;22: 100745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang Z, Mo H, He Z, Chen A, Cheng P. Extracellular vesicles as an emerging drug delivery system for cancer treatment: current strategies and recent advances. Biomed Pharmacother. 2022;153: 113480. [DOI] [PubMed] [Google Scholar]
- 27.Yang T, Fogarty B, LaForge B, Aziz S, Pham T, Lai L, et al. Delivery of small interfering RNA to inhibit vascular endothelial growth factor in zebrafish using natural brain endothelia cell-secreted exosome nanovesicles for the treatment of brain cancer. AAPS J. 2017;19:475–86. [DOI] [PubMed] [Google Scholar]
- 28.Irmer B, Chandrabalan S, Maas L, Bleckmann A, Menck K. Extracellular vesicles in liquid biopsies as biomarkers for solid tumors. Cancers. 2023;15:1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Beetler DJ, Di Florio DN, Bruno KA, Ikezu T, March KL, Cooper LT, et al. Extracellular vesicles as personalized medicine. Mol Aspects Med. 2023;91: 101155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Terstappen GC, Meyer AH, Bell RD, Zhang W. Strategies for delivering therapeutics across the blood–brain barrier. Nat Rev Drug Discov. 2021;20:362–83. [DOI] [PubMed] [Google Scholar]
- 31.Palazzolo S, Memeo L, Hadla M, Duzagac F, Steffan A, Perin T, et al. Cancer extracellular vesicles: next-generation diagnostic and drug delivery nanotools. Cancers. 2020;12:3165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jayaseelan VP. Emerging role of exosomes as promising diagnostic tool for cancer. Cancer Gene Ther. 2020;27:395–8. [DOI] [PubMed] [Google Scholar]
- 33.Han J, Kim S, Hwang YH, Kim SA, Lee Y, Kim J, et al. Novel personalized cancer vaccine using tumor extracellular vesicles with attenuated tumorigenicity and enhanced immunogenicity. Adv Sci. 2024;11:2308662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ding Z, Jiang Y, Liu X. Nanoemulsions-Based Drug Delivery for Brain Tumors. In: Nanotechnology-Based Targeted Drug Delivery Systems for Brain Tumors. Elsevier; 2018. p. 327–58.
- 35.Mushtaq A, Mohd Wani S, Malik AR, Gull A, Ramniwas S, Ahmad Nayik G, et al. Recent insights into nanoemulsions: their preparation, properties and applications. Food Chemistry: X. 2023;18: 100684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Din FU, Aman W, Ullah I, Qureshi OS, Mustapha O, Shafique S, et al. Effective use of nanocarriers as drug delivery systems for the treatment of selected tumors. Int J Nanomed. 2017;12:7291–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ganta S, Talekar M, Singh A, Coleman TP, Amiji MM. Nanoemulsions in translational research-opportunities and challenges in targeted cancer therapy. AAPS PharmSciTech. 2014;15:694–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ganta S, Singh A, Rawal Y, Cacaccio J, Patel NR, Kulkarni P, et al. Formulation development of a novel targeted theranostic nanoemulsion of docetaxel to overcome multidrug resistance in ovarian cancer. Drug Deliv. 2016;23:968–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kaur H, Kaur K, Singh A, Bedi N, Singh B, Alturki MS, et al. Frankincense oil-loaded nanoemulsion formulation of paclitaxel and erucin: a synergistic combination for ameliorating drug resistance in breast cancer: In vitro and in vivo study. Front Pharmacol. 2022;13:1020602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bugno J, Hsu H-J, Hong S. Tweaking dendrimers and dendritic nanoparticles for controlled nano-bio interactions: potential nanocarriers for improved cancer targeting. J Drug Target. 2015;23:642–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pérez-Ferreiro M, Abelairas AM, Criado A, Gómez IJ, Mosquera J. Dendrimers: exploring their wide structural variety and applications. Polymers. 2023;15:4369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Madaan K, Kumar S, Poonia N, Lather V, Pandita D. Dendrimers in drug delivery and targeting: drug-dendrimer interactions and toxicity issues. J Pharm Bioallied Sci. 2014;6:139–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McMahon MT, Bulte JWM. Two decades of dendrimers as versatile MRI agents: a tale with and without metals. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2018;10: e1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kaurav M, Ruhi S, Al-Goshae HA, Jeppu AK, Ramachandran D, Sahu RK, et al. Dendrimer: an update on recent developments and future opportunities for the brain tumors diagnosis and treatment. Front Pharmacol. 2023;14:1159131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kharwade R, More S, Warokar A, Agrawal P, Mahajan N. Starburst pamam dendrimers: synthetic approaches, surface modifications, and biomedical applications. Arab J Chem. 2020;13:6009–39. [Google Scholar]
- 46.Pavelić K, Kraljević Pavelić S, Bulog A, Agaj A, Rojnić B, Čolić M, et al. Nanoparticles in medicine: current status in cancer treatment. Int J Mol Sci. 2023;24:12827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vines JB, Yoon J-H, Ryu N-E, Lim D-J, Park H. Gold nanoparticles for photothermal cancer therapy. Front Chem. 2019;7:167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bharti C, Nagaich U, Pal AK, Gulati N. Mesoporous silica nanoparticles in target drug delivery system: a review. Int J Pharm Investig. 2015;5:124–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Huang K-W, Hsu F-F, Qiu JT, Chern G-J, Lee Y-A, Chang C-C, et al. Highly efficient and tumor-selective nanoparticles for dual-targeted immunogene therapy against cancer. Sci Adv. 2020;6: eaax5032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pugazhendhi A, Edison TNJI, Karuppusamy I, Kathirvel B. Inorganic nanoparticles: a potential cancer therapy for human welfare. Int J Pharm. 2018;539:104–11. [DOI] [PubMed] [Google Scholar]
- 51.Uner M, Yener G. Importance of solid lipid nanoparticles (SLN) in various administration routes and future perspectives. Int J Nanomed. 2007;2:289–300. [PMC free article] [PubMed] [Google Scholar]
- 52.Sivadasan D, Ramakrishnan K, Mahendran J, Ranganathan H, Karuppaiah A, Rahman H. Solid lipid nanoparticles: applications and prospects in cancer treatment. Int J Mol Sci. 2023;24:6199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Khizar S, Alrushaid N, Alam Khan F, Zine N, Jaffrezic-Renault N, Errachid A, et al. Nanocarriers based novel and effective drug delivery system. Int J Pharm. 2023;632: 122570. [DOI] [PubMed] [Google Scholar]
- 54.Raut ID, Doijad RC, Mohite SK, Manjappa AS. Preparation and characterization of solid lipid nanoparticles loaded with cisplatin. J Drug Delive Ther. 2018;8:125–31. [Google Scholar]
- 55.Aldawsari HM, Singh S. Rapid microwave-assisted cisplatin-loaded solid lipid nanoparticles: synthesis characterization and anticancer study. Nanomaterials. 2020;10:510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.German-Cortés J, Vilar-Hernández M, Rafael D, Abasolo I, Andrade F. Solid lipid nanoparticles: multitasking nano-carriers for cancer treatment. Pharmaceutics. 2023;15:831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Son KH, Hong JH, Lee JW. Carbon nanotubes as cancer therapeutic carriers and mediators. Int J Nanomed. 2016;11:5163–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang W, Zhang Z, Zhang Y. The application of carbon nanotubes in target drug delivery systems for cancer therapies. Nanoscale Res Lett. 2011;6:555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hernández-Rivera M, Zaibaq NG, Wilson LJ. Toward carbon nanotube-based imaging agents for the clinic. Biomaterials. 2016;101:229–40. [DOI] [PubMed] [Google Scholar]
- 60.Singh R, Torti SV. Carbon nanotubes in hyperthermia therapy. Adv Drug Deliv Rev. 2013;65:2045–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sheikhpour M, Department of Mycobacteriology and Pulmonary Research, Pasteur Institute of Iran, Tehran, Iran, Sadeghizadeh M, Department of Genetics, Faculty of Biological Sciences, Tarbiat Modares University, Tehran, Iran, Yazdian F, Department of Life Science Engineering, Faculty of New Sciences and Technologies, University of Tehran, Tehran, Iran, et al. Co-Administration of Curcumin and Bromocriptine Nano-liposomes for Induction of Apoptosis in Lung Cancer Cells. ibj. 2020;24:24–9. [DOI] [PMC free article] [PubMed]
- 62.Kamazani FM, Sotoodehnejad Nematalahi F, Siadat SD, Pornour M, Sheikhpour M. A success targeted nano delivery to lung cancer cells with multi-walled carbon nanotubes conjugated to bromocriptine. Sci Rep. 2021;11:24419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Muzi L, Ménard-Moyon C, Russier J, Li J, Chin CF, Ang WH, et al. Diameter-dependent release of a cisplatin pro-drug from small and large functionalized carbon nanotubes. Nanoscale. 2015;7:5383–94. [DOI] [PubMed] [Google Scholar]
- 64.Naief MF, Mohammed SN, Mayouf HJ, Mohammed AM. A review of the role of carbon nanotubes for cancer treatment based on photothermal and photodynamic therapy techniques. J Organomet Chem. 2023;999: 122819. [Google Scholar]
- 65.Yousefi Rizi HA, Hoon Shin D, Yousefi RS. Polymeric nanoparticles in cancer chemotherapy: a narrative review. Iran J Public Health. 2022;51:226–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yao Y, Zhou Y, Liu L, Xu Y, Chen Q, Wang Y, et al. Nanoparticle-based drug delivery in cancer therapy and its role in overcoming drug resistance. Front Mol Biosci. 2020;7:193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gao Y, Wang K, Zhang J, Duan X, Sun Q, Men K. Multifunctional nanoparticle for cancer therapy. MedComm. 2020;2023(4): e187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ferreira Soares DC, Domingues SC, Viana DB, Tebaldi ML. Polymer-hybrid nanoparticles: current advances in biomedical applications. Biomed Pharmacother. 2020;131: 110695. [DOI] [PubMed] [Google Scholar]
- 69.Makadia HK, Siegel SJ. Poly lactic-co-glycolic acid (PLGA) as biodegradable controlled drug delivery carrier. Polymers. 2011;3:1377–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cotta MA. Quantum dots and their applications: what lies ahead? ACS Appl Nano Mater. 2020;3:4920–4. [Google Scholar]
- 71.Fang M, Peng C-W, Pang D-W, Li Y. Quantum dots for cancer research: current status, remaining issues, and future perspectives. Cancer Biol Med. 2012;9:151–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhao M-X, Zhu B-J. The research and applications of quantum dots as nano-carriers for targeted drug delivery and cancer therapy. Nanoscale Res Lett. 2016;11:207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dhas N, Pastagia M, Sharma A, Khera A, Kudarha R, Kulkarni S, et al. Organic quantum dots: an ultrasmall nanoplatform for cancer theranostics. J Control Release. 2022;348:798–824. [DOI] [PubMed] [Google Scholar]
- 74.Mishra S, Das K, Chatterjee S, Sahoo P, Kundu S, Pal M, et al. Facile and green synthesis of novel fluorescent carbon quantum dots and their silver heterostructure: an in vitro anticancer activity and imaging on colorectal carcinoma. ACS Omega. 2023;8:4566–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang M, Lan S, Zhang W, Jin Q, Du H, Sun X, et al. Anti-cancer potency of copper-doped carbon quantum dots against breast cancer progression. IJN. 2024;19:1985–2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Soltani M, Moradi Kashkooli F, Souri M, Zare Harofte S, Harati T, Khadem A, et al. Enhancing clinical translation of cancer using nanoinformatics. Cancers. 2021;13:2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhang B, Shi H, Wang H. Machine learning and AI in cancer prognosis, prediction, and treatment selection: a critical approach. J Multidiscip Healthc. 2023;16:1779–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Iqbal MJ, Javed Z, Sadia H, Qureshi IA, Irshad A, Ahmed R, et al. Clinical applications of artificial intelligence and machine learning in cancer diagnosis: looking into the future. Cancer Cell Int. 2021;21:270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fjelland R. Why general artificial intelligence will not be realized. Humanit Soc Sci Commun. 2020;7:10. [Google Scholar]
- 80.Sharma NK, Sarode SC. Artificial intelligence vs. evolving super-complex tumor intelligence: critical viewpoints. Front Artif Intell. 2023;6:1220744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kochanny SE, Pearson AT. Academics as leaders in the cancer artificial intelligence revolution. Cancer. 2021;127:664–71. [DOI] [PubMed] [Google Scholar]
- 82.Dlamini Z, Francies FZ, Hull R, Marima R. Artificial intelligence (AI) and big data in cancer and precision oncology. Comput Struct Biotechnol J. 2020;18:2300–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Turnbull J, MacLellan J, Churruca K, Ellis LA, Prichard J, Browne D, et al. A multimethod study of NHS 111 online. Health Soc Care Deliv Res. 2023. 10.3310/YTRR9821. [DOI] [PubMed] [Google Scholar]
- 84.Bajwa J, Munir U, Nori A, Williams B. Artificial intelligence in healthcare: transforming the practice of medicine. Future Healthc J. 2021;8:e188–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Undru TR, Uday U, Lakshmi JT, Kaliappan A, Mallamgunta S, Nikhat SS, et al. Integrating artificial intelligence for clinical and laboratory diagnosis - a review. Maedica. 2022;17:420–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Haleem A, Javaid M, Singh RP, Rab S, Suman R. Applications of nanotechnology in medical field: a brief review. Glob Health J. 2023;7:70–7. [Google Scholar]
- 87.Jia Y, Jiang Y, He Y, Zhang W, Zou J, Magar KT, et al. Approved nanomedicine against diseases. Pharmaceutics. 2023;15:774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Barenholz Y. Doxil® — The first FDA-approved nano-drug: lessons learned. J Control Release. 2012;160:117–34. [DOI] [PubMed] [Google Scholar]
- 89.Leonard RCF, Williams S, Tulpule A, Levine AM, Oliveros S. Improving the therapeutic index of anthracycline chemotherapy: focus on liposomal doxorubicin (Myocet™). The Breast. 2009;18:218–24. [DOI] [PubMed] [Google Scholar]
- 90.Allen TM, Martin FJ. Advantages of liposomal delivery systems for anthracyclines. Semin Oncol. 2004;31:5–15. [DOI] [PubMed] [Google Scholar]
- 91.Saif MW. U.S. Food and drug administration approves paclitaxel protein-bound particles (Abraxane®) in combination with gemcitabine as first-line treatment of patients with metastatic pancreatic cancer. JOP J Pancreas. 2013;14:686–8. [DOI] [PubMed] [Google Scholar]
- 92.Milano G, Innocenti F, Minami H. Liposomal irinotecan (Onivyde): exemplifying the benefits of nanotherapeutic drugs. Cancer Sci. 2022;113:2224–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Blair HA. Daunorubicin/cytarabine liposome: a review in acute myeloid leukaemia. Drugs. 2018;78:1903–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bonvalot S, Rutkowski PL, Thariat J, Carrère S, Ducassou A, Sunyach M-P, et al. NBTXR3, a first-in-class radioenhancer hafnium oxide nanoparticle, plus radiotherapy versus radiotherapy alone in patients with locally advanced soft-tissue sarcoma (Act.In.Sarc): a multicentre, phase 2–3, randomised, controlled trial. Lancet Oncol. 2019;20:1148–59. [DOI] [PubMed] [Google Scholar]
- 95.Rodríguez F, Caruana P, De la Fuente N, Español P, Gámez M, Balart J, et al. Nano-based approved pharmaceuticals for cancer treatment: present and future challenges. Biomolecules. 2022;12:784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Liu X, Zhang Y, Wang Y, Zhu W, Li G, Ma X, et al. Comprehensive understanding of magnetic hyperthermia for improving antitumor therapeutic efficacy. Theranostics. 2020;10:3793–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Silverman JA, Deitcher SR. Marqibo® (vincristine sulfate liposome injection) improves the pharmacokinetics and pharmacodynamics of vincristine. Cancer Chemother Pharmacol. 2013;71:555–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Domínguez AR, Hidalgo DO, Garrido RV, Sánchez ET. Liposomal cytarabine (DepoCyte®) for the treatment of neoplastic meningitis. Clin Transl Oncol. 2005;7:232–8. [DOI] [PubMed] [Google Scholar]
- 99.Kager L, Pötschger U, Bielack S. Review of mifamurtide in the treatment of patients with osteosarcoma. Ther Clin Risk Manag. 2010;6:279–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Oerlemans C, Bult W, Bos M, Storm G, Nijsen JFW, Hennink WE. Polymeric micelles in anticancer therapy: targeting, imaging and triggered release. Pharm Res. 2010;27:2569–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Meneguetti GP, Santos JHPM, Obreque KMT, Barbosa CMV, Monteiro G, Farsky SHP, et al. Novel site-specific PEGylated L-asparaginase. PLoS ONE. 2019;14: e0211951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.von Maltzahn G, Park J-H, Lin KY, Singh N, Schwöppe C, Mesters R, et al. Nanoparticles that communicate in vivo to amplify tumour targeting. Nat Mater. 2011;10:545–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sonali, Singh RP, Sharma G, Kumari L, Koch B, Singh S, et al. RGD-TPGS decorated theranostic liposomes for brain targeted delivery. Colloids Surf B Biointerfaces. 2016;147:129–41. [DOI] [PubMed] [Google Scholar]
- 104.Cao X, Zheng Y, Liao H, Guo X, Li Y, Wang Z, et al. A clinical nomogram and heat map for assessing survival in patients with stage I non-small cell lung cancer after complete resection. Ther Adv Med Oncol. 2020;12:1758835920970063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yaqoob A, Musheer Aziz R, Verma NK. Applications and techniques of machine learning in cancer classification: a systematic review. Hum-Cent Intell Syst. 2023;3:588–615. [Google Scholar]
- 106.Jiang X, Hu Z, Wang S, Zhang Y. Deep learning for medical image-based cancer diagnosis. Cancers. 2023;15:3608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hassan SU, Abdulkadir SJ, Zahid MSM, Al-Selwi SM. Local interpretable model-agnostic explanation approach for medical imaging analysis: a systematic literature review. Comput Biol Med. 2025;185: 109569. [DOI] [PubMed] [Google Scholar]
- 108.Rana M, Bhushan M. Machine learning and deep learning approach for medical image analysis: diagnosis to detection. Multimed Tools Appl. 2022;82:1–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Yamazaki K, Vo-Ho V-K, Bulsara D, Le N. Spiking Neural Networks and Their Applications: A Review. Brain Sci. 2022;12:863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Madan S, Lentzen M, Brandt J, Rueckert D, Hofmann-Apitius M, Fröhlich H. Transformer models in biomedicine. BMC Med Inform Decis Mak. 2024;24:214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Xu Y, Liu X, Cao X, Huang C, Liu E, Qian S, et al. Artificial intelligence: a powerful paradigm for scientific research. Innovation. 2021;2: 100179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Liu C, Liu X, Wu F, Xie M, Feng Y, Hu C. Using artificial intelligence (Watson for Oncology) for treatment recommendations amongst Chinese patients with lung cancer: feasibility study. J Med Internet Res. 2018;20: e11087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ahmed Z, Mohamed K, Zeeshan S, Dong X. Artificial intelligence with multi-functional machine learning platform development for better healthcare and precision medicine. Database. 2020;2020: baaa10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Thompson RF, Valdes G, Fuller CD, Carpenter CM, Morin O, Aneja S, et al. Artificial intelligence in radiation oncology: a specialty-wide disruptive transformation? Radiother Oncol. 2018;129:421–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Applying machine learning to radiotherapy planning for head & neck cancer. DeepMind. 2016.
- 116.Raza K, Dey N, editors. Translational bioinformatics applications in healthcare. 1st ed. Boca Raton: CRC Press; 2021. [Google Scholar]
- 117.Mashraqi AM, Allehyani B. Current trends on the application of artificial intelligence in medical sciences. Bioinformation. 2022;18:1050–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Baxi V, Edwards R, Montalto M, Saha S. Digital pathology and artificial intelligence in translational medicine and clinical practice. Mod Pathol. 2022;35:23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Osipov A, Nikolic O, Gertych A, Parker S, Hendifar A, Singh P, et al. The Molecular Twin artificial-intelligence platform integrates multi-omic data to predict outcomes for pancreatic adenocarcinoma patients. Nat Cancer. 2024;5:299–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Chen M, Decary M. Artificial intelligence in healthcare: an essential guide for health leaders. Healthc Manage Forum. 2020;33:10–8. [DOI] [PubMed] [Google Scholar]
- 121.Visions P. Through the prism of innovation. J Pathol Inform. 2020;2021(12):37. [Google Scholar]
- 122.Bonaparte I, Fragnoli F, Gregucci F, Carbonara R, Di Guglielmo FC, Surgo A, et al. Improving quality assurance in a radiation oncology using ARIA visual care path. J Pers Med. 2024;14:416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Askin S, Burkhalter D, Calado G, El Dakrouni S. Artificial intelligence applied to clinical trials: opportunities and challenges. Health Technol. 2023;13:203–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Chopra H, Annu, Shin DK, Munjal K, Priyanka, Dhama K, et al. Revolutionizing clinical trials: the role of AI in accelerating medical breakthroughs. Int J Surg. 2023;109:4211–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ho D, Wang P, Kee T. Artificial intelligence in nanomedicine. Nanoscale Horiz. 2019;4:365–77. [DOI] [PubMed] [Google Scholar]
- 126.Russo V, Lallo E, Munnia A, Spedicato M, Messerini L, D’Aurizio R, et al. Artificial intelligence predictive models of response to cytotoxic chemotherapy alone or combined to targeted therapy for metastatic colorectal cancer patients: a systematic review and meta-analysis. Cancers. 2022;14:4012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ho D, Zarrinpar A, Chow EK-H. Diamonds, digital health, and drug development: optimizing combinatorial nanomedicine. ACS Nano. 2016;10:9087–92. [DOI] [PubMed] [Google Scholar]
- 128.Zarrinpar A, Lee D-K, Silva A, Datta N, Kee T, Eriksen C, et al. Individualizing liver transplant immunosuppression using a phenotypic personalized medicine platform. Sci Transl Med. 2016. 10.1126/scitranslmed.aac5954. [DOI] [PubMed] [Google Scholar]
- 129.Weiss A, Berndsen RH, Ding X, Ho C-M, Dyson PJ, Van Den Bergh H, et al. A streamlined search technology for identification of synergistic drug combinations. Sci Rep. 2015;5:14508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Weiss A, Ding X, Van Beijnum JR, Wong I, Wong TJ, Berndsen RH, et al. Rapid optimization of drug combinations for the optimal angiostatic treatment of cancer. Angiogenesis. 2015;18:233–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Mukhopadhyay A, Sumner J, Ling LH, Quek RHC, Tan ATH, Teng GG, et al. Personalised dosing using the CURATE.AI algorithm: protocol for a feasibility study in patients with hypertension and type II diabetes mellitus. Int J Environ Res Public Health. 2022;19:8979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Blasiak A, Khong J, Kee T. CURATE.AI: optimizing personalized medicine with artificial intelligence. SLAS Technol. 2020;25:95–105. [DOI] [PubMed] [Google Scholar]
- 133.Pantuck AJ, Lee D, Kee T, Wang P, Lakhotia S, Silverman MH, et al. Modulating BET bromodomain inhibitor ZEN-3694 and enzalutamide combination dosing in a metastatic prostate cancer patient using CURATE.AI, an artificial intelligence platform. Adv Ther. 2018;1:1800104. [Google Scholar]
- 134.Tan P, Chen X, Zhang H, Wei Q, Luo K. Artificial intelligence aids in development of nanomedicines for cancer management. Semin Cancer Biol. 2023;89:61–75. [DOI] [PubMed] [Google Scholar]
- 135.Alshawwa SZ, Kassem AA, Farid RM, Mostafa SK, Labib GS. Nanocarrier drug delivery systems: characterization, limitations, future perspectives and implementation of artificial intelligence. Pharmaceutics. 2022;14:883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Chow JCL. Nanomaterial-based molecular imaging in cancer: advances in simulation and AI integration. Biomolecules. 2025;15:444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kulkarni S, Lin B, Radhakrishnan R. Machine learning enabled multiscale model for nanoparticle margination and physiology based pharmacokinetics. Comput Chem Eng. 2025;198: 109081. [Google Scholar]
- 138.Shirzad M, Salahvarzi A, Razzaq S, Javid-Naderi MJ, Rahdar A, Fathi-karkan S, et al. Revolutionizing prostate cancer therapy: artificial intelligence – based nanocarriers for precision diagnosis and treatment. Crit Rev Oncol Hematol. 2025;208: 104653. [DOI] [PubMed] [Google Scholar]
- 139.Bhange M, Telange D. Convergence of nanotechnology and artificial intelligence in the fight against liver cancer: a comprehensive review. Discov Onc. 2025;16:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Vora LK, Gholap AD, Jetha K, Thakur RRS, Solanki HK, Chavda VP. Artificial intelligence in pharmaceutical technology and drug delivery design. Pharmaceutics. 2023;15:1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Govindan B, Sabri MA, Hai A, Banat F, Haija MA. A review of advanced multifunctional magnetic nanostructures for cancer diagnosis and therapy integrated into an artificial intelligence approach. Pharmaceutics. 2023;15:868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Xu M, Qin Z, Chen Z, Wang S, Peng L, Li X, et al. Nanorobots mediated drug delivery for brain cancer active targeting and controllable therapeutics. Discover Nano. 2024;19:183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Wasilewski T, Kamysz W, Gębicki J. AI-assisted detection of biomarkers by sensors and biosensors for early diagnosis and monitoring. Biosensors. 2024;14:356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Das KP. Nanoparticles and convergence of artificial intelligence for targeted drug delivery for cancer therapy: current progress and challenges. Front Med Technol. 2023;4:1067144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Bohr A, Memarzadeh K. The rise of artificial intelligence in healthcare applications. In: Artificial Intelligence in Healthcare. Elsevier; 2020. p. 25–60.
- 146.Konstantinova J, Jiang A, Althoefer K, Dasgupta P, Nanayakkara T. Implementation of tactile sensing for palpation in robot-assisted minimally invasive surgery: a review. IEEE Sensors J. 2014;14:2490–501. [Google Scholar]
- 147.Hu M, Ge X, Chen X, Mao W, Qian X, Yuan W-E. Micro/Nanorobot: a promising targeted drug delivery system. Pharmaceutics. 2020;12:665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Sanchez-Martinez S, Camara O, Piella G, Cikes M, González-Ballester MÁ, Miron M, et al. Machine learning for clinical decision-making: challenges and opportunities in cardiovascular imaging. Front Cardiovasc Med. 2022;8: 765693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Bush B, Nifong LW, Alwair H, Chitwood WR. Robotic mitral valve surgery-current status and future directions. Ann Cardiothorac Surg. 2013;2:814–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Nobbenhuis MAE, Gul N, Barton-Smith P, O’Sullivan O, Moss E, Ind TEJ, et al. Robotic surgery in gynaecology: Scientific Impact Paper No. 71 (July 2022). BJOG. 2023;130: e1. [DOI] [PubMed] [Google Scholar]
- 151.Garisto J, Ramakrishnan VM, Bertolo R, Kaouk J. The re-discovery of alternative access to the pelvic fossa: the role of the single-port robotic platform. In: Single-Port Robotic Surgery in Urology. Elsevier; 2022. p. 35–59.
- 152.You Y, Lai X, Pan Y, Zheng H, Vera J, Liu S, et al. Artificial intelligence in cancer target identification and drug discovery. Sig Transduct Target Ther. 2022;7:156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Vijayakumar M, Shetty R. Robotic surgery in oncology. Indian J Surg Oncol. 2020;11:549–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Yang Y, Song L, Huang J, Cheng X, Luo Q. A uniportal right upper lobectomy by three-arm robotic-assisted thoracoscopic surgery using the da Vinci (Xi) Surgical System in the treatment of early-stage lung cancer. Transl Lung Cancer Res. 2021;10:1571–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Liu G, Zhang S, Zhang Y, Fu X, Liu X. Robotic surgery in rectal cancer: potential, challenges, and opportunities. Curr Treat Options in Oncol. 2022;23:961–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Mehta CH, Narayan R, Nayak UY. Computational modeling for formulation design. Drug Discov Today. 2019;24:781–8. [DOI] [PubMed] [Google Scholar]
- 157.Mohammadzadeh N, Safdari R. Robotic surgery in cancer care: opportunities and challenges. Asian Pac J Cancer Prev. 2014;15:1081–3. [DOI] [PubMed] [Google Scholar]
- 158.Malhotra K, Wong BNX, Lee S, Franco H, Singh C, Cabrera Silva LA, et al. Role of artificial intelligence in global surgery: a review of opportunities and challenges. Cureus. 2023. 10.7759/cureus.43192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Munshi A. Do not use robotic surgery in oncology patients when conventional surgical approaches are equally effective. Lancet Oncol. 2019;20: e240. [DOI] [PubMed] [Google Scholar]
- 160.Van Der Meijden OAJ, Schijven MP. The value of haptic feedback in conventional and robot-assisted minimal invasive surgery and virtual reality training: a current review. Surg Endosc. 2009;23:1180–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Yadav N, Pandey S, Gupta A, Dudani P, Gupta S, Rangarajan K. Data privacy in healthcare: in the era of artificial intelligence. Indian Dermatol Online J. 2023;14:788–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Mittermaier M, Raza MM, Kvedar JC. Bias in AI-based models for medical applications: challenges and mitigation strategies. Npj Digit Med. 2023;6:113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Adegbesan A, Akingbola A, Aremu O, Adewole O, Amamdikwa JC, Shagaya U. From scalpels to algorithms: the risk of dependence on artificial intelligence in surgery. J Med Surg Public Health. 2024;3: 100140. [Google Scholar]
- 164.Hassija V, Chamola V, Mahapatra A, Singal A, Goel D, Huang K, et al. Interpreting black-box models: a review on explainable artificial intelligence. Cogn Comput. 2024;16:45–74. [Google Scholar]
- 165.Habli I, Lawton T, Porter Z. Artificial intelligence in health care: accountability and safety. Bull World Health Organ. 2020;98:251–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Olawade DB, David-Olawade AC, Wada OZ, Asaolu AJ, Adereni T, Ling J. Artificial intelligence in healthcare delivery: prospects and pitfalls. J Medi Surg Public Health. 2024;3: 100108. [Google Scholar]
- 167.Lee H, Chen Y-PP. Image based computer aided diagnosis system for cancer detection. Expert Syst Appl. 2015;42:5356–65. [Google Scholar]
- 168.Duong MT, Rauschecker AM, Mohan S. Diverse applications of artificial intelligence in neuroradiology. Neuroimaging Clin N Am. 2020;30:505–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Holodny AI. Am I about to Lose my Job?!”: a comment on “computer-extracted texture features to distinguish cerebral radiation necrosis from recurrent brain tumors on multiparametric MRI: a feasibility study. AJNR Am J Neuroradiol. 2016;37:2237–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Belge Bilgin G, Bilgin C, Burkett BJ, Orme JJ, Childs DS, Thorpe MP, et al. Theranostics and artificial intelligence: new frontiers in personalized medicine. Theranostics. 2024;14:2367–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Ng AY, Oberije CJG, Ambrózay É, Szabó E, Serfőző O, Karpati E, et al. Prospective implementation of AI-assisted screen reading to improve early detection of breast cancer. Nat Med. 2023;29:3044–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Gupta N, Verma VK. Next-generation sequencing and its application: empowering in public health beyond reality. In: Arora PK, editor. Microbial technology for the welfare of society. Singapore: Springer Singapore; 2019. p. 313–41. [Google Scholar]
- 173.Nair SV, Madhulaxmi, Thomas G, Ankathil R. Next-generation sequencing in cancer. J Maxillofac Oral Surg. 2021;20:340–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Patel SK, George B, Rai V. Artificial intelligence to decode cancer mechanism: beyond patient stratification for precision oncology. Front Pharmacol. 2020;11:1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Abraham J, Heimberger AB, Marshall J, Heath E, Drabick J, Helmstetter A, et al. Machine learning analysis using 77,044 genomic and transcriptomic profiles to accurately predict tumor type. Transl Oncol. 2021;14: 101016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Wang X, Zou C, Zhang Y, Li X, Wang C, Ke F, et al. Prediction of BRCA Gene mutation in breast cancer based on deep learning and histopathology images. Front Genet. 2021;12: 661109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Mei W-J, Mi M, Qian J, Xiao N, Yuan Y, Ding P-R. Clinicopathological characteristics of high microsatellite instability/mismatch repair-deficient colorectal cancer: a narrative review. Front Immunol. 2022;13:1019582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Niu Y, Wang L, Zhang X, Han Y, Yang C, Bai H, et al. Predicting tumor mutational burden from lung adenocarcinoma histopathological images using deep learning. Front Oncol. 2022;12: 927426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Batra U, Nathany S, Nath SK, Jose JT, Sharma T, et al. AI-based pipeline for early screening of lung cancer: integrating radiology, clinical, and genomics data. Lancet Region Health - Southeast Asia. 2024;24: 100352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Mayer C, Ofek E, Fridrich DE, Molchanov Y, Yacobi R, Gazy I, et al. Direct identification of ALK and ROS1 fusions in non-small cell lung cancer from hematoxylin and eosin-stained slides using deep learning algorithms. Mod Pathol. 2022;35:1882–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Darmofal M, Suman S, Atwal G, Chen J-F, Chang JC, Toomey M, et al. Deep Learning Model for Tumor Type Prediction using Targeted Clinical Genomic Sequencing Data. medRxiv. 2023;:2023.09.08.23295131. [DOI] [PMC free article] [PubMed]
- 182.Kang J, Lee A, Lee YS. Prediction of PIK3CA mutations from cancer gene expression data. PLoS ONE. 2020;15: e0241514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Liu S, Shah Z, Sav A, Russo C, Berkovsky S, Qian Y, et al. Isocitrate dehydrogenase (IDH) status prediction in histopathology images of gliomas using deep learning. Sci Rep. 2020;10:7733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Wang H, Guo M, Wei H, Chen Y. Targeting p53 pathways: mechanisms, structures, and advances in therapy. Sig Transduct Target Ther. 2023;8:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Perez-Villatoro F, Oikkonen J, Casado J, Chernenko A, Gulhan DC, Tumiati M, et al. Optimized detection of homologous recombination deficiency improves the prediction of clinical outcomes in cancer. Npj Precis Onc. 2022;6:96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Abiodun OI, Jantan A, Omolara AE, Dada KV, Mohamed NA, Arshad H. State-of-the-art in artificial neural network applications: a survey. Heliyon. 2018;4: e00938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Al-Thelaya K, Gilal NU, Alzubaidi M, Majeed F, Agus M, Schneider J, et al. Applications of discriminative and deep learning feature extraction methods for whole slide image analysis: a survey. J Pathol Inform. 2023;14: 100335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Rifaioglu AS, Atas H, Martin MJ, Cetin-Atalay R, Atalay V, Doğan T. Recent applications of deep learning and machine intelligence on in silico drug discovery: methods, tools and databases. Brief Bioinform. 2019;20:1878–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Sadybekov AV, Katritch V. Computational approaches streamlining drug discovery. Nature. 2023;616:673–85. [DOI] [PubMed] [Google Scholar]
- 190.Campanella G, Hanna MG, Geneslaw L, Miraflor A, Werneck Krauss Silva V, Busam KJ, et al. Clinical-grade computational pathology using weakly supervised deep learning on whole slide images. Nat Med. 2019;25:1301–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Arvaniti E, Fricker KS, Moret M, Rupp N, Hermanns T, Fankhauser C, et al. Automated Gleason grading of prostate cancer tissue microarrays via deep learning. Sci Rep. 2018;8:12054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Litjens G, Kooi T, Bejnordi BE, Setio AAA, Ciompi F, Ghafoorian M, et al. A survey on deep learning in medical image analysis. Med Image Anal. 2017;42:60–88. [DOI] [PubMed] [Google Scholar]
- 193.Chen H, Engkvist O, Wang Y, Olivecrona M, Blaschke T. The rise of deep learning in drug discovery. Drug Discov Today. 2018;23:1241–50. [DOI] [PubMed] [Google Scholar]
- 194.Zhavoronkov A, Ivanenkov YA, Aliper A, Veselov MS, Aladinskiy VA, Aladinskaya AV, et al. Deep learning enables rapid identification of potent DDR1 kinase inhibitors. Nat Biotechnol. 2019;37:1038–40. [DOI] [PubMed] [Google Scholar]
- 195.Esteva A, Robicquet A, Ramsundar B, Kuleshov V, DePristo M, Chou K, et al. A guide to deep learning in healthcare. Nat Med. 2019;25:24–9. [DOI] [PubMed] [Google Scholar]
- 196.Torkamannia A, Omidi Y, Ferdousi R. SYNDEEP: a deep learning approach for the prediction of cancer drugs synergy. Sci Rep. 2023;13:6184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Preuer K, Lewis RPI, Hochreiter S, Bender A, Bulusu KC, Klambauer G. DeepSynergy: predicting anti-cancer drug synergy with deep learning. Bioinformatics. 2018;34:1538–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Sebastian AM, Peter D. Artificial intelligence in cancer research: trends, challenges and future directions. Life. 2022;12:1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Liang M, Cowley AW, Greene AS. High throughput gene expression profiling: a molecular approach to integrative physiology. J Physiol. 2004;554(Pt 1):22–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Warnat-Herresthal S, Perrakis K, Taschler B, Becker M, Baßler K, Beyer M, et al. Scalable prediction of acute myeloid leukemia using high-dimensional machine learning and blood transcriptomics. iScience. 2020;23: 100780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Ben Azzouz F, Michel B, Lasla H, Gouraud W, François A-F, Girka F, et al. Development of an absolute assignment predictor for triple-negative breast cancer subtyping using machine learning approaches. Comput Biol Med. 2021;129: 104171. [DOI] [PubMed] [Google Scholar]
- 202.Alkhateeb A, Rezaeian I, Singireddy S, Cavallo-Medved D, Porter LA, Rueda L. Transcriptomics signature from next-generation sequencing data reveals new transcriptomic biomarkers related to prostate cancer. Cancer Inform. 2019;18:1176935119835522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Long NP, Park S, Anh NH, Nghi TD, Yoon SJ, Park JH, et al. High-throughput omics and statistical learning integration for the discovery and validation of novel diagnostic signatures in colorectal cancer. Int J Mol Sci. 2019;20:296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Yassi M, Chatterjee A, Parry M. Application of deep learning in cancer epigenetics through DNA methylation analysis. Brief Bioinform. 2023;24: bbadd411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Ye Y, Wu X, Wang H, Ye H, Zhao K, Yao S, et al. Artificial intelligence-assisted analysis for tumor-immune interaction within the invasive margin of colorectal cancer. Ann Med. 2023;55:2215541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Ottaiano A, Ianniello M, Santorsola M, Ruggiero R, Sirica R, Sabbatino F, et al. From chaos to opportunity: decoding cancer heterogeneity for enhanced treatment strategies. Biology. 2023;12:1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Ravindran U, Gunavathi C. A survey on gene expression data analysis using deep learning methods for cancer diagnosis. Prog Biophys Mol Biol. 2023;177:1–13. [DOI] [PubMed] [Google Scholar]
- 208.Papageorgiou EI, Spyridonos P, Stylios C, Ravazoula P, Groumpos PP, Nikiforidis G. A Fuzzy Cognitive Map model for grading urinary bladder tumors. 2003.
- 209.Papageorgiou EI, Spyridonos PP, Stylios CD, Nikiforidis GC, Groumpos PP. Grading urinary bladder tumors using unsupervised Hebbian Algorithm for Fuzzy Cognitive Maps. 2003.
- 210.Liu S, See KC, Ngiam KY, Celi LA, Sun X, Feng M. Reinforcement learning for clinical decision support in critical care: comprehensive review. J Med Internet Res. 2020;22: e18477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Elia S, Pompeo E, Santone A, Rigoli R, Chiocchi M, Patirelis A, et al. Radiomics and artificial intelligence can predict malignancy of solitary pulmonary nodules in the elderly. Diagnostics. 2023;13:384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.DiMaio S, Hanuschik M, Kreaden U. The da Vinci surgical system. In: Rosen J, Hannaford B, Satava RM, editors. Surgical robotics. Boston, MA: Springer, US; 2011. p. 199–217. [Google Scholar]
- 213.Tokai Y, Yoshio T, Aoyama K, Horie Y, Yoshimizu S, Horiuchi Y, et al. Application of artificial intelligence using convolutional neural networks in determining the invasion depth of esophageal squamous cell carcinoma. Esophagus. 2020;17:250–6. [DOI] [PubMed] [Google Scholar]
- 214.Knight SR, Ng N, Tsanas A, Mclean K, Pagliari C, Harrison EM. Mobile devices and wearable technology for measuring patient outcomes after surgery: a systematic review. Npj Digit Med. 2021;4:157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Cabitza F, Rasoini R, Gensini GF. Unintended consequences of machine learning in medicine. JAMA. 2017;318:517. [DOI] [PubMed] [Google Scholar]
- 216.Fenech M, Strukelj N, Buston O. Ethical, social, and political challenges of artificial intelligence in health. 2018.
- 217.Cowls J, Floridi L. Prolegomena to a white paper on an ethical framework for a good AI society. SSRN J. 2018. 10.2139/ssrn.3198732. [Google Scholar]
- 218.Holzinger A, Biemann C, Pattichis CS, Kell DB. What do we need to build explainable AI systems for the medical domain? 2017.
- 219.Sen D, Chakrabarti R, Chatterjee S, Grewal DS, Manrai K. Artificial intelligence and the radiologist: the future in the Armed Forces Medical Services. BMJ Mil Health. 2020;166:254–6. [DOI] [PubMed] [Google Scholar]
- 220.Carter SM, Rogers W, Win KT, Frazer H, Richards B, Houssami N. The ethical, legal and social implications of using artificial intelligence systems in breast cancer care. The Breast. 2020;49:25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Farasati FB. Artificial intelligence ethics in precision oncology: balancing advancements in technology with patient privacy and autonomy. Explor Target Antitumor Ther. 2023;4:685–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Majumder A, Sen D. Artificial intelligence in cancer diagnostics and therapy: current perspectives. Indian J Cancer. 2021;58:481. [DOI] [PubMed] [Google Scholar]
- 223.Zhou N, Zhang C-T, Lv H-Y, Hao C-X, Li T-J, Zhu J-J, et al. Concordance study between IBM Watson for Oncology and clinical practice for patients with cancer in China. Oncologist. 2019;24:812–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Alum EU. AI-driven biomarker discovery: enhancing precision in cancer diagnosis and prognosis. Discov Oncol. 2025;16:313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Beaubier N, Bontrager M, Huether R, Igartua C, Lau D, Tell R, et al. Integrated genomic profiling expands clinical options for patients with cancer. Nat Biotechnol. 2019;37:1351–60. [DOI] [PubMed] [Google Scholar]
- 226.Dougherty E. Artificial intelligence decodes cancer pathology images.
- 227.Boston M, Tampa F. PathAI and Aster Insights Partner to Deliver Combined AI-Derived Structured Pathology and Multimodal Real-World Data Solutions. 2024.
- 228.Wiklund P, Medson K. Use of a deep learning algorithm for detection and triage of cancer-associated incidental pulmonary embolism. Radiol Artif Intell. 2023;5: e220286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Sako C, Duan C, Maresca K, Kent S, Schmidt TG, Aerts HJWL, et al. Real-World and clinical trial validation of a deep learning radiomic biomarker for PD-(L)1 immune checkpoint inhibitor response in advanced non-small cell lung cancer. JCO Clin Cancer Inform. 2024;8: e2400133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Đorđević S, Gonzalez MM, Conejos-Sánchez I, Carreira B, Pozzi S, Acúrcio RC, et al. Current hurdles to the translation of nanomedicines from bench to the clinic. Drug Deliv Transl Res. 2022;12:500–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Liu Y, Zhang Y, Li H, Hu TY. Recent advances in the bench-to-bedside translation of cancer nanomedicines. Acta Pharmaceutica Sinica B. 2025;15:97–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Hanna MG, Pantanowitz L, Jackson B, Palmer O, Visweswaran S, Pantanowitz J, et al. Ethical and bias considerations in artificial intelligence/machine learning. Mod Pathol. 2025;38: 100686. [DOI] [PubMed] [Google Scholar]
- 233.Aravazhi PS, Gunasekaran P, Benjamin NZY, Thai A, Chandrasekar KK, Kolanu ND, et al. The integration of artificial intelligence into clinical medicine: trends, challenges, and future directions. Dis Mon. 2025. 10.1016/j.disamonth.2025.101882. [DOI] [PubMed] [Google Scholar]
- 234.Singh Y, Patel H, Vera-Garcia DV, Hathaway QA, Sarkar D, Quaia E. Beyond the hype: navigating bias in AI-driven cancer detection. Oncotarget. 2024;15:764–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Parambil MMA, Rustamov J, Ahmed SG, Rustamov Z, Awad AI, Zaki N, et al. Integrating AI-based and conventional cybersecurity measures into online higher education settings: challenges, opportunities, and prospects. Comput Educ Artif Intell. 2024;7: 100327. [Google Scholar]
- 236.Harishbhai Tilala M, Kumar Chenchala P, Choppadandi A, Kaur J, Naguri S, Saoji R, et al. Ethical considerations in the use of artificial intelligence and machine learning in health care: a comprehensive review. Cureus. 2024. 10.7759/cureus.62443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Lutton L. FDA-approved AI algorithm more likely to detect false positive breast cancer cases in black women. 2024.
- 238.Echefu G, Shah R, Sanchez Z, Rickards J, Brown S-A. Artificial intelligence: applications in cardio-oncology and potential impact on racial disparities. Am Heart J Plus: Cardiol Res Pract. 2024;48: 100479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Das A, Tariq A, Batalini F, Dhara B, Banerjee I. Exposing vulnerabilities in clinical LLMs through data poisoning attacks: case study in breast cancer. 2024.
- 240.Khalid N, Qayyum A, Bilal M, Al-Fuqaha A, Qadir J. Privacy-preserving artificial intelligence in healthcare: techniques and applications. Comput Biol Med. 2023;158: 106848. [DOI] [PubMed] [Google Scholar]
- 241.Fountzilas E, Pearce T, Baysal MA, Chakraborty A, Tsimberidou AM. Convergence of evolving artificial intelligence and machine learning techniques in precision oncology. NPJ Digit Med. 2025;8:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets/information used for this study is available on within the manuscript.