Abstract
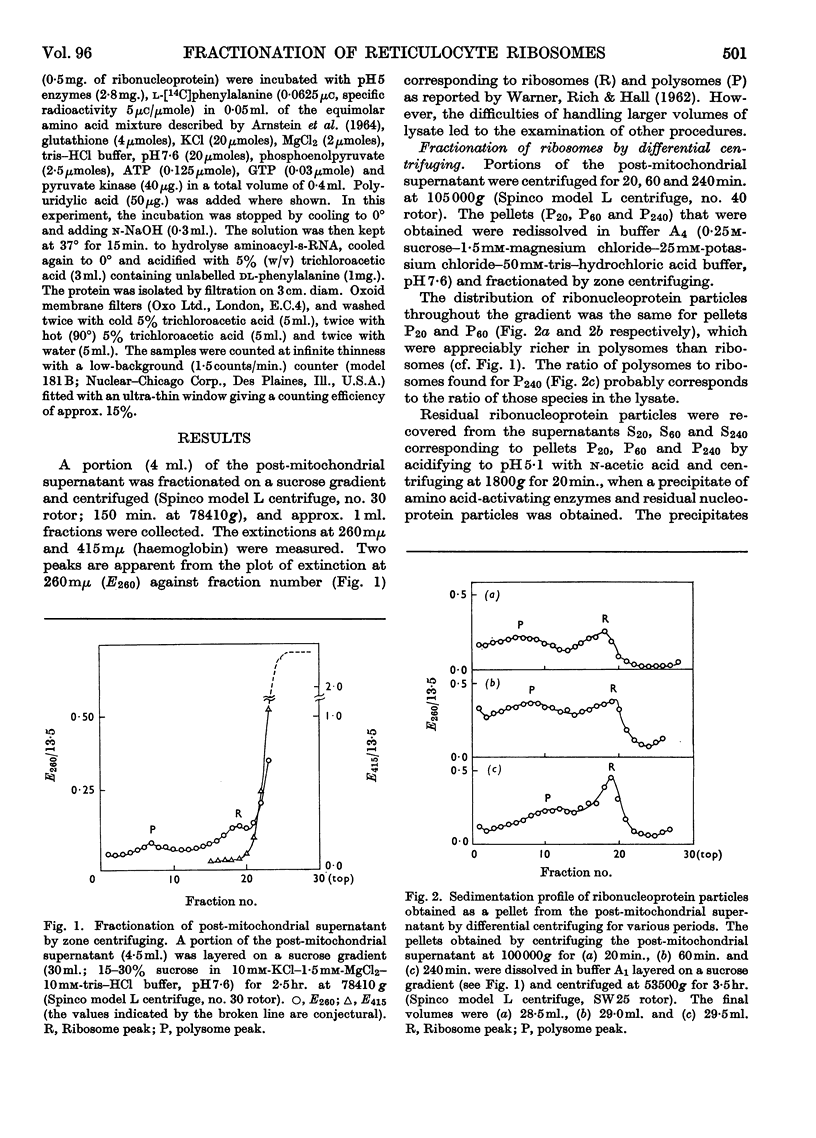
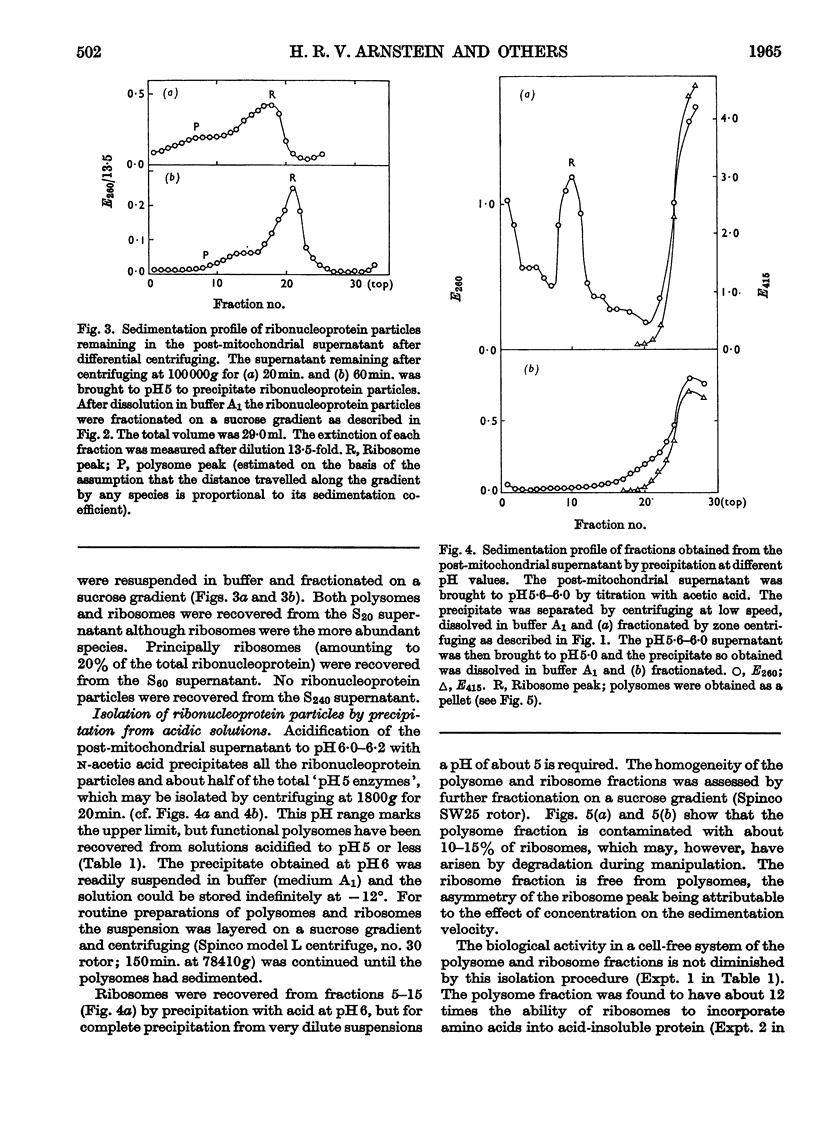
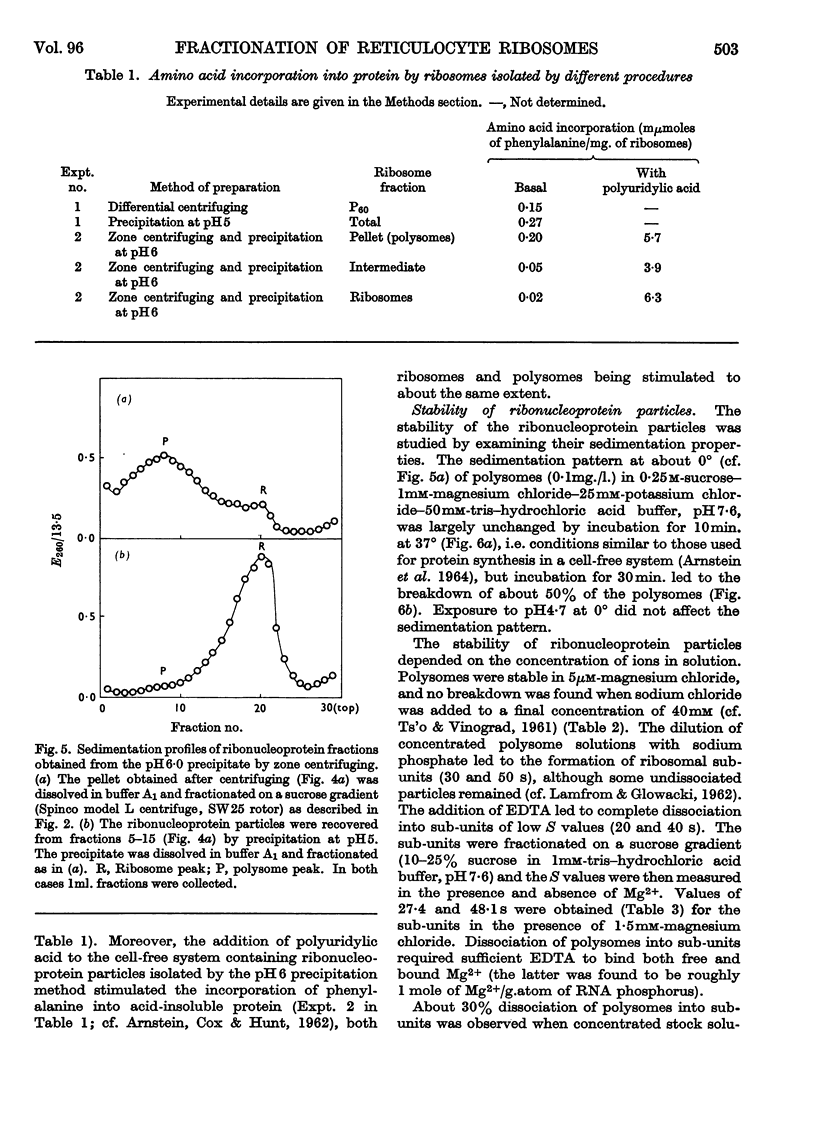
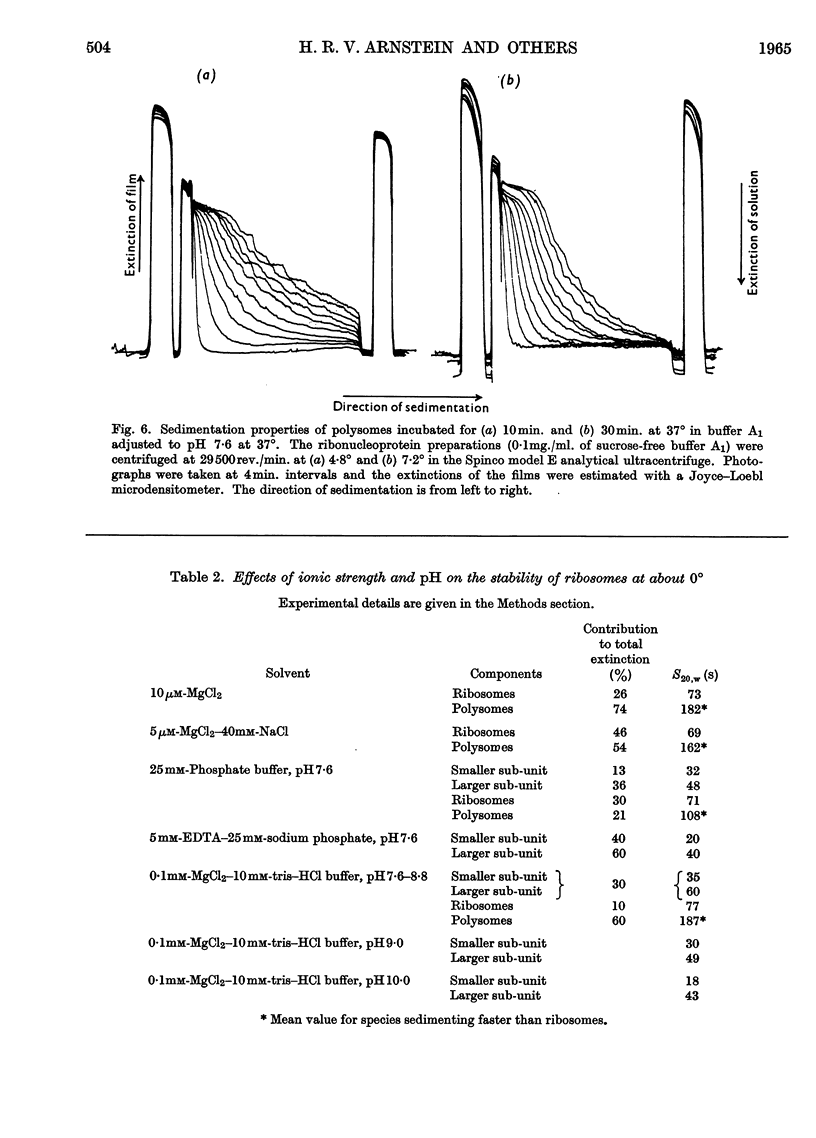
1. Polysomes, ribosomes and pH5 enzymes were isolated from rabbit reticulocytes by acidifying the post-mitochondrial supernatant to pH6·0 to precipitate all ribonucleoprotein particles and about half the pH5 enzymes; the precipitate was redissolved in buffer, pH7·6, and fractionated by zone centrifuging. 2. The isolation of polysome-rich and ribosome-rich fractions from the post-mitochondrial supernatant was also examined. 3. Studies of the stability of polysomes revealed that dissociation into sub-units occurred when both bound and free Mg2+ was chelated by EDTA or when the pH was increased above pH8·8.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARNSTEIN H. R., COX R. A., HUNT J. A. Function of polyuridylic acid and ribonucleic acid in protein biosynthesis by ribosomes from mammalian reticulocytes. Nature. 1962 Jun 16;194:1042–1044. doi: 10.1038/1941042a0. [DOI] [PubMed] [Google Scholar]
- Arnstein H. R., Cox R. A., Hunt J. A. The function of high-molecular-weight ribonucleic acid from rabbit reticulocytes in haemoglobin biosynthesis. Biochem J. 1964 Sep;92(3):648–661. doi: 10.1042/bj0920648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BORSOOK H., FISCHER E. H., KEIGHLEY G. Factors affecting protein synthesis in vitro in rabbit reticulocytes. J Biol Chem. 1957 Dec;229(2):1059–1070. [PubMed] [Google Scholar]
- GROS F., HIATT H., GILBERT W., KURLAND C. G., RISEBROUGH R. W., WATSON J. D. Unstable ribonucleic acid revealed by pulse labelling of Escherichia coli. Nature. 1961 May 13;190:581–585. doi: 10.1038/190581a0. [DOI] [PubMed] [Google Scholar]
- HAMILTON M. G., PETERMANN M. L. Ultracentrifugal studies on ribonucleoprotein from rat liver microsomes. J Biol Chem. 1959 Jun;234(6):1441–1446. [PubMed] [Google Scholar]
- Schweet R., Lamfrom H., Allen E. THE SYNTHESIS OF HEMOGLOBIN IN A CELL-FREE SYSTEM. Proc Natl Acad Sci U S A. 1958 Oct 15;44(10):1029–1035. doi: 10.1073/pnas.44.10.1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TS'O P. O., VINOGRAD J. Studies on ribosomes from reticulocytes. Biochim Biophys Acta. 1961 Apr 29;49:113–129. doi: 10.1016/0006-3002(61)90875-7. [DOI] [PubMed] [Google Scholar]
- WARNER J. R., KNOPF P. M., RICH A. A multiple ribosomal structure in protein synthesis. Proc Natl Acad Sci U S A. 1963 Jan 15;49:122–129. doi: 10.1073/pnas.49.1.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WETTSTEIN F. O., STAEHELIN T., NOLL H. Ribosomal aggregate engaged in protein synthesis: characterization of the ergosome. Nature. 1963 Feb 2;197:430–435. doi: 10.1038/197430a0. [DOI] [PubMed] [Google Scholar]
- Warner J. R., Rich A., Hall C. E. Electron Microscope Studies of Ribosomal Clusters Synthesizing Hemoglobin. Science. 1962 Dec 28;138(3548):1399–1403. doi: 10.1126/science.138.3548.1399. [DOI] [PubMed] [Google Scholar]


