Abstract
The human T-cell leukemia virus type 1 Tax protein transforms human T lymphocytes, which can lead to the development of adult T-cell leukemia. Tax transformation is related to its ability to activate gene expression via the ATF/CREB and the NF-κB pathways. Transcriptional activation of these pathways is mediated by the actions of the related coactivators CREB binding protein (CBP) and p300. In this study, immunocytochemistry and confocal microscopy were used to localize CBP and p300 in cells expressing wild-type Tax or Tax mutants that are able to selectively activate gene expression from either the NF-κB or ATF/CREB pathway. Wild-type Tax colocalized with both CBP and p300 in nuclear bodies which also contained ATF-1 and the RelA subunit of NF-κB. However, a Tax mutant that selectively activates gene expression from only the ATF/CREB pathway colocalized with CBP but not p300, while a Tax mutant that selectively activates gene expression from only the NF-κB pathway colocalized with p300 but not CBP. In vitro and in vivo protein interaction studies indicated that the integrity of two independent domains of Tax delineated by these mutants was involved in the direct interaction of Tax with either CBP or p300. These studies are consistent with a model in which activation of either the NF-κB or the ATF/CREB pathway by specific Tax mutants is mediated by distinct interactions with related coactivator proteins.
The human T-cell leukemia virus type 1 (HTLV-1) is a retrovirus which is the etiologic agent of adult T-cell leukemia (36, 51, 65). Adult T-cell leukemia is characterized by the presence in the peripheral blood of malignant lymphoid cells which contain the HTLV-1 provirus (38, 85). HTLV-1 encodes a protein, Tax, which is a potent activator of viral transcription (15, 22, 77) and is also involved in transforming cells of both lymphoid and nonlymphoid origin (30, 31, 54, 66, 76, 79). Tax also activates the expression of specific cellular genes involved in normal T-cell activation and proliferation, including the gene coding for interleukin-2, the gene for interleukin-2 receptor, and the proto-oncogene c-fos (5, 23, 46, 72). These latter patterns of transcriptional activation result from Tax-mediated increases in the nuclear levels of NF-κB (14, 37, 41, 42, 45, 50) and direct interactions of Tax with the serum response factor (24).
Tax activates HTLV-1 gene expression via direct interactions with members of the ATF/CREB family of transcription factors, which bind to three 21-bp repeat regulatory elements present in the viral long terminal repeat (LTR) (12, 25, 26, 33, 58, 60, 62, 71, 80, 83, 84, 86). Interactions of Tax with members of the ATF/CREB family of transcription factors including CREB and CREM (3, 7, 62, 71, 78, 80, 86) and ATF-1 (3, 80, 86) have been demonstrated. Complex formation between Tax and ATF/CREB proteins results in increased binding affinity of these factors to the HTLV-1 21-bp repeats (7, 13, 62, 80, 84). However, a more complex set of interactions is probably required for Tax activation of gene expression. The binding of Tax to the cellular coactivator CREB binding protein (CBP) and evidence demonstrating that ternary complexes form between Tax, CREB, and CBP on the HTLV-1 21-bp repeats suggest that the complex between Tax and CREB may act as a scaffold to recruit additional regulatory proteins to the HTLV-1 LTR (28, 44).
Tax is also capable of increasing gene expression via the NF-κB pathway by regulating multiple steps in NF-κB activation (reviewed in reference 35). Increased nuclear levels of NF-κB are present in HTLV-1-transformed lymphocytes (45), and this effect is probably mediated by the ability of Tax to increase the phosphorylation of both IκBα and IκBβ (14, 41, 50). The phosphorylated IκB proteins are targets for subsequent ubiquitination and proteasomal degradation, with resultant nuclear translocation of RelA (17). Tax may either directly or indirectly increase the activity of kinases which phosphorylate the amino terminus of both IκBα and IκBβ. In addition, Tax can directly associate in the cytoplasm with NF-κB2 or p100, which acts as an inhibitor of RelA nuclear localization. The interaction of Tax and p100 relieves p100 inhibition to result in the nuclear translocation of RelA (8, 35, 40, 59). Whether the interactions between Tax and NF-κB family members are mediated by additional factors that associate with NF-κB such as the coactivators CBP and p300 (63) remains to be determined. Finally, Tax colocalizes in nuclear structures with the NF-κB p50 and RelA subunits in addition to specific transcripts from a promoter containing NF-κB binding sites, which is activated by Tax (10, 61). Thus, Tax probably modulates several distinct processes which are important for the activation of gene expression via the NF-κB pathway.
The coactivator proteins CBP and p300 are involved in the regulation of gene expression via both the ATF/CREB and NF-κB pathways (27, 43, 44, 63). CBP is a protein of 265 kDa which was first identified as a factor that interacts with the phosphorylated form of CREB (18, 43). The homologous coactivator p300 was identified as a cellular protein which binds to the adenovirus E1A protein (20, 49). Both CBP and p300 belong to a conserved family of coactivators (1) which have a large network of interactions with a variety of cellular and viral proteins (39). These include oncogenic proteins such as c-jun and c-fos (40), c-Myb (19, 55), E1A (2, 20), the simian virus 40 (SV40) large T antigen (21), Tax (44), and the tumor suppressor p53 (32, 48); steroid hormone receptors (16, 40, 74); the sterol regulatory element binding proteins (57); and transcription factors including the RelA subunit of NF-κB (27, 63), the phosphorylated form of CREB (18, 43, 44, 59), and TFIIB (43). In addition, CBP has intrinsic histone acetylase activity (6, 56) and interacts with another cellular factor, P/CAF, that also possesses histone acetylase activity (82). CBP and p300 function as coactivators since they participate in the activation of gene expression by bridging upstream transcription factor complexes with the general transcription machinery to potentially increase the state of nucleosome acetylation. However, previous studies have not addressed whether these two related coactivator proteins have unique functional properties.
Tax colocalizes in discrete nuclear bodies with transcription factors, components of the spliceosome, and specific transcripts of genes that are activated by Tax (10, 70). To further characterize the role of these nuclear structures in Tax-mediated activation of gene expression, we addressed whether CBP and p300 and members of the ATF/CREB and NF-κB families of transcription factors were present with Tax in these nuclear structures. We found that the endogenous CBP and p300 were associated with distinct nuclear speckles in cells that do not express Tax. However, both CBP and p300 colocalized with wild-type Tax in nuclear bodies. In contrast, a Tax mutant which was defective in its ability to activate gene expression via the ATF/CREB but not the NF-κB pathway colocalized in nuclear structures with p300 and RelA but not with CBP. Another Tax mutant which activated gene expression via the ATF/CREB but not the NF-κB pathway formed nuclear structures containing CBP but not p300 and RelA. In vitro and in vivo protein interaction studies demonstrated that independent domains of Tax delineated by these mutations were required for Tax binding to either p300 or CBP. Thus, differential interactions with related coactivator proteins mediate the ability of Tax mutants to activate gene expression via either the ATF/CREB or the NF-κB pathways.
MATERIALS AND METHODS
Cell culture.
Cell lines were obtained from the American Type Culture Collection or the NIH AIDS Research and Reagent Program. BHK21 cells (hamster kidney, ATCC CRL 8544) were grown in Glasgow minimum essential medium (Gibco BRL, Gaithersburg, Md.) supplemented with 10% tryptose phosphate, 20 mM HEPES buffer, and 5% fetal bovine serum (FBS); HOS cells (human osteogenic sarcoma, ATCC CRL 1543) were grown in Dulbecco modified Eagle medium (Gibco BRL) supplemented with 10% FBS; and MT2 cells (human T-cell leukemia cells isolated from cord blood lymphocytes cocultured with cells from a patient with T-cell leukemia, obtained from the National Institutes of Health AIDS Research and Reagent Program) were grown in RPMI medium (Gibco BRL) supplemented with 10% FBS, 100 U of penicillin G sodium salt per ml, 100 μg of streptomycin sulfate per ml, and 2 mM glutamine.
Plasmids.
Cloning of the cDNA fragment carrying the wild-type tax gene or the tax gene carrying the F1 mutation (Ser113 and Leu128 converted into Ala and Pro, respectively) in the pSFV3 vector (47) was described previously (10). Mutations M47 (Leu319-Leu320 converted into Arg-Ser) (75) and M148 (Gly148 converted into Val) (81) were introduced into the tax gene by site-directed mutagenesis and cloned in the vector pSFV3. Wild-type Tax and the F1, M47, and M148 cDNAs were also inserted in the bacterial expression vector pQE60 (Qiagen). The human immunodeficiency virus (HIV-1) LTR chloramphenicol acetyltransferase (CAT) reporter plasmid, which contains the EcoRV (−343)-HindIII (+80) fragment from the HIV-1 LTR of ARV2 (34) and the HTLV-1 LTR CAT (68), was described previously.
Establishment of SFV-Tax recombinant stocks.
The Semliki Forest virus (SFV) expression system was described previously (9, 47). Briefly, SFV vector RNA and wild-type or mutant recombinant SFV-Tax RNAs were synthesized with an SP6 in vitro transcription system. Each of these RNAs was cotransfected with SFV helper 2 RNA by electroporation into BHK21 cells with an electroporation device (Bio-Rad, Hercules, Calif.). This generated virus stocks for control SFV, SFV-Tax, SFV-Tax F1, SFV-Tax M47, and SFV-Tax M148, which were concentrated 100-fold by ultracentrifugation, resuspended in TNE buffer (50 mM Tris-HCl [pH 7.4], 100 mM NaCl, 0.5 mM EDTA), and stored at −80°C. The virus stocks were activated by chymotrypsin treatment before use (9), and their titers were determined by immunofluorescence staining of BHK21 cells infected with dilutions of the different stocks. Typically, the recombinant virus stocks which ranged from 109 to 1010 PFU/ml were used to infect cells at a multiplicity of infection of 5.
Antibodies.
Monoclonal antibody 168-A51, recognizing a C-terminal epitope of Tax (NIH AIDS Research and Reagent Program), or an anti-Tax polyclonal serum generated by immunizing a rabbit with a purified glutathione S-transferase (GST)–Tax fusion protein was used in the immunofluorescence and Western blot analyses to detect the Tax protein. The monoclonal antibody directed against Sm, Y12 (64), the monoclonal antibody directed against p300, RW128 (20), and the anti-CREM rabbit polyclonal antibody were kind gifts from J. A. Steitz, S. Bhattacharya, and G. Schutz, respectively. Antibodies recognizing NF-κB p65 (C20), CREB (24H4B), CBP (A22), and ATF-1 (C41-5.1) were purchased from Santa Cruz Biotechnology, Inc., Santa Cruz, Calif., and used in the immunofluorescence studies. Additional antibodies for CBP (C20), CREB (C21), and ATF-1 (FI-1) were also purchased from Santa Cruz Biotechnology or generated by immunizing rabbits with GST fusions (CBP and CREB); these antibodies were used to confirm the antibody specificity seen in the immunofluorescence staining. The specificity of these antibodies and their ability to recognize the corresponding antigens in hamster (BHK21) and human (HOS) cells were tested by Western blot analysis. The secondary antibodies, goat anti-rabbit fluorescein isothiocyanate (FITC), goat anti-mouse lissamine rhodamine sulfchloride (LRSC), goat anti-mouse FITC, and goat anti-rabbit Texas Red conjugates, were purchased from Jackson ImmunoResearch, West Grove, Pa. Nuclei were stained with TO-PRO-3 (35), a fluorescent DNA stain purchased from Molecular Probes, Eugene, Oreg.
Immunocytochemistry and confocal microscopy.
Cells cultured on coverslips were infected at a multiplicity of infection of 5 with the different SFV recombinants, washed with phosphate-buffered saline (PBS), and fixed with methanol at −20°C for 6 to 15 min or alternatively with 3.7% paraformaldehyde followed by a brief permeabilization with 0.5% Triton X-100 at 4°C. The cells were washed twice with PBS, blocked for 30 min in PBS containing 0.5% gelatin and 0.25% bovine serum albumin, and incubated for 1 h at room temperature with the primary antibodies (diluted 1:50 to 1:150 in the blocking solution). The preparations were washed three times with 0.2% gelatin in PBS and incubated for 1 h with the secondary antibodies. Samples were washed three times and then mounted in a solution of 1 mg of p-phenylenediamine per ml in 90% glycerol.
For dual immunofluorescence staining with the rabbit polyclonal antibody directed against Tax and a monoclonal antibody directed against endogenous cellular proteins (ATF-1, p300, CREB, and Sm), the secondary antibodies used were anti-rabbit Texas Red and anti-mouse FITC conjugates. For dual immunofluorescence staining with the anti-Tax monoclonal antibody and rabbit polyclonal antibodies directed against endogenous cellular proteins (CBP, RelA, CREB, and CREM), the secondary antibodies used were anti-mouse LRSC and anti-rabbit FITC conjugates. For dual immunofluorescence staining with two monoclonal antibodies (Sm and p300), immunofluorescence staining was performed successively with each pair of primary and secondary antibodies. Incubation for 30 min with goat anti-mouse immunoglobulins was performed after staining by the first pair of antibodies to prevent nonspecific staining by the second pair of antibodies. Omission of the primary antibody in the second pair demonstrated minimal background staining. The preparations were examined on an MRC1024 laser scanning confocal microscope (Microscience Division, Bio-Rad, Cambridge, Mass.) equipped with a 15-mW air-cooled krypton-argon laser light source (Ion Laser Technology, Salt Lake City, Utah) as a light source. The emission filters were 522DF32 for FITC, 605DF32 for LRSC and Texas Red, and 680DF32 for TO-PRO-3. The single and two-color merged images were constructed from gray-scale confocal fluorescent images with Adobe software.
Transfections and CAT assays.
BHK21 cells (106 cells) were transfected with 1 μg of either the HIV-1 LTR CAT or HTLV-1 LTR CAT reporter plasmids by lipofection (Lipofectamine; Gibco BRL). After 5 h of incubation, the transfection medium was removed and the cells were infected with the different recombinant SFV-Tax stocks. The cells were collected at 24 h postinfection, and 10 μg of protein (typically 1/10) of the cell extract was assayed for determination of the CAT activity by separation of acetylated chloramphenicol by thin-layer chromatography. The percentage of acetylated chloramphenicol was quantitated with a phosphorimager.
Expression and purification of bacterially produced Tax proteins.
The full-length histidine-tagged wild-type and mutant Tax cDNAs which were cloned into the pQE60 vector were transformed into Escherichia coli M15(pREP 4). The plasmid for bacterial production of the GST-CBP (amino acids [aa] 450 to 682) fusion was constructed by inserting a PCR fragment containing nucleotides 1351 to 2046 of CBP into the BamHI and HindIII restriction sites of vector pGEX-KG (73). PCR was performed with plasmid RSV/CBP (a gift from R. Goodman) as a template. The plasmid for bacterial expression of GST-p300 (aa 436 to 662) was constructed in the same way with p300 cDNA (20) (a gift from D. Livingston) as a template for PCR amplification of nucleotides 1306 to 1986. The resultant plasmids were transformed into E. coli BL21.
Expression of the GST fusion proteins was induced by adding 0.5 mM isopropyl-β-d-thiogalactopyranoside for 3 h to the bacterial culture in the exponential phase of growth. The bacteria were pelleted, resuspended in buffer A (50 mM Tris-HCl [pH 8.0], 100 mM NaCl, 0.1 mM phenylmethylsulfonyl fluoride), and mildly sonicated, and the debris was pelleted. The proteins were purified after binding to glutathione-agarose beads and elution with glutathione as described previously (73) and visualized by Coomassie blue staining. The cell lysates containing the histidine-tagged Tax proteins were incubated with 1 ml of Ni-nitrilotriacetic acid agarose (Qiagen) for 60 min at 4°C. The proteins were eluted with buffer (5 mM Tris-HCl [pH 8.0], 100 mM NaCl, 1 mM dithiothreitol, 1 mM EDTA) containing 300 mM imidazole, and the Tax proteins were visualized by Coomassie blue staining.
In vitro protein-protein binding.
GST-CBP (aa 450 to 682) or GST-p300 (aa 436 to 662) fusion proteins (20 μg) were incubated with glutathione-agarose beads for 1 h at 4°C. Approximately 500 ng of purified wild-type or mutant Tax proteins was then added in TIB buffer (10% glycerol, 20 mM HEPES [pH 7.5], 50 mM KCl, 50 nM ZnCl2, 2.5 mM MgCl2, 0.1% Nonidet P-40, 1 mM dithiothreitol, 0.1 mM phenylmethylsulfonyl fluoride) for 1 h at 4°C. The protein-protein complexes were washed five times each with 10 volumes of TIB buffer at 4°C. The Tax proteins which remained bound to the beads were resolved on sodium dodecyl sulfate (SDS)–10% polyacrylamide gels and were detected by Western blot analysis with a monoclonal antibody directed against Tax.
Immunoprecipitation of Tax with p300 and CBP.
BHK21 cells which were infected for 18 h with SFV-Tax, SFV-Tax F1, SFV-Tax M47, or SFV-Tax M148 were resuspended in 500 μl of lysis buffer containing 50 mM Tris-HCl (pH 8.0), 100 mM NaCl, 0.2% Nonidet P-40, 1 mM phenylmethylsulfonyl fluoride, 0.5 μg of leupeptin per ml, and 1 μg of aprotinin per ml. The cell suspensions were incubated on ice for 20 min and then homogenized. The lysates were centrifuged twice at 17,000 × g for 30 min at 4°C. The supernatants were incubated overnight at 4°C with 100 ng of a monoclonal antibody to Tax. Then a 20-μl bed volume of protein A-agarose (Bio-Rad) was added, and the mixture was incubated at 4°C for 1 h. The immunoprecipitated complexes were washed five times with 10 volumes of lysis buffer. The components of the complexes were resolved on an SDS-polyacrylamide gel (either 10% [Tax] or 6% [CBP and p300] polyacrylamide) and detected by Western blot analysis.
RESULTS
Differential activation of the NF-κB and ATF/CREB pathways by Tax mutants.
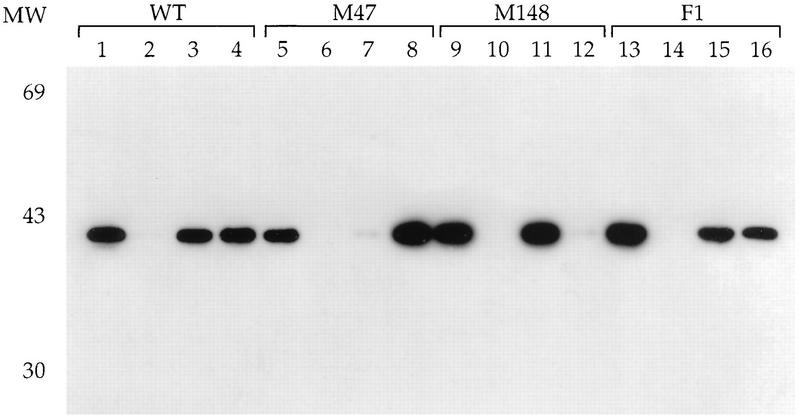
We have previously demonstrated that expression of Tax with an SFV vector results in the same punctate nuclear distribution of Tax observed in HTLV-1-transformed lymphocytes (10). This expression system was used to express wild-type and mutant Tax proteins F1 (Ser113 and Leu128 converted into Ala and Pro) (10), M148 (Gly148 converted into Val) (81), and M47 (Leu319 and Leu320 converted into Arg and Ser) (75) (Fig. 1A). These Tax mutants are defective in the activation of gene expression via either the ATF/CREB (M47), the NF-κB (M148), or both (F1) pathways. Western blot analysis of cellular extracts from BHK21 cells infected for increasing times with the recombinant SFV expressing wild-type Tax indicated that Tax is present at 6 h postinfection and further accumulates in the cells during the next 18 h (Fig. 1B). The steady-state levels of the Tax F1, M47, and M148 mutant proteins were identical to that of wild-type Tax (Fig. 1B).
FIG. 1.
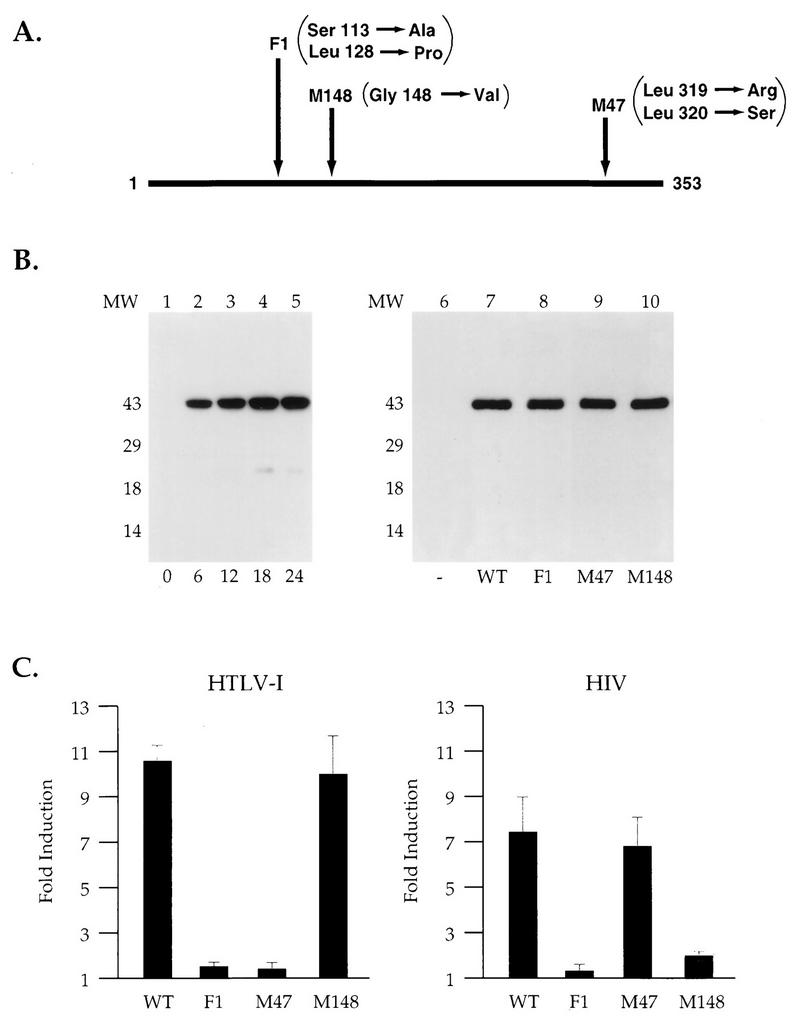
Differential activation of NF-κB and ATF/CREB pathways by Tax mutants. (A) A schematic of the 353-aa Tax protein. The positions of the amino acid changes for the three Tax mutants F1, M148, and M47 are shown. (B) Western blot analysis was performed on extracts prepared from BHK21 cells infected with SFV-Tax for 0 h (lane 1), 6 h (lane 2), 12 h (lane 3), 18 h (lane 4), or 24 h (lane 5). BHK21 cells were infected for 18 h with either control SFV (lane 6), SFV-Tax WT (lane 7), SFV-Tax F1 (lane 8), SFV-Tax M47 (lane 9), or SFV-Tax M148 (lane 10). Cellular extracts were subjected to electrophoresis on an SDS–15% polyacrylamide gel, and Western blot analysis was performed with a monoclonal antibody directed against Tax. Molecular weights (MW) in thousands are shown on the left. (C) BHK21 cells were transfected with either 1 μg of HTLV-1 CAT or HIV-1 CAT reporter plasmids; 5 h after transfection, the cells were infected with the control SFV (WT), SFV-Tax WT, SFV-Tax F1, SFV-Tax M47, or SFV-Tax M148, and they were harvested 24 h later. The CAT activity of the cell extracts was then determined, and the fold induction by either wild-type or mutant Tax was calculated for three independent experiments with the standard deviation indicated.
Next we characterized the ability of wild-type and mutant Tax proteins to activate gene expression via either the NF-κB or the ATF/CREB pathway (Fig. 1C). An HIV-1 LTR CAT reporter which contains two NF-κB sites was used to assay for the effects of Tax on the NF-κB pathway, and an HTLV-1 LTR CAT reporter which contains three unique CRE sites known as 21-bp repeats was used to assay for the effects of Tax on the ATF/CREB pathway. Similar reporter constructs have been used in previous studies to define Tax activation via either the ATF/CREB or NF-κB pathway (10, 69, 75, 81). BHK21 cells were transfected with the HTLV-1 LTR CAT or HIV-1 LTR CAT reporter plasmids and subsequently infected with the control SFV or SFV expressing the wild-type Tax or the Tax mutants M47, M148, and F1. These studies demonstrated that wild-type Tax could activate gene expression from both the HIV-1 and the HTLV-1 LTR whereas the Tax mutant F1 was defective in activating gene expression from both promoters (Fig. 1C). In contrast, the Tax mutant M47 was defective in the activation of gene expression from the HTLV-1 LTR but not the HIV-1 LTR (75) whereas the Tax mutant M148 activated gene expression from the HTLV-1 LTR but not the HIV-1 LTR (81). These studies demonstrate that the transfection/infection system accurately reflected the transcriptional activity of previously described Tax mutants.
CBP and p300 associate with distinct nuclear speckles.
Since Tax has been demonstrated to bind to the coactivators CBP and p300 (44), which are involved in the regulation of gene expression via the ATF/CREB and NF-κB pathways (27, 43, 44, 63), we addressed whether CBP and/or p300 was present in discrete nuclear structures with Tax. First, it was important to determine the intracellular localization of CBP and p300 by using immunofluorescence staining of uninfected BHK21 cells. Antibodies that were able to specifically recognize each of these proteins were first analyzed by Western blot analysis of BHK21 cells. As shown in Fig. 2, these antibodies specifically reacted with CBP and p300. Dual immunofluorescence staining with a rabbit polyclonal serum directed against CBP and TO-PRO-3 (35), a fluorescent DNA stain, indicated that CBP was present predominantly in the nuclei of the cells (Fig. 3A), as previously noted (18). CBP was distributed in a nuclear speckled structure; its distribution resembled the nuclear distribution of splicing factor Sm. Dual immunofluorescence staining of these cells with antibodies directed against CBP and Sm indicated that CBP colocalized with Sm in the nuclear speckled structures (Fig. 3B).
FIG. 2.
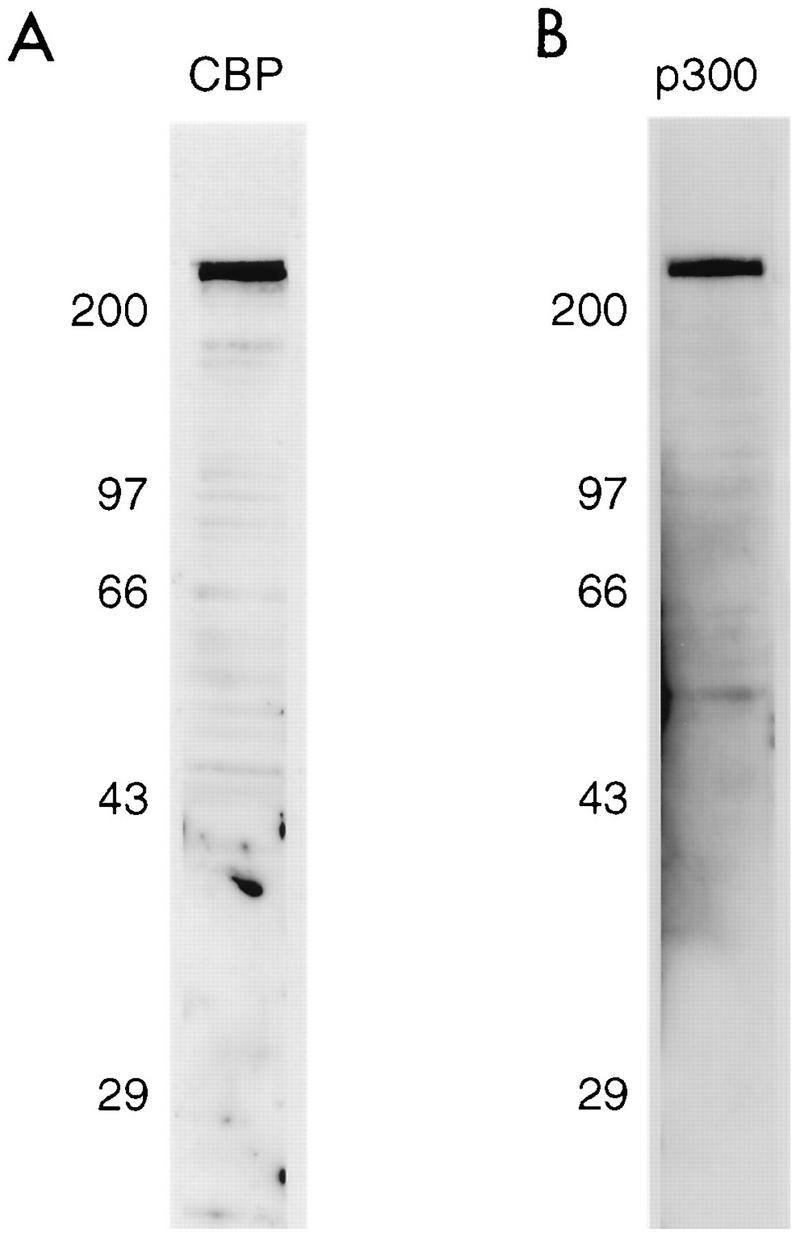
Specificity of the antibodies to CBP and p300. Western blot analysis was performed on BHK21 cell lysates with the rabbit polyclonal antibody to CBP (A) or the monoclonal antibody to p300 (B).
FIG. 3.
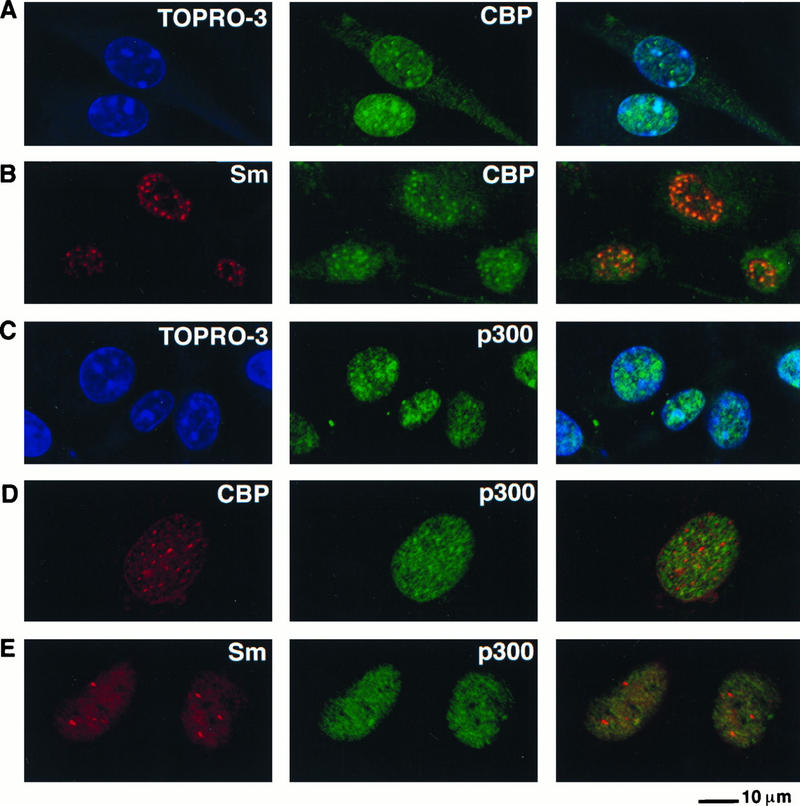
CBP and p300 are distributed in distinct nuclear speckles. Uninfected BHK21 cells were fixed and analyzed by single immunofluorescence staining with either a rabbit polyclonal antibody that recognizes CBP (A) or a monoclonal antibody to p300 (C). The anti-CBP antibody was also used in combination with the monoclonal antibody to splicing factor Sm (B) or the monoclonal antibody to p300 (D). The anti-p300 antibody was used in combination with the monoclonal antibody to Sm (E). The DNA stain TO-PRO-3 was used to stain the nuclei (A and C). The panel on the right demonstrates the overlay of the blue and green staining or the red and green staining.
Like CBP, p300 also localized predominantly in the nucleus and displayed a speckled distribution in both uninfected BHK21 cells (Fig. 3C) and HOS cells (data not shown). However, the p300 and CBP speckles formed two populations of predominantly nonoverlapping nuclear structures, although the techniques do not exclude the possibility that a subset of the speckles contained both CBP and p300 (Fig. 3D). In contrast to the colocalization of CBP and Sm, the p300 nuclear speckle was predominantly distinct from the Sm speckle (Fig. 3E). Infection of BHK21 or HOS cells with the control SFV and fixation of the cells with paraformaldehyde instead of methanol gave the same distribution of both CBP and p300 (data not shown).
Tax mutants exhibit differential colocalization with CBP and p300.
Next we determined whether Tax colocalized with either CBP or p300 by performing dual immunofluorescence staining of BHK21 cells infected with SFV-Tax. De novo expression of Tax led to the formation of nuclear bodies in which both CBP (Fig. 4A) and p300 (Fig. 4B) colocalized with Tax. These results demonstrated that both CBP and p300 were distributed predominantly in distinct nuclear speckles and that both of these proteins were able to colocalize in nuclear bodies with wild-type Tax.
FIG. 4.
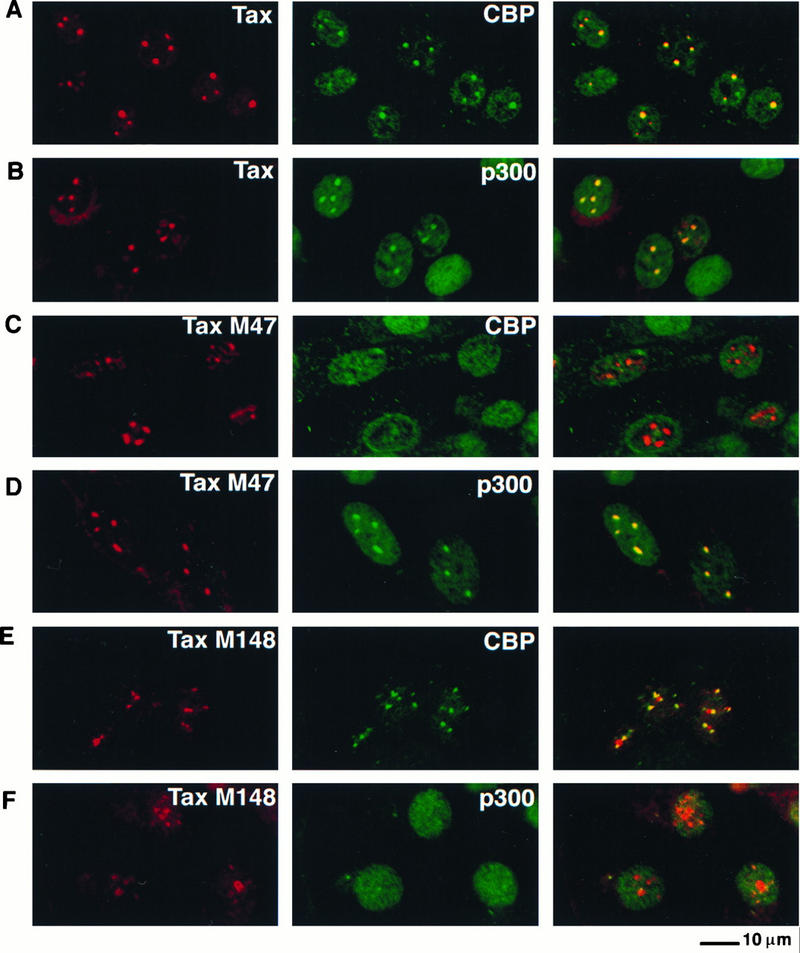
CBP and p300 colocalize with wild-type Tax in nuclear bodies. BHK21 cells were infected for 18 h with SFV-Tax (A and B), SFV-Tax M47 (C and D), or SFV-Tax M148 (E and F). The cells were fixed and stained by dual immunofluorescence with either a monoclonal antibody to Tax in combination with a rabbit polyclonal antibody that specifically recognizes CBP (A, C, and E) or a rabbit polyclonal antibody to Tax and a monoclonal antibody to p300 (B, D, and F). The panel on the right demonstrates the overlay of the red and green fluorescence staining.
The Tax mutants M47 and M148 are selectively altered in their ability to activate gene expression via the ATF/CREB and NF-κB pathways, respectively. M47 is defective in activation of gene expression via the ATF/CREB but not the NF-κB pathway, while M148 is defective in the activation of gene expression via the NF-κB pathway but not the ATF/CREB pathway (Fig. 1C). Each of these Tax mutants was next assayed for its ability to colocalize with CBP and p300 after infection of BHK21 cells with recombinant SFV. Although the Tax M47 mutant formed nuclear structures which contained Tax, CBP did not colocalize in these structures (Fig. 4C). In contrast, expression of the Tax M47 mutant resulted in colocalization of this protein with p300 in nuclear bodies, as observed with wild-type Tax (Fig. 4D). The Tax M148 mutant which activates HTLV-1 gene expression via the ATF/CREB pathway colocalized with CBP in nuclear foci (Fig. 4E). In contrast, this mutant did not colocalize with p300 (Fig. 4F). In addition to the nuclear structures containing both the M148 Tax protein and CBP, other Tax-containing structures devoid of CBP were observed (Fig. 4E). This result indicates that this mutant may have a defect in the formation of the Tax-containing nuclear structures. The correlation between the differential ability of the two Tax mutants to colocalize with CBP or p300 and their ability to selectively activate gene expression via the ATF/CREB or NF-κB pathway suggests that CBP may be a critical factor in Tax activation via the ATF/CREB pathway while p300 may be crucial for Tax activation via the NF-κB pathway.
Two independent domains of Tax mediate interaction with either CBP or p300.
The Tax protein has been demonstrated to interact with the KIX domains of CBP and p300. The KIX domain is also the site of interaction with the phosphorylated form of CREB (44). The results presented here suggest that the Tax mutants M47 and M148 may be selectively defective for interaction with CBP and p300. Using in vitro protein interaction assays, we next tested whether the ability of the F1, M47, and M148 Tax mutants to interact with the KIX domains of CBP and p300 was altered from that of wild-type Tax. Either GST-CBP (aa 450 to 682) or GST-p300 (aa 436 to 662) was bound to glutathione-agarose beads and incubated with bacterially produced wild-type or mutant Tax proteins. After extensive washing, the Tax proteins which remained attached to the GST fusion proteins were resolved by SDS-polyacrylamide gel electrophoresis and visualized following Western blot analysis with a monoclonal antibody to Tax (Fig. 5).
FIG. 5.
Independent domains in Tax mediate the interaction with CBP and p300. GST alone (lanes 2, 6, 10, and 14), GST-CBP (aa 450 to 682) (lanes 3, 7, 11, and 15), or GST-p300 (aa 436 to 662) (lanes 4, 8, 12, and 16) was bound to glutathione-agarose beads and incubated with 500 ng of purified wild-type (WT) Tax (lanes 2 to 4), Tax M47 (lanes 6 to 8), Tax M148 (lanes 10 to 12), or Tax F1 (lanes 14 to 16) protein. After extensive washing, the Tax proteins which remained attached to the beads were resolved by SDS-polyacrylamide gel electrophoresis and analyzed by Western blot analysis with a monoclonal antibody to Tax. Lanes 1, 5, 9, and 13 contain approximately 10% of the input Tax proteins. Molecular weights (in thousands) are shown on the left.
Wild-type Tax did not interact with glutathione-agarose beads containing GST alone but bound strongly to both GST-CBP and GST-p300, confirming that Tax binds strongly to the KIX domains of CBP and p300 (44) (Fig. 5, lanes 1 to 4). Tax mutant M47 bound only weakly to GST-CBP but exhibited wild-type binding to GST-p300 (lanes 7 and 8). In contrast, Tax mutant M148 exhibited wild-type binding to GST-CBP but only weak binding to GST-p300 (lanes 11 and 12). Binding of Tax mutant F1 resulted in only a slight reduction in the quantity of Tax bound to both GST-CBP and GST-p300 (lanes 15 and 16). These results confirm that wild-type Tax interacts directly with both CBP and p300. They also demonstrate that two domains of Tax, a central domain and a carboxy-terminal domain delineated by mutations M148 and M47, mediate interactions with either p300 or CBP. Thus, differential interactions of Tax with the KIX domains of p300 and CBP correlate with the ability of Tax to activate gene expression via either the NF-κB or the ATF/CREB pathway, respectively.
In vivo association of Tax with CBP and p300.
We next asked whether the in vitro interactions between Tax and the KIX domains of both CBP and p300 correlated with the formation of in vivo complexes between Tax and the endogenous pools of CBP and p300. A monoclonal antibody to Tax was used in immunoprecipitation studies with cells expressing either wild-type Tax or the M148, M47, and F1 Tax mutants. The presence of CBP and p300 in the immunoprecipitated complexes was then analyzed by Western blot analysis.
First, Western blot analysis was performed on lysates from SFV-infected cells expressing wild-type or mutant Tax proteins to demonstrate whether equivalent amounts of Tax, CBP, and p300 were present. Equivalent quantities of these proteins were found to be present (Fig. 6A to C). Immunoprecipitation of an extract prepared from mock-infected BHK21 cells with Tax antibody followed by Western blot analysis resulted in no detectable CBP or p300 (Fig. 6D and E). Immunoprecipitation of an extract prepared from BHK21 cells infected with SFV-Tax or SFV-Tax F1 with Tax antibody followed by Western blot analysis with antibodies to CBP or p300 demonstrated the presence of both of these proteins (Fig. 6D and E). In contrast, immunoprecipitation with Tax antibody followed by Western blot analysis of an extract prepared from cells infected with SFV-Tax M47 demonstrated the presence of p300 but not CBP (Fig. 6D and E). Immunoprecipitation with Tax antibody followed by Western blot analysis of extract prepared from cells infected with SFV-Tax M148 demonstrated the presence of CBP but not p300 (Fig. 6D and E). These results indicated that wild-type Tax interacts with both CBP and p300 whereas mutants M148 and M47 selectively associate with CBP and p300, respectively. Thus, the ability of different Tax mutants to colocalize in nuclear bodies with CBP and p300 correlated not only with their ability to interact in vitro with the KIX domains of CBP or p300 but also with their ability to form in vivo complexes with these related coactivator proteins.
FIG. 6.
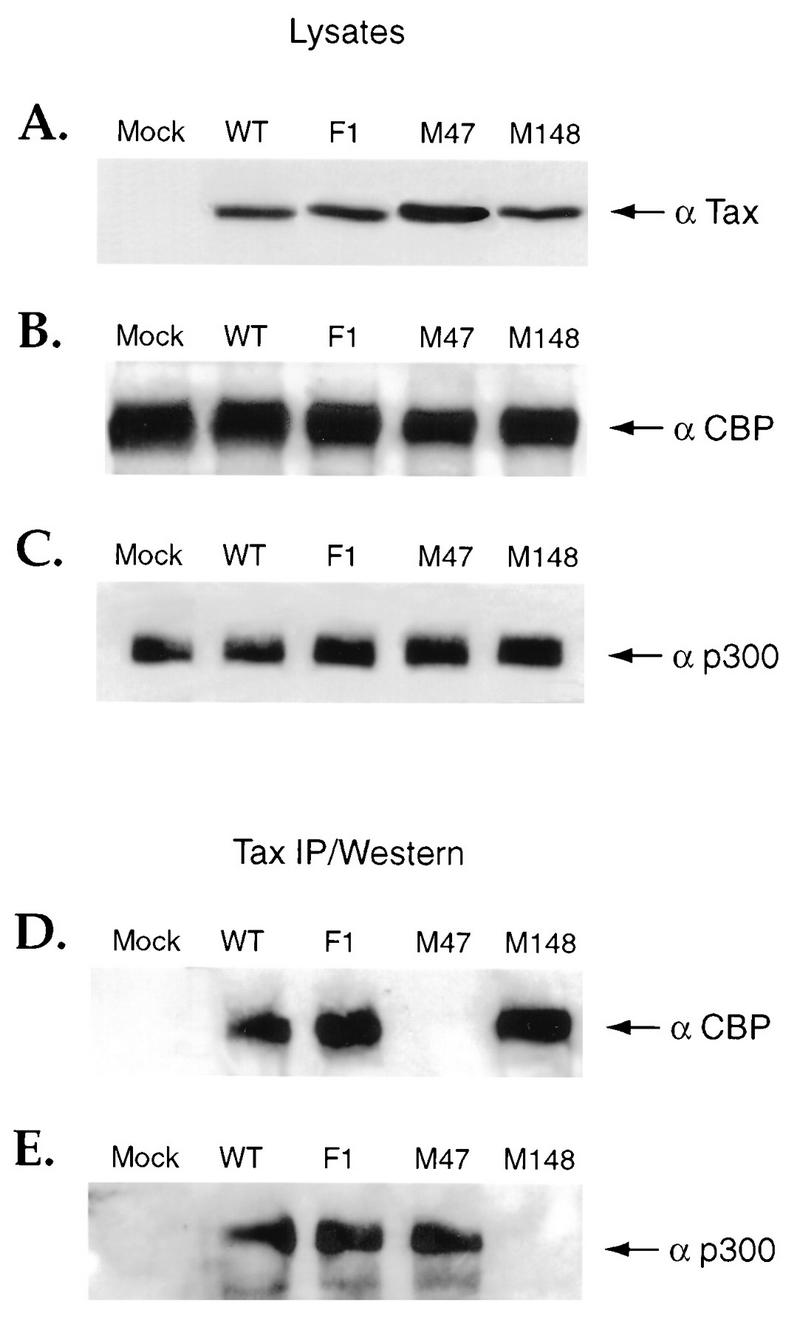
In vivo association of Tax with CBP and p300. BHK21 cells were infected for 18 h with the control SFV (Mock) or with SFV expressing wild-type (WT) Tax or Tax mutants F1, M47, and M148. The cells were collected, and coimmunoprecipitation assays were performed with a monoclonal antibody to Tax. Western blot analysis was then performed with the cell lysates, using antibodies to Tax (A), CBP (B), and p300 (C), or on the immunoprecipitated complexes (IP), using antibodies to CBP (D) or p300 (E).
Nuclear structures containing the Tax M148 mutant protein do not contain the RelA subunit of NF-κB.
The nuclear translocation of the RelA subunit of NF-κB is critical for the activation of NF-κB-responsive promoters (67). Since RelA interacts with CBP and p300 (27, 63), it was important to test whether the presence of p300 in the nuclear structures containing Tax correlated with the presence of RelA. We determined whether the Tax mutants M47 and M148 were able to induce RelA to colocalize in nuclear structures with these Tax mutant proteins. BHK21 cells expressing either wild-type Tax or the Tax mutants F1, M47, or M148 were analyzed by dual immunofluorescence staining with antibodies to Tax and RelA. Wild-type Tax (Fig. 7B) but not an SFV control vector (Fig. 7A) induced RelA translocation from the cytoplasm and resulted in Tax and RelA colocalization in nuclear structures (Fig. 7B), as we demonstrated previously (10). Tax mutant F1, which was predominantly cytoplasmic and did not form nuclear bodies (10), was nevertheless able to induce the translocation of RelA into the nucleus (Fig. 7C). Tax mutant M47 colocalized in nuclear structures with RelA (Fig. 7D). However, the nuclear structures containing the Tax mutant M148 did not contain RelA, which remained localized predominantly in the cytoplasm (Fig. 7E). A collection of images from horizontal sections (Z series and projections) demonstrated that Tax and RelA colocalized (Fig. 8). Z series were collected for each of the factors colocalizing with Tax, including CBP, p300, ATF-1, and Sm, and gave similar results (data not shown).
FIG. 7.

NF-κB RelA exhibits differential colocalization with the Tax mutants M47 and M148. BHK21 cells were infected for 18 h with control SFV (A), SFV-Tax (B), SFV-Tax F1 (C), SFV-Tax M47 (D), or SFV-Tax M148 (E). The cells were fixed and stained by dual immunofluorescence with a monoclonal antibody to Tax (left panel) and a rabbit polyclonal antibody to the RelA subunit of NF-κB (middle panel). The panel on the right demonstrates the overlay of the red and green fluorescence staining.
FIG. 8.
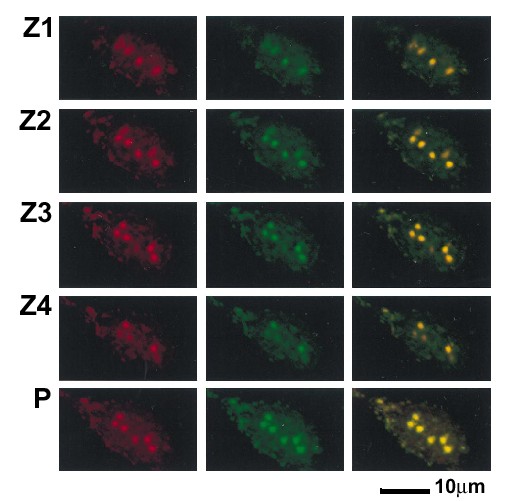
Assessment of Tax and RelA colocalization. Images from horizontal sections (Z series) were collected from a specimen of BHK21 cells infected with SFV-Tax. The cells were analyzed by dual immunofluorescence staining with antibodies to Tax and RelA. Z1 to Z4 are four of these sections, which were collected with 1-μm intervals. P is the projection of the Z series. The left panel displays the LRSC fluorescence of Tax, the middle panel displays the FITC fluorescence of RelA, and the right panel is the overlay of the red and green fluorescence staining.
Tax mutant M47, which activates the NF-κB pathway, colocalized with both p300 and RelA, while Tax mutant M148, which does not activate this pathway, did not colocalize with either p300 or RelA. These results are consistent with the observations of Yamaoka et al. demonstrating that RelA is not translocated into the nucleus in cells expressing Tax mutant M148 (81). Since Tax mutant M148 did not colocalize with either p300 or RelA, the inability to activate gene expression via the NF-κB pathway could be due to the failure of Tax to induce the translocation of RelA into the nucleus and/or to the inability of this mutant to interact with p300. However, Tax mutant F1 induced RelA nuclear translocation (Fig. 7C) but was defective in the activation of gene expression via the NF-κB pathway (Fig. 1C). This result suggests that specific activation of gene expression by Tax via the NF-κB pathway is probably dependent on both Tax-mediated nuclear translocation of RelA and direct interaction of Tax with p300 in nuclear bodies.
Transcription factor ATF-1, but not CREB and CREM, colocalizes with Tax in nuclear foci.
We then addressed whether members of the ATF/CREB family were able to colocalize with Tax in nuclear structures. Tax has been demonstrated to directly interact in vitro with the bZIP domains of the related ATF/CREB factors CREB, ATF-1, and CREM and to increase the affinity of binding of these factors to the HTLV-1 LTR (7, 13, 58, 62, 78, 83, 84, 86). To study whether the localization of these ATF/CREB factors correlated with Tax-mediated increases in HTLV-1 gene expression, we used immunofluorescence staining and confocal microscopy to assay for the presence of these factors in nuclear bodies containing either wild-type or mutant Tax proteins.
Dual immunofluorescence staining of BHK21 cells infected with SFV-Tax was performed with an antibody to Tax and antibodies that specifically recognized either ATF-1, CREB, or CREM. ATF-1 was present as a speckled and diffuse pattern in the nuclei of cells infected with an SFV vector lacking the tax gene (Fig. 9A) and in uninfected cells (data not shown). The expression of wild-type Tax resulted in colocalization of ATF-1 with Tax in nuclear bodies (Fig. 9B). The Tax F1 mutant protein is localized predominantly in the cytoplasm and does not result in the formation of nuclear bodies (Fig. 7C). The staining of both ATF-1 and the Tax F1 mutant protein was detected throughout the cytoplasm in addition to nuclear staining of ATF-1 (Fig. 9C). Both the M47 and M148 Tax mutants colocalized with ATF-1 in discrete nuclear structures (Fig. 9D and E). Thus, de novo expression of Tax results in the formation of nuclear bodies which contain ATF-1 in addition to Tax. Furthermore, Tax mutant M47, which did not activate gene expression via ATF/CREB pathway, colocalized with ATF-1 but not with CBP. This result suggests that inclusion of ATF-1 in the nuclear bodies may not be sufficient for Tax-mediated activation of gene expression via the ATF/CREB pathway but that inclusion of CBP is probably also required.
FIG. 9.
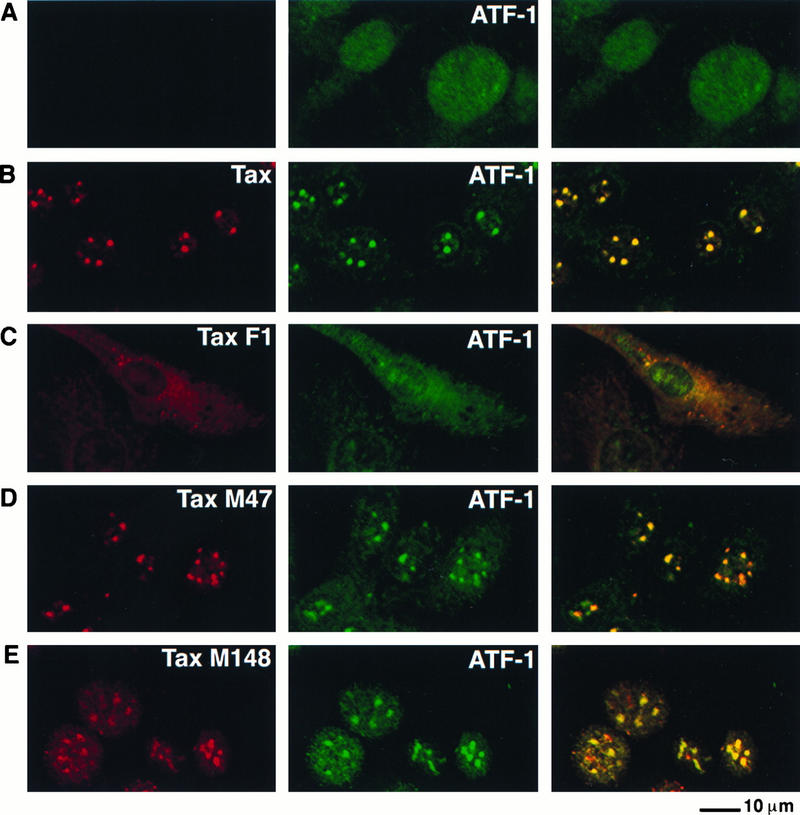
ATF-1 colocalizes with Tax in nuclear foci. BHK21 cells were infected for 18 h with control SFV (A), SFV-Tax (B), SFV-Tax F1 (C), SFV-Tax M47 (D), or SFV-Tax M148 (E). The cells were then fixed and stained by dual immunofluorescence with a rabbit polyclonal serum to Tax (left panel) and a monoclonal antibody that specifically recognizes ATF-1 (middle panel). The panel on the right demonstrates the overlay of the FITC and Texas Red fluorescence.
Next we assayed the ability of both CREB and CREM to colocalize with Tax in discrete nuclear bodies. In contrast to the results with ATF-1, no colocalization of CREB and CREM with wild-type Tax was observed when antibodies specifically recognizing these proteins were used in dual staining in conjunction with an antibody to Tax. CREB displayed a cytoplasmic filamentous distribution in addition to forming discrete nuclear foci, and CREM had a granular perinuclear distribution in cells that do not express Tax (Fig. 10A and C). A similar distribution of CREB and CREM was noted in uninfected cells (Fig. 10A and C) and in cells infected with an SFV vector lacking the tax gene (data not shown). Expression of Tax did not modify the distribution of CREB and CREM, and there was no colocalization of Tax with these two proteins (Fig. 10B and D). The same distribution of CREB was seen when three different antibodies that specifically recognized CREB were used and when other cell lines were analyzed (data not shown). Treatment of cells with forskolin, which stimulates protein kinase A-dependent phosphorylation of CREB, did not result in colocalization of CREB with Tax. Thus, the three homologous factors CREB, CREM, and ATF-1 display differential cellular distribution and only one of these factors, ATF-1, colocalized with Tax in nuclear bodies.
FIG. 10.
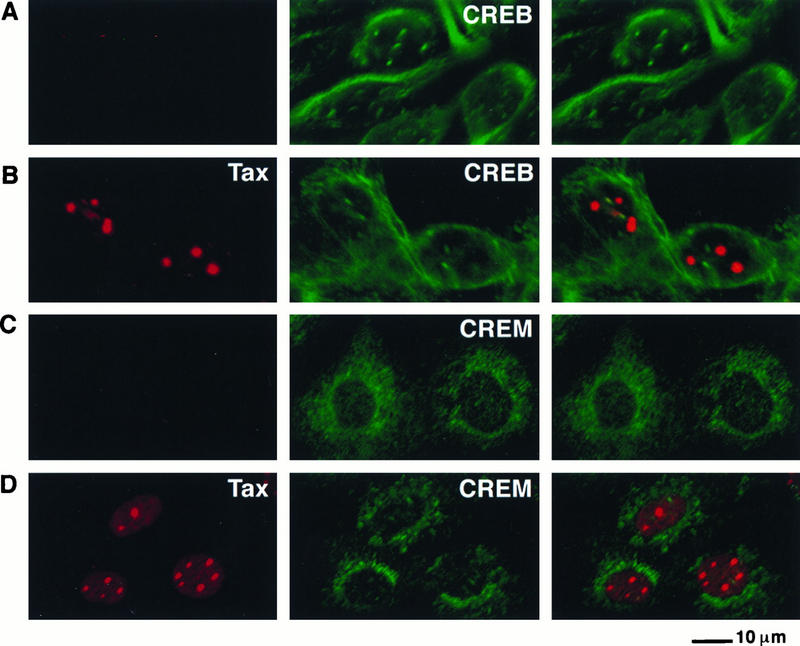
Tax does not colocalize with the ATF/CREB proteins CREB and CREM. Either uninfected BHK21 cells (A and C) or BHK21 cells infected for 18 h with SFV-Tax (B and D) were fixed and analyzed by dual immunofluorescence staining with a Tax monoclonal antibody (left panel) and specific polyclonal antibodies that recognize either CREB (A and B) or CREM (C and D) (middle panel). The panel on the right demonstrates the overlay of the red and green fluorescence.
CBP, p300, and ATF-1 colocalize with Tax in nuclear foci of HTLV-1-transformed lymphocytes.
It was important to address whether Tax colocalized with CBP and p300 in HTLV-1-transformed lymphocytes. We have previously demonstrated that Tax displays both a nuclear punctate distribution and a diffuse cytoplasmic staining in MT2 cells, which are HTLV-1-transformed lymphocytes (10). Both MT2 and Jurkat cells were analyzed by dual immunofluorescence staining for colocalization of either CBP or p300 with Tax. CBP displayed a punctate nuclear distribution in both Jurkat cells and MT2 cells (Fig. 11A and B), and the nuclear foci present in MT2 cells contained both CBP and Tax (Fig. 11B). In addition, Tax and p300 colocalized in the nuclei of MT2 cells (Fig. 11D).
FIG. 11.
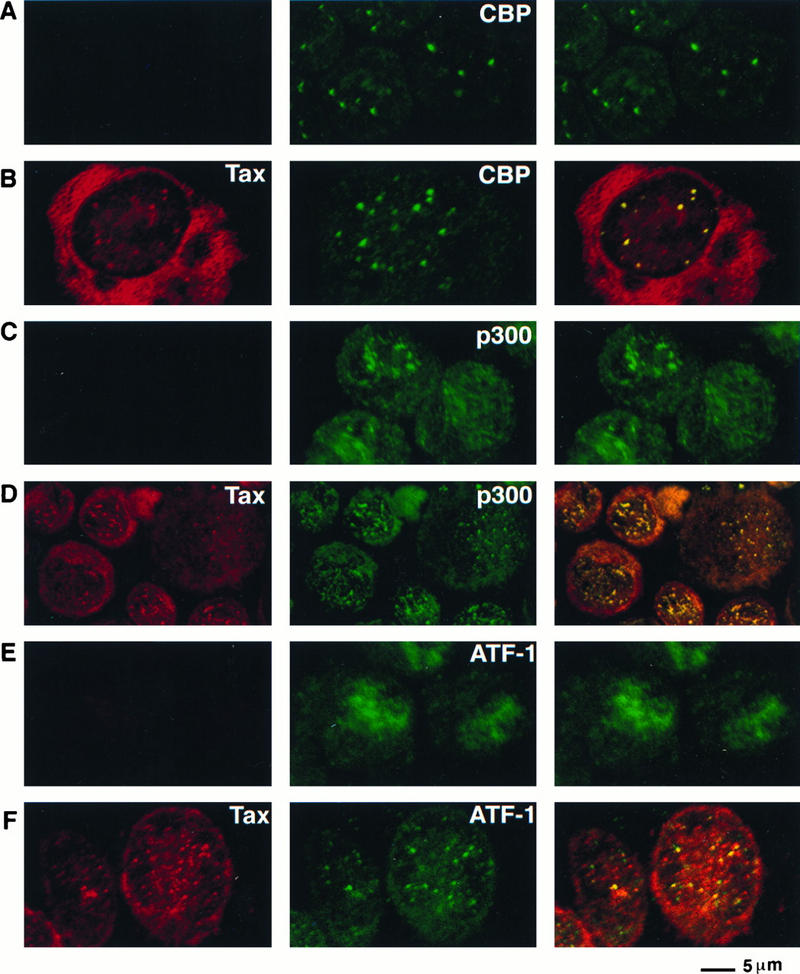
Tax colocalizes with CBP, p300, and ATF-1 in discrete nuclear foci present in HTLV-1-transformed lymphocytes. Either the HTLV-1-transformed MT2 lymphocytes (B, D, and F) or the T-lymphocyte Jurkat cells (A, C, and E) were centrifuged on microscope slides, fixed, and analyzed by dual immunofluorescence staining with a monoclonal antibody to Tax (left panel) and antibodies that specifically recognize either CBP (A and B) or p300 (C and D) (middle panel). The rabbit polyclonal antibody to Tax was used in conjunction with the monoclonal antibody to ATF-1 (E and F). The panel on the right demonstrates the overlay of the red and green fluorescence.
Finally, we determined the distribution of ATF-1, CREB, and CREM in MT2 and Jurkat cells. In MT2 cells, ATF-1 was present in a punctate distribution in the nucleus (Fig. 11F). Such a distribution was not observed in Jurkat cells; instead, ATF-1 displayed a diffuse distribution (Fig. 11E). Tax colocalized with ATF-1 in a subset of the nuclear foci in MT2 cells (Fig. 11F). Neither CREB nor CREM colocalized with Tax in MT2 cells (data not shown). Thus, ATF-1, CBP, and p300 colocalize with Tax in nuclear foci in both HTLV-1-transformed lymphocytes and BHK21 cells following de novo expression of Tax.
DISCUSSION
Recent studies have demonstrated that two related coactivators, CBP and p300, are involved in a variety of protein-protein interactions which activate gene expression (39). These proteins may regulate gene expression by bridging upstream transcription factor complexes with the general transcriptional machinery to result in nucleosome remodeling. Here we demonstrate that de novo expression of Tax results in the colocalization of the two coactivators CBP and p300 with members of the ATF/CREB and NF-κB families of transcription factors in nuclear bodies that also contain Tax. Endogenous CBP and p300 are both present in nuclear speckles in a variety of different cell lines tested (human lymphocytes, human osteosarcoma cells, and hamster fibroblasts). However, these two related factors display differential localization. The CBP and p300 nuclear speckles are predominantly nonoverlapping, and CBP, but not p300, associates with the Sm speckle. CBP and p300 colocalize with Tax in nuclear bodies both in cells expressing Tax via infection with SFV-Tax and in HTLV-1-transformed lymphocytes. We previously demonstrated that RNA polymerase II, splicing factors (Sm and SC35), and mRNA encoded by a promoter specifically activated by Tax are also present in nuclear bodies with Tax (10).
In this study, we established a correlation between the ability of Tax mutants to interact with the related coactivators CBP and p300, the inclusion of these factors with Tax in nuclear structures, and the ability of these Tax mutants to activate gene expression via either the ATF/CREB or the NF-κB pathway. These data suggest that nuclear localization of distinct sets of regulatory proteins is crucial for Tax activation of gene expression via the ATF/CREB and NF-κB pathways. The relevance of these observations, which were generated with an SFV vector system for expression of Tax, is supported by the fact that expression of Tax by infection with the SFV-Tax vector results in the same pattern of transcriptional activity for wild-type and mutant Tax proteins previously observed in plasmid transfection system assays (69, 75, 81). Furthermore, the transient-expression system used in our study also demonstrated the punctate nuclear distribution of Tax and its colocalization with cellular factors such as Sm, CBP, p300, and ATF-1 which is seen in HTLV-1-transformed lymphocytes.
Subcellular localization of related transcriptional regulatory factors.
The differential subcellular localization of related factors belonging to the ATF/CREB and CBP/p300 families was addressed in this study. Among the three related ATF/CREB factors, ATF-1, CREB, and CREM, only ATF-1 colocalizes with Tax in nuclear bodies. These three proteins have a high degree of sequence homology in their carboxy-terminal basic leucine zipper (bZIP) domains, which is both necessary and sufficient for their interaction with Tax (62, 80, 83). In addition, they form a subclass of ATF/CREB factors, with each protein containing serine residues in the KID domain (29) which are phosphorylated in response to extracellular signaling and are involved in interactions with CBP (18, 43, 44, 59). Thus, these factors contain a common bZIP domain, which enables interaction with Tax, and a specific domain, which may account for their differential subcellular localization and their differential redistribution in response to Tax. In vivo, differential compartmentalization and/or higher-order complex formation with CBP/p300 or other factors may control the specific colocalization of ATF-1 with Tax. It is important to note that colocalization of Tax with ATF-1 in the nuclear bodies is not sufficient for Tax activation of the ATF/CREB pathway, since the M47 Tax mutant, which is unable to activate gene expression via this pathway, is nevertheless able to colocalize with ATF-1 in nuclear bodies. Thus, colocalization of ATF-1 with other factors such as coactivators is probably required for Tax activation of gene expression from the ATF/CREB pathway.
Mechanisms regulating the specificity of Tax interaction with CBP and p300.
In vitro and in vivo interactions of Tax with the two related coactivators CBP and p300 and the differential response of these factors to the expression of mutant Tax proteins suggest that specific interactions of related cellular factors may be important for Tax transcriptional activity. Two independent domains of Tax delineated by mutations M47 and M148 mediate selective interactions with CBP or p300, respectively. Wild-type Tax is able to interact with both CBP and p300 to result in the inclusion of these factors in intracellular complexes and colocalization in nuclear bodies. The Tax mutants M47 and M148 are selectively defective in their ability to interact and colocalize with CBP and p300, respectively, and to activate gene expression via either ATF/CREB or the NF-κB pathway. Mutation M47 identifies a carboxy-terminal domain of Tax which is required for Tax-mediated activation of gene expression via the ATF/CREB pathway (74). This domain is also required for the in vitro and in vivo interactions of Tax with CBP and for the inclusion of CBP in nuclear bodies containing Tax. However, this domain is dispensable for interaction of Tax with the related coactivator p300. In contrast, the Tax mutant M148 identifies a central domain of Tax which is necessary for the ability of Tax to activate gene expression via the NF-κB pathway (81) and is important for the interaction of Tax with p300. However, this domain is dispensable for the interaction of Tax with CBP.
It is important to note that the Tax mutant M148 is also defective in inducing the nuclear translocation of RelA. Thus, we cannot rule out that the failure of this Tax mutant to activate NF-κB gene expression is due to its inability to induce the nuclear translocation of RelA. The fact that the Tax mutant F1 is defective in NF-κB activation but induces RelA translocation suggests that the nuclear translocation of RelA alone is not sufficient for Tax activation of gene expression via the NF-κB pathway. The differential interactions with CBP and p300 may explain in part how the M47 and M148 Tax mutants specifically alter the activation of gene expression via either the ATF/CREB or the NF-κB pathway while leaving the activation of the other pathway unaffected.
Both CBP and p300 contain a domain known as KIX (aa 443 to 670 for CBP and aa 427 to 649 for p300), which is involved in direct interaction with Tax and binding of the phosphorylated form of CREB (59). Comparison of the amino acid sequences in the KIX domain of CBP and p300 indicate that it contains a conserved region (aa 596 to 670 for CBP and aa 576 to 649 for p300; 95% amino acid similarity) and an adjacent, more divergent domain (aa 443 to 595 for CBP and aa 427 to 575 for p300; 44% amino acid similarity). The different sequences in the KIX domains of p300 and CBP may account for the differential in vitro and in vivo interactions of these two factors with the central and carboxy-terminal domains of Tax.
Wild-type Tax can transform human T lymphocytes and rat fibroblasts (30, 76, 81), while both Tax mutants M47 and M148 are unable to induce cellular transformation (76, 81). A common property of these two mutants is their inability to form nuclear structures containing both CBP and p300. Thus, the presence of both CBP and p300 in unique nuclear structures with Tax may be important for the transforming function of Tax. Alterations in both the CBP and p300 genes are found in human cancers (11, 53), and mutations in the adenovirus E1A protein and the SV40 T antigen that fail to interact with CBP and p300 result in defects in the induction of cellular DNA synthesis and transformation (21, 52). The p53 tumor suppressor also interacts with CBP and p300, and this interaction modulates p53 activation of transcription and cell cycle arrest (4, 32, 48). Thus, both CBP and p300 are targets for interaction with a variety of viral transforming proteins including E1A (52), SV40 T antigen (21), and Tax. Additional studies on the pathways leading to the nuclear localization of Tax with CBP and p300 will be critical in defining the role of these factors on Tax-mediated activation of viral and cellular gene expression as well as on cellular transformation induced by Tax.
ACKNOWLEDGMENTS
We thank Richard Goodman for providing the CBP clone, David Livingston for providing p300 cDNA and the p300 monoclonal antibody, Joan Steitz and Gunther Schutz for providing the Sm and CREM antibodies, and the NIH AIDS Research and Reagent Program for providing the MT2 cells and the Tax monoclonal antibody. In addition, we acknowledge Caroline Vanhulle for technical assistance and Sharon Johnson and Anthony Cancel for the preparation of the manuscript and the figures.
This work was supported by grants from the Belgian Fonds National de la Recherche Scientifique and Télévie, from the Fonds de Financement de la Recherche Cancérologique de la CGER Assurances, from the NIH, and from the Council of Tobacco Research.
REFERENCES
- 1.Arany Z, Sellers W R, Livingston D M, Eckner R. E1A-associated p300 and CREB-associated CBP belong to a conserved family of coactivators. Cell. 1994;77:799–800. doi: 10.1016/0092-8674(94)90127-9. [DOI] [PubMed] [Google Scholar]
- 2.Arany Z, Newsome D, Oldread E, Livingston D M, Eckner R. A family of transcriptional adaptor proteins targeted by the E1A oncoprotein. Nature. 1995;374:81–84. doi: 10.1038/374081a0. [DOI] [PubMed] [Google Scholar]
- 3.Armstrong A P, Franklin A A, Henbogaard M N, Giebler H A, Nyborg J K. Pleiotropic effect of the human T-cell leukemia virus TAX protein on the DNA binding activity of eukaryotic transcription factors. Proc Natl Acad Sci USA. 1993;90:7303–7307. doi: 10.1073/pnas.90.15.7303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Avantaggiati M L, Ogryzko V, Gardner K, Giordano A, Levine A S, Kelly K. Recruitment of p300/CBP in p53-dependent signal pathways. Cell. 1997;89:1175–1184. doi: 10.1016/s0092-8674(00)80304-9. [DOI] [PubMed] [Google Scholar]
- 5.Ballard D W, Bohnlein E, Lowenthal J W, Wano Y, Franza B R, Greene W C. HTLV-1 Tax induces cellular proteins that activate the κB element in the IL-2 receptor α gene. Science. 1988;241:1652–1655. doi: 10.1126/science.241.4873.1652. [DOI] [PubMed] [Google Scholar]
- 6.Bannister A J, Kouzarides T. The CBP co-activator is a histone acetyltransferase. Nature. 1996;384:641–643. doi: 10.1038/384641a0. [DOI] [PubMed] [Google Scholar]
- 7.Baranger A M, Palmer C R, Hamm M K, Glebler H A, Brauweiler A, Nyborg J K, Schepartz A. Mechanism of DNA-binding enhancement by the human T-cell leukaemia virus transactivator Tax. Nature. 1995;376:606–608. doi: 10.1038/376606a0. [DOI] [PubMed] [Google Scholar]
- 8.Beraud C, Sun S-C, Ganchi P, Ballard D W, Greene W C. Human T-cell leukemia virus type I Tax associates with and is negatively regulated by the NF-κB2 p100 gene product: implication for viral latency. Mol Cell Biol. 1994;14:1374–1382. doi: 10.1128/mcb.14.2.1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berglund P, Sjöberg M, Garoff H, Atkins G J, Sheahan B J, Liljeström P. Semliki Forest virus expression system: production of conditionally infectious recombinant particles. Bio/Technology. 1993;11:916–920. doi: 10.1038/nbt0893-916. [DOI] [PubMed] [Google Scholar]
- 10.Bex F, McDowall A, Burny A, Gaynor R B. The human T-cell leukemia virus transactivator protein Tax colocalizes with NF-κB proteins in unique nuclear bodies. J Virol. 1997;71:3484–3497. doi: 10.1128/jvi.71.5.3484-3497.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Borrow J, Stanton V P, Jr, Andersen J M, Becher R, Behm F G, Chaganti R S, Civin C I, Disteche C, Dube I, Frichauf A M, Horsman D, Mitelman F, Volinia S, Watmore A E, Housman D E. The translocation t(8;16) (p11;p13) of acute myeloid leukaemia fuses a putative acetyltransferase to the CREB-binding protein. Nat Genet. 1996;14:33–41. doi: 10.1038/ng0996-33. [DOI] [PubMed] [Google Scholar]
- 12.Brady J, Jeang K T, Duvall J, Khoury G. Identification of p40x-responsive regulatory sequences within the human T-cell leukemia virus type I long terminal repeat. J Virol. 1987;61:2175–2181. doi: 10.1128/jvi.61.7.2175-2181.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brauweiler A, Garl P, Franklin A A, Giebler H A, Nyborg J K. A molecular mechanism for human T-cell leukemia virus latency and Tax transactivation. J Biol Chem. 1995;270:12814–12822. doi: 10.1074/jbc.270.21.12814. [DOI] [PubMed] [Google Scholar]
- 14.Brockman J A, Scherer D C, McKinsey T A, Hall S M, Qi X, Lee W Y, Ballard D W. Coupling of a signal response domain in IκBα to multiple pathways for NF-κB activation. Mol Cell Biol. 1995;15:2809–2818. doi: 10.1128/mcb.15.5.2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cann A J, Rosenblatt J D, Wachsman W, Shah N P, Chen I S Y. Identification of the gene responsible for human T-cell leukemia virus transcriptional regulation. Nature. 1985;318:571–574. doi: 10.1038/318571a0. [DOI] [PubMed] [Google Scholar]
- 16.Chakravarti D, LaMorte V J, Nelson M C, Nakajima T, Schulman I G, Juguilon H, Montminy M, Evans R M. Role of CBP/p300 in nuclear receptor signaling. Nature. 1996;383:99–103. doi: 10.1038/383099a0. [DOI] [PubMed] [Google Scholar]
- 17.Chen Z, Hagler J, Palombella V J, Melandri F, Scherer D, Ballard D, Maniatis T. Signal-induced site-specific phosphorylation targets IκBα to the ubiquitin-proteasome pathway. Genes Dev. 1995;9:1586–1597. doi: 10.1101/gad.9.13.1586. [DOI] [PubMed] [Google Scholar]
- 18.Chrivia J C, Kwok R P S, Lamb N, Hagiwara M, Montminy M R, Goodman R H. Phosphorylated CREB binds specifically to the nuclear protein CBP. Nature. 1993;365:855–859. doi: 10.1038/365855a0. [DOI] [PubMed] [Google Scholar]
- 19.Dai P, Akimaru H, Tanaka Y, Hou D-X, Yasukawa T, Kanei-Ishii C, Takahashi T, Ishii S. CBP as a transcriptional coactivator of c-Myb. Genes Dev. 1996;10:528–540. doi: 10.1101/gad.10.5.528. [DOI] [PubMed] [Google Scholar]
- 20.Eckner R, Ewen M E, Newsome D, Gerdes M, DeCaprio J A, Lawrence J B, Livingston D M. Molecular cloning and functional analysis of the adenovirus E1A-associated 300-kD protein (p300) reveals a protein with properties of a transcriptional adaptor. Genes Dev. 1994;8:869–884. doi: 10.1101/gad.8.8.869. [DOI] [PubMed] [Google Scholar]
- 21.Eckner R, Ludlow J W, Lill N L, Oldread E, Arany Z, Modjtahedi N, DeCaprio J A, Livingston D M, Morgan J A. Association of p300 and CBP with simian virus 40 large T antigen. Mol Cell Biol. 1996;16:3454–3464. doi: 10.1128/mcb.16.7.3454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Felber B K, Paskalis H, Kleinman-Ewing D, Wong-Staal F, Pavlakis G N. The pX protein of HTLV-1 is a transcriptional activator of its long terminal repeats. Science. 1985;229:54–58. doi: 10.1126/science.2992082. [DOI] [PubMed] [Google Scholar]
- 23.Fujii M, Sassone-Corsi P, Verma I M. c-fos promoter trans-activation by the tax1 protein of human T-cell leukemia virus type I. Proc Natl Acad Sci USA. 1988;85:8526–8530. doi: 10.1073/pnas.85.22.8526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fujii M, Tsuchiya H, Chuhjo T, Akizawa T, Seiki M. Interaction of HTLV-I Tax1 with p67SRF causes the aberrant induction of cellular immediate early genes through CArG boxes. Genes Dev. 1992;6:2066–2076. doi: 10.1101/gad.6.11.2066. [DOI] [PubMed] [Google Scholar]
- 25.Fujisawa J, Seiki M, Sato M, Yoshida M. A transcriptional enhancer sequence of HTLV-I is responsible for trans-activation mediated by p40x of HTLV-I. EMBO J. 1986;5:713–718. doi: 10.1002/j.1460-2075.1986.tb04272.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fujisawa J-I, Toita M, Yoshida M. Tax of HTLV-1 interacts with cAMP-responsive element (CRE) binding and CRE modulator proteins that bind to the 21 base-pair enhancer of HTLV-1. Proc Natl Acad Sci USA. 1994;90:610–614. doi: 10.1073/pnas.90.2.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gerritsen M E, Williams A J, Neish A S, Moore S, Shi Y, Collins T. CREB-binding protein/p300 are transcriptional coactivators of p65. Proc Natl Acad Sci USA. 1997;94:2927–2932. doi: 10.1073/pnas.94.7.2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Giebler H A, Loring J E, van Orden K, Colgin M A, Garrus J E, Escudero K W, Brauweiler A, Nyborg J K. Anchoring of CREB binding protein to the human T-cell leukemia virus type 1 promoter: a molecular mechanism of Tax transactivation. Mol Cell Biol. 1997;17:5156–5164. doi: 10.1128/mcb.17.9.5156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gonzalez G A, Menzel P, Leonard J, Fischer W H, Montminy M R. Characterization of motifs which are critical for activity of the cyclic AMP-responsive transcription factor CREB. Mol Cell Biol. 1991;11:1306–1312. doi: 10.1128/mcb.11.3.1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grassmann R, Dengler C, Muller-Fleckenstein I, Fleckenstein B, McGuire K, Dokhelar M, Sodroski J, Haseltine W. Transformation to continuous growth of primary human T lymphocytes by human T-cell leukemia virus type I X-region genes transduced by a Herpesvirus saimiri vector. Proc Natl Acad Sci USA. 1989;86:3351–3355. doi: 10.1073/pnas.86.9.3351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grossman W J, Kimata J T, Wong F-H, Zutter M, Ley T J, Ratner L. Development of leukemia in mice transgenic for the tax gene of human T-cell leukemia virus type I. Proc Natl Acad Sci USA. 1995;92:1057–1061. doi: 10.1073/pnas.92.4.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gu W, Shl X-L, Roeder R G. Synergistic activation of transcription by CBP and p53. Nature. 1997;387:819–823. doi: 10.1038/42972. [DOI] [PubMed] [Google Scholar]
- 33.Hai T W, Liu F, Coukos W J, Green M R. Transcription factor ATF cDNA clones: an extensive family of leucine zipper proteins able to selectively form DNA-binding heterodimers. Genes Dev. 1989;3:2083–2090. doi: 10.1101/gad.3.12b.2083. [DOI] [PubMed] [Google Scholar]
- 34.Harrich D, Garcia J, Mitsuyasu R, Gaynor R. TAR independent activation of the human immunodeficiency virus in phorbol ester stimulated T lymphocytes. EMBO J. 1990;9:4417–4423. doi: 10.1002/j.1460-2075.1990.tb07892.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Haugland R P. Handbook of fluorescent probes and research chemicals. Eugene, Oreg: Molecular Probes, Inc.; 1996. [Google Scholar]
- 36.Hinuma Y, Komoda H, Chosa T, Kondo T, Kohakura M, Takenaka T, Kikuchi M, Ichimaru M, Yunoki K, Sato I, Matsuo R, Takiuchi Y, Uchino H, Hanaoka M. Antibodies to adult T-cell leukemia-virus-associated antigen (ATLA) in sera from patients with ATL and controls in Japan: a nation-wide seroepidemiologic study. Int J Cancer. 1982;29:631–635. doi: 10.1002/ijc.2910290606. [DOI] [PubMed] [Google Scholar]
- 37.Hiscott J, Petropoulos L, Lacoste J. Molecular interactions between HTLV-1 Tax protein and the NF-kappa B/kappa B transcription complex. Virology. 1995;214:3–11. doi: 10.1006/viro.1995.9960. [DOI] [PubMed] [Google Scholar]
- 38.Hoshino H, Esumi H, Miwa M. Establishment and characterization of 10 cell lines derived from patients with adult T-cell leukemia. Proc Natl Acad Sci USA. 1983;80:6061–6065. doi: 10.1073/pnas.80.19.6061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Janknecht R, Hunter T. Transcription. A growing coactivator network. Nature. 1996;383:22–23. doi: 10.1038/383022a0. [DOI] [PubMed] [Google Scholar]
- 40.Kamei Y, Xu L, Heinzel T, Torchia J, Kurokawa R, Gloss B, Lin S-C, Heyman R A, Rose D W, Glass C K, Rosenfeld M G. A CBP integrator complex mediates transcriptional activation and AP-1 inhibition by nuclear receptors. Cell. 1996;85:403–414. doi: 10.1016/s0092-8674(00)81118-6. [DOI] [PubMed] [Google Scholar]
- 41.Kanno T, Brown K, Franzoso G, Siebenlist U. Kinetic analysis of human T-cell leukemia virus type I tax-mediated activation of NF-κB. Mol Cell Biol. 1994;14:6443–6451. doi: 10.1128/mcb.14.10.6443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kanno T, Franzoso G, Siebenlist U. Human T-cell leukemia virus type I Tax-protein-mediated activation of NF-κB from p100 (NF-κB2)-inhibited reservoirs. Proc Natl Acad Sci USA. 1994;91:12634–12638. doi: 10.1073/pnas.91.26.12634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kwok R P S, Lundblad J R, Chrivia J C, Richards J P, Bachinger H P, Brennan R G, Roberts S G E, Green M R, Goodman R H. Nuclear protein CBP is a coactivator for the transcription factor CREB. Nature. 1994;370:223–226. doi: 10.1038/370223a0. [DOI] [PubMed] [Google Scholar]
- 44.Kwok R P S, Laurance M E, Lundblad J R, Goldman P S, Shih H-M, Connor L M, Marriott S J, Goodman R H. Control of cAMP-regulated enhancers by the viral transactivator Tax through CREB and the co-activator CBP. Nature. 1996;380:642–646. doi: 10.1038/380642a0. [DOI] [PubMed] [Google Scholar]
- 45.Lacoste J, Petropoulos L, Pépin N, Hiscott J. Constitutive phosphorylation and turnover of IκBα in human T-cell leukemia virus type I-infected and tax-expressing T cells. J Virol. 1995;69:564–569. doi: 10.1128/jvi.69.1.564-569.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Leung K, Nabel G J. HTLV-1 transactivator induces interleukin-2 receptor expression through an NF-kappa B-like factor. Nature. 1988;333:776–778. doi: 10.1038/333776a0. [DOI] [PubMed] [Google Scholar]
- 47.Liljeström P, Garoff H. A new generation of animal cell expression vectors based on the Semliki Forest virus replicon. Bio/Technology. 1991;9:1356–1361. doi: 10.1038/nbt1291-1356. [DOI] [PubMed] [Google Scholar]
- 48.Lill N L, Grossman S R, Ginsberg D, DeCaprio J, Livingston D M. Binding and modulation of p53 by p300/CBP coactivators. Nature. 1997;387:823–827. doi: 10.1038/42981. [DOI] [PubMed] [Google Scholar]
- 49.Lundblad J R, Kwok R P S, Laurance M E, Harter M L, Goodman R H. Adenoviral E1A-associated protein p300 as a functional homologue of the transcriptional co-activator CBP. Nature. 1995;374:85–88. doi: 10.1038/374085a0. [DOI] [PubMed] [Google Scholar]
- 50.McKinsey T A, Brockman J A, Scherer D C, Al-Murrani S W, Green P L, Ballard D W. Inactivation of IκBβ by the Tax protein of human T-cell leukemia virus type 1: a potential mechanism for constitutive induction of NF-κB. Mol Cell Biol. 1996;16:2083–2090. doi: 10.1128/mcb.16.5.2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Miyoshi I, Kubonishi I, Yoshimoto S, Akagi T, Ohtsuki Y, Shiraishi Y, Nagata K, Hinuma Y. Type C virus particles in a cord T-cell line derived by co-cultivating normal human cord leukocytes and human leukeamic T-cells. Nature. 1981;294:770–771. doi: 10.1038/294770a0. [DOI] [PubMed] [Google Scholar]
- 52.Moran E. DNA tumor virus transforming proteins and the cell cycle. Curr Opin Genet Dev. 1993;3:63–70. doi: 10.1016/s0959-437x(05)80342-9. [DOI] [PubMed] [Google Scholar]
- 53.Muraoka M, Konishi M, Yanoshita R, Tanaka K, Shitara N, Chong J M, Iwama T, Miyaki M. p300 gene alterations in colorectal and gastric carcinomas. Oncogene. 1996;12:1565–1569. [PubMed] [Google Scholar]
- 54.Nerenberg M, Hinrichs S H, Reynolds R K, Khoury G, Jay G. The tat gene of human T-lymphotropic virus type 1 induces mesenchymal tumors in transgenic mice. Science. 1987;237:1324–1329. doi: 10.1126/science.2888190. [DOI] [PubMed] [Google Scholar]
- 55.Oelgeschlager M, Janknecht R, Krieg J, Schreek S, Luscher B. Interaction of the co-activator CBP with Myb proteins: effects on Myb-specific transactivation and on the cooperativity with NF-M. EMBO J. 1996;15:2771–2780. [PMC free article] [PubMed] [Google Scholar]
- 56.Ogryzko V V, Schiltz R L, Russanova V, Howard B H, Nakatani Y. The transcriptional coactivators p300 and CBP are histone acetyltransferases. Cell. 1996;87:953–959. doi: 10.1016/s0092-8674(00)82001-2. [DOI] [PubMed] [Google Scholar]
- 57.Oliner J D, Andresen J M, Hansen S K, Zhou S, Tjian R. SREBP transcriptional activity is mediated through an interaction with the CREB-binding protein. Genes Dev. 1996;10:2903–2911. doi: 10.1101/gad.10.22.2903. [DOI] [PubMed] [Google Scholar]
- 58.Paca-Uccaralertkun S, Zhao L J, Adya N, Cross J V, Cullen B R, Boros I M, Giam C. In vitro selection of DNA elements highly responsive to the human T-cell lymphotropic virus type I transcriptional activator, Tax. Mol Cell Biol. 1994;14:456–462. doi: 10.1128/mcb.14.1.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Parker D, Ferreri K, Nakajima T, LaMorte V J, Evans R, Koerber S C, Hoeger C, Montminy M R. Phosphorylation of CREB at Ser-133 induces complex formation with CREB-binding protein via a direct mechanism. Mol Cell Biol. 1996;16:694–703. doi: 10.1128/mcb.16.2.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Paskalis H, Felber B K, Pavlakis G N. cis-acting sequences responsible for the transcriptional activation of a human T-cell leukemia virus type I constitute a conditional enhancer. Proc Natl Acad Sci USA. 1986;83:6558–6562. doi: 10.1073/pnas.83.17.6558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pepin N, Roulston A, Lacoste J, Lin R, Hiscott J. Subcellular redistribution of HTLV-I Tax protein by NF-κB/Rel transcription factors. Virology. 1994;204:706–716. doi: 10.1006/viro.1994.1586. [DOI] [PubMed] [Google Scholar]
- 62.Perini G, Wagner S, Green M B. Recognition of bZIP proteins by the human T-cell leukemia virus transactivator Tax. Nature. 1995;376:602–605. doi: 10.1038/376602a0. [DOI] [PubMed] [Google Scholar]
- 63.Perkins N D, Felzien L K, Betts J C, Leung K, Beach D H, Nabel G J. Regulation of NF-κB by cyclin-dependent kinases associated with the p300 coactivator. Science. 1997;275:523–527. doi: 10.1126/science.275.5299.523. [DOI] [PubMed] [Google Scholar]
- 64.Pettersson I, Hinterberger M, Mimori T, Gottlieb E, Steitz J A. The structure of mammalian small nuclear ribonucleoproteins: identification of multiple protein components reactive with (U1) ribonucleoprotein and anti-Sm autoantibodies. J Biol Chem. 1984;259:5907–5914. [PubMed] [Google Scholar]
- 65.Poiesz B J, Ruscetti R W, Gazdar A F, Bunn P A, Minna J D, Gallo R C. Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc Natl Acad Sci USA. 1980;77:7415–7419. doi: 10.1073/pnas.77.12.7415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pozzatti R, Vogel J, Jay G. The human T-lymphotropic virus type I tax gene can cooperate with the ras oncogene to induce neoplastic transformation of cells. Mol Cell Biol. 1990;10:413–417. doi: 10.1128/mcb.10.1.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schmitz M L, Baeuerle P A. The p65 subunit is responsible for the strong transcription activating potential of NF-kappa B. EMBO J. 1991;10:3805–3817. doi: 10.1002/j.1460-2075.1991.tb04950.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Seeler J S, Muchardt C, Podar M, Gaynor R B. Regulatory elements involved in tax-mediated transactivation of the HTLV-1 LTR. Virology. 1993;196:442–450. doi: 10.1006/viro.1993.1500. [DOI] [PubMed] [Google Scholar]
- 69.Semmes O J, Jeang K-T. Mutational analysis of human T-cell leukemia virus type I Tax: regions necessary for function determined with 47 mutant proteins. J Virol. 1992;66:7183–7192. doi: 10.1128/jvi.66.12.7183-7192.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Semmes O J, Jeang K-T. Localization of human T-cell leukemia virus type 1 Tax to subnuclear compartments that overlap with interchromatin speckles. J Virol. 1996;70:6347–6357. doi: 10.1128/jvi.70.9.6347-6357.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shimotohno K, Takano M, Teruchi T, Miwa M. Requirement of multiple copies of a 21-nucleotide sequence in the U3 region of human T-cell leukemia virus type I and type II long terminal repeats for trans-acting activation of transcription. Proc Natl Acad Sci USA. 1986;83:8112–8116. doi: 10.1073/pnas.83.21.8112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Siekevitz M, Feinberg M B, Holbrook N, Wong-Staal F, Greene W C. Activation of interleukin 2 and interleukin 2 receptor (Tac) promoter expression by the transactivator (tat) gene product of human T-cell leukemia virus type 1. Proc Natl Acad Sci USA. 1987;84:5389–5393. doi: 10.1073/pnas.84.15.5389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Smith D B, Johnson K S. Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene. 1988;67:31–40. doi: 10.1016/0378-1119(88)90005-4. [DOI] [PubMed] [Google Scholar]
- 74.Smith C L, Onate S A, Tsai M J, O’Malley B W. CREB binding protein acts synergistically with steroid receptor coactivator-1 to enhance steroid receptor-dependent transcription. Proc Natl Acad Sci USA. 1996;93:8884–8888. doi: 10.1073/pnas.93.17.8884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Smith M R, Greene W C. Identification of HTLV-I tax trans-activator mutants exhibiting novel transcriptional phenotypes. Genes Dev. 1990;4:1875–1885. doi: 10.1101/gad.4.11.1875. [DOI] [PubMed] [Google Scholar]
- 76.Smith M R, Greene W C. Type I human T-cell leukemia tax protein transforms rat fibroblasts through the cyclic adenosine monophosphate response element binding protein/activating transcription factor pathway. J Clin Invest. 1991;88:1038–1042. doi: 10.1172/JCI115364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sodroski J, Rosen C, Goh W C, Haseltine W. A transcriptional activator protein encoded by the x-lor region of the human T-cell leukemia virus. Science. 1985;228:1430–1434. doi: 10.1126/science.2990028. [DOI] [PubMed] [Google Scholar]
- 78.Suzuki T, Fujisawa J I, Toita M, Yoshida M. The trans-activator tax of human T-cell leukemia virus type 1 (HTLV-I) interacts with cAMP-responsive element (CRE) binding and CRE modulator proteins that bind to the 21-base-pair enhancer of HTLV-I. Proc Natl Acad Sci USA. 1993;90:610–614. doi: 10.1073/pnas.90.2.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tanaka A, Takahashi C, Yamaoka S, Nosaka T, Maki M, Hatanaka M. Oncogenic transformation by the tax gene of human T-cell leukemia virus type I in vitro. Proc Natl Acad Sci USA. 1990;87:1071–1075. doi: 10.1073/pnas.87.3.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wagner S, Green M R. HTLV-I Tax protein stimulation of DNA binding of bZIP proteins by enhancing dimerization. Science. 1993;262:395–399. doi: 10.1126/science.8211160. [DOI] [PubMed] [Google Scholar]
- 81.Yamaoka S, Inoue H, Sakurai M, Sugiyama T, Hazama M, Yamada T, Hatanaka M. Constitutive activation of NF-κB is essential for transformation of rat fibroblasts by the human T-cell leukemia virus type I Tax protein. EMBO J. 1996;15:873–887. [PMC free article] [PubMed] [Google Scholar]
- 82.Yang X-J, Ogryzko V V, Nishikawa J-I, Howard B H, Nakatani Y. A p300/CBP-associated factor that competes with the adenoviral oncoprotein E1A. Nature. 1996;382:319–324. doi: 10.1038/382319a0. [DOI] [PubMed] [Google Scholar]
- 83.Yin M, Paulssen E, Seeler J, Gaynor R. Chimeric proteins comprised of Jun and CREB define domains required for interaction with the HTLV-I Tax protein. J Virol. 1995;69:6209–6218. doi: 10.1128/jvi.69.10.6209-6218.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yin M-J, Gaynor R B. HTLV-I 21bp repeat sequences facilitate stable association between Tax and CREB to increase CREB binding affinity. J Mol Biol. 1996;264:20–31. doi: 10.1006/jmbi.1996.0620. [DOI] [PubMed] [Google Scholar]
- 85.Yoshida M, Miyoshi I, Hinuma Y. Isolation and characterization of retrovirus from cell lines of human adult T-cell leukemia and its implication in the disease. Proc Natl Acad Sci USA. 1982;79:2031–2035. doi: 10.1073/pnas.79.6.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhao L J, Giam C Z. Interaction of the human T-cell lymphotropic virus type I (HTLV-I) transcriptional activator Tax with cellular factors that bind specifically to the 21-base-pair repeats in the HTLV-I enhancer. Proc Natl Acad Sci USA. 1991;88:11445–11449. doi: 10.1073/pnas.88.24.11445. [DOI] [PMC free article] [PubMed] [Google Scholar]