Abstract
Proteolytic processing of the amyloid precursor protein by β-secretase generates C99, which subsequently is cleaved by γ-secretase, yielding the amyloid β peptide (Aβ). This γ-cleavage occurs within the transmembrane domain (TMD) of C99 and is similar to the intramembrane cleavage of Notch. However, Notch and C99 differ in their site of intramembrane cleavage. The main γ-cleavage of C99 occurs in the middle of the TMD, whereas the cleavage of Notch occurs close to the C-terminal end of the TMD, making it unclear whether both are cleaved by the same protease. To investigate whether γ-cleavage always occurs in the middle of the TMD of C99 or may also occur at the end of the TMD, we generated C99-mutants with an altered length of the TMD and analyzed their γ-cleavage in COS7 cells. The C terminus of Aβ and thus the site of γ-cleavage were determined by using monoclonal antibodies and mass spectrometry. Compared with C99-wild type (wt), most mutants with an altered length of the TMD changed the cleavage site of γ-secretase, whereas control mutants with mutations outside the TMD did not. Thus, the length of the whole TMD is a major determinant for the cleavage site of γ-secretase. Moreover, the C99-mutants were not only cleaved at one site but at two sites within their TMD. One cleavage site was located around the middle of the TMD, regardless of its actual length. An additional cleavage occurred within the N-terminal half of their TMD and thus at the opposite side of the Notch cleavage site.
Regulated intramembrane proteolysis of type I membrane proteins has been shown to play an important role in the pathogenesis of Alzheimer's disease and in cell differentiation (for a review, see ref. 1). The corresponding proteins involved in these biological processes are the amyloid precursor protein (APP) and Notch, respectively.
The intramembrane proteolysis of APP leads to the generation of the amyloid β peptide (Aβ), which is deposited in the brains of patients with Alzheimer's disease (for a review, see ref. 2). To undergo intramembrane proteolysis, APP is first proteolytically processed by one of the proteases termed α- and β-secretase (for a review, see ref. 3). The α-secretase seems to be a member of the ADAM (a disintegrin and metalloprotease)-family of disintegrin-metalloproteases (4–6), whereas the recently identified β-secretase is a novel aspartyl protease called BACE (beta-site APP cleaving enzyme; for a review, see ref. 7). The α- and β-secretases cleave APP within its ectodomain at a short distance to the transmembrane domain (TMD), shearing off most of the ectodomain (secretory APP). Subsequently, the remaining membrane-bound C-terminal fragments C83 (through α-cleavage) and C99 (through β-cleavage) may undergo intramembrane proteolysis by a protease called γ-secretase, which has not yet been unequivocally identified. However, the γ-secretase cleavage depends on the presence of the polytopic membrane protein presenilin 1, which itself may be γ-secretase (for reviews, see refs. 8 and 9). Because presenilin 1 is part of a high molecular weight protein complex, it may require additional proteins in this complex to carry out its proteolytic function. One such protein could be the recently identified Nicastrin, which has been found to bind both presenilin 1 and the substrate C99 (10). The γ-cleavage of C99 and C83 generates the N-terminal peptide fragments Aβ and p3, which are secreted by cultured cells (11–13), and the short-lived C-terminal fragment p7 (CTFγ), which may stimulate the transcription of so far unknown target genes (14). Because the γ-cleavage occurs at different peptide bonds—mainly after residue 40 and to a lower extent after residue 42 of C99—the Aβ peptides have 40 or 42 residues (Aβ40, Aβ42); the same C termini are found for the p3 peptides (p340, p342) (15, 16). The γ-cleavage seems to occur with broad substrate specificity because Aβ is still generated when residues within the TMD of C99 are mutated (15, 17–21). In contrast, the specific cleavage site used by γ-secretase (e.g., cleavage after residue 40 or 42) and thus the C terminus of Aβ strongly depends on the amino acid composition of the TMD (15, 17, 18, 20, 21).
The proteolytic processing of APP is similar to the processing of the cell surface receptor Notch (for an overview, see ref. 8). On ligand binding, Notch is proteolytically processed within its ectodomain. The remaining C-terminal, membrane-bound fragment may then undergo intramembrane proteolysis within its TMD at the so-called S3-site. As for the APP-derived fragment C99, the intramembrane cleavage of Notch depends on the presence of presenilin 1. Despite these similarities, the site within the TMDs of C99 and Notch, where the intramembrane cleavage occurs, is different: C99 is mainly cleaved in the middle of its TMD, whereas Notch is cleaved close to the C-terminal end of its TMD (22). These differences of the cleavage site raise doubt about whether both cleavage events share the same proteolytic mechanism and whether both are carried out by the same protease.
To address this question we analyzed in this study which sequence elements of C99 direct the γ-cleavage to occur mainly in the middle of the TMD and whether the γ-cleavage of C99 may also occur close to the end of the TMD similar to Notch. By using C99-mutants with an altered length of the TMD we show that the γ-cleavage site strongly depends on the length of the whole TMD but not on sequence elements outside of the TMD. The cleavage occurs in the middle of the TMD but may even occur close to the N-terminal end of the TMD and thus at the opposite side of the Notch cleavage site.
Methods
Cell Culture and Transfections.
COS7 cells were cultured according to the protocol described previously (17). Cell culture media were obtained from Sigma. The pCEP4 vector (Invitrogen) with the SPC99 cDNA inserts was transfected into COS7 cells by using Lipofectin (GIBCO/BRL) as described in the manufacturer's protocol. For each construct, two or more independent transfections were analyzed in cell culture with respect to γ-secretase activity.
Antibodies.
The monoclonal antibodies W02 (for the precipitation of all Aβ peptides regardless of the specific C terminus), G2-10 (specific for Aβ ending at residue 40), and G2-11 (specific for Aβ ending at residue 42) were raised against synthetic peptides (23).
Metabolic Labeling and Immunoprecipitation.
After 45 min of preincubation in methionine-free MEM, stably transfected COS7 cells were incubated for 16 h in methionine-free MEM containing 10% FBS and 133 μCi/ml [35S]methionine (Amersham Pharmacia). The conditioned media were centrifuged at 4°C for 1 min at 4000 × g and divided into three samples with a volume of 1 ml each. Aβ and p3 were immunoprecipitated with antibody W02 (5 μg/ml), G2-10 (12.5 μg/ml), or G2-11 (17.3 μg/ml) and 100 μg protein-G Agarose (Boehringer Mannheim), respectively. The immunoprecipitated proteins were separated on 10% Tris/Tricine gels (24). The intensity of the bands was quantified by using a Fuji PhosphorImager (BAS 1000).
To determine whether the introduced mutations altered the amount of Aβ generated from C99, Aβ and C99 were immunoprecipitated from the conditioned medium and the cell lysate, respectively, by using antibody W02 (concentration as above). After gel electrophoresis and Western Blotting using antibody W02, the amounts of Aβ and C99 were measured. Each experiment was carried out in three or more independent experiments. For the inhibition of Aβ-generation, the experiments were carried out as described above. The specific γ-secretase inhibitor {1S-benzyl-4R-[1-(1S-carbamoyl-2-phenylethylcarbamoyl)-1S-3-methylbutylcarbamoyl]-2R-hydroxy-5-phenylpentyl}carbamic acid tert-butyl ester, referred to as Merck A in this study and described in detail elsewhere (25), was dissolved in DMSO and was used at a concentration of 10 μM. The control cells without inhibitor were treated with DMSO alone.
Plasmid Construction.
Plasmids pUC18/SPC99-ΔNT, ΔCT, and CTI were generated as described earlier for pUC/C99-T43A (15), by using suited oligonucleotides and plasmid pSP65/SPC99 (26) as template. The KpnI/SpeI fragments of the pUC18/SPC99-ΔNT and CTI constructs were cloned into the pCEP4 vector digested with KpnI/NheI to generate the mutated expression plasmids pCEP4/SPC99 plasmids. pCEP4/SPC99-ΔCT was generated by cloning the NruI/SpeI fragment of pUC18/SPC99-ΔCT into the pCEP4 vector that was digested with PvuII/NheI.
Plasmids pBS/SPC99 with the mutations K28E, ΔSN, and Δ1725 were generated by using the Quik Change Site-Directed Mutagenesis Kit (Stratagene), suited oligonucleotides and pBS/SPC99 reverse as DNA-template (15). The KpnI/SpeI fragments of these constructs were cloned into the pCEP4 vector that was digested with KpnI/NheI. Plasmid pSP65/SPC99-NTI was obtained by using the Quik Change Site-Directed Mutagenesis Kit (Stratagene), the corresponding sense and antisense primers, and pSP65/SPC99 as DNA-template (26). The SmaI/SpeI fragment of pSP65/SPC99-NTI was cloned into the pCEP4 vector that was digested with NheI/PvuII.
The identity of the constructs obtained by PCR was confirmed by DNA sequencing.
Surface Enhanced Laser Desorption/Ionization Time-of-Flight (SELDI-TOF) Mass Spectrometry.
Immunocapture of amyloid peptides from conditioned media by using monoclonal antibody 6E10 (Senetec PLC, Napa, CA) covalently coupled to a SELDI protein chip (Ciphergen Biosystems, Fremont, CA) was done as described previously (25). Briefly, COS7 cells were incubated over night in DMEM (Sigma), conditioned media diluted with equal volumes of 25 mM Hepes (pH 7.3) and 1 mM EDTA and incubated overnight at 4°C with a 6E10-coupled SELDI protein chip. After extensive washing of the chip, a bovine insulin standard for internal calibration was dissolved in a 1:5 diluted saturated α-cyano-4-hydroxycinnamic acid solution in 40% acetonitrile, 0.25% trifluoroacetic acid. The standard (0.5 μl, 25 fmol bovine insulin) was applied to each spot of the SELDI chip, and, after air drying, samples were analyzed on a SELDI mass analyzer PBS II with a linear time-of-flight mass spectrometer (Ciphergen Biosystems) using time-lag focusing. All spectra were calibrated internally by using the single and double positively charged species of the bovine insulin peptide.
Results
To analyze whether specific sequence elements of C99 influence the γ-cleavage site, a systematic series of C99-mutants was generated with an altered length of the TMD or with deletions covering the whole ectodomain and cytoplasmic domain of C99. Mutations into the N-terminal 16 residues of C99 were not introduced, because C83—which lacks the N-terminal 16 residues of C99 because of cleavage by α-secretase—is cleaved by γ-secretase at the same site as C99 (15–17), and, thus, the N-terminal 16 residues of C99 do not influence the γ-cleavage site. With four mutants, we analyzed possible sequence elements in the ectodomain and the cytoplasmic domain of C99 (Fig. 1): a deletion of the whole cytoplasmic domain of C99 (Δcyto), a deletion of nine residues in the ectodomain (Leu-17 to Gly-25, Δ1725), a deletion of two residues in the ectodomain (Ser-26 to Asn-27, ΔSN), and a substitution of residue Lys-28 by Glu (K28E). Because Lys-28 may function as a membrane anchor for C99, it was not deleted, but substituted by a residue of opposite charge (Glu).
Figure 1.
SPC99 mutations used for the study of γ-secretase cleavage. SPC99 consists of the signal peptide (SP) of APP followed by Leu and Glu and the C-terminal 99 aa (C99) of APP. Amino acids are shown in the one letter code and are numbered according to the C99-sequence (1 to 99). The vertical bars within the C99-sequence indicate the cleavage sites of γ-secretase after residues 40 and 42. The shaded area represents the TMD. N and C, N- and C-terminal halves of the TMD.
With four additional mutants, we analyzed whether the length of the TMD of C99 influences the cleavage site of γ-secretase. In two mutants, two residues were inserted at the N-terminal (C99-NTI) or C-terminal (CTI) end of the TMD of C99 (Fig. 1). In two additional mutants, two residues were deleted at the N-terminal (C99-ΔNT) or C-terminal (ΔCT) end of the TMD. Larger alterations of the length of the TMD were not used, because such mutations could interfere strongly with protein transport: proteins with short TMDs might not be transported to later compartments of the secretory pathway (27), where at least a part of the γ-secretase activity occurs. In support of this notion, a recent study showed that several deletions and insertions of four or more residues strongly reduced the turnover of APP to Aβ (18).
Processing of C99-Mutants by γ-Secretase.
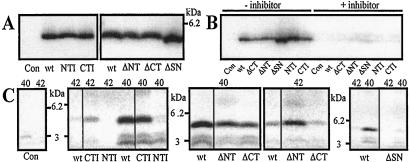
All C99-constructs were stably transfected as SPC99 (APP-signal-peptide-Leu-Glu-C99, Fig. 1) in COS7 cells. After signal peptide removal in the endoplasmic reticulum, the mature C99 starts at its N terminus with the two additional residues Leu and Glu. Western Blot analysis of Aβ immunoprecipitated from the conditioned medium shows that C99-wild type (wt) and all mutants were processed to similar amounts of the 4.5-kDa Aβ peptide (Fig. 2A, shown for the mutants with an altered length of the TMD). Notably, Aβ generated from the mutant C99-ΔSN shows a slightly faster electrophoretic mobility than the other Aβ peptides. No Aβ was detected in vector-transfected control cells. This finding demonstrates that neither the specific residues that were mutated or deleted nor the specific sequence of residues into which amino acids had been introduced (NTI, CTI) are required for general γ-secretase cleavage.
Figure 2.
Influence of the SPC99 mutations on substrate turnover and on the γ-secretase cleavage site. COS7 cells were stably transfected with the SPC99 constructs indicated below the panels. (A and B) Western Blot analysis of Aβ from the conditioned medium. Antibody W02 was used for the immunoprecipitation and Western Blot detection. (A) The COS7 cells expressed similar amounts of C99. (B) The specific γ-secretase inhibitor Merck A (10 μM) suppressed the generation of Aβ from wild-type and mutant C99. In this experiment, COS7 cells were used that did not express equal amounts of C99, which explains why different levels of Aβ were observed for the different mutants. (C) Generation of Aβ40 and Aβ42 from C99. Proteins from the conditioned medium of COS7 cells labeled with [35S]methionine were immunoprecipitated with the antibodies specific for Aβ ending in residue 40 or 42 (indicated above the panels). Both antibodies also precipitate the 3-kDa p3 peptides. 40 and 42, antibodies specific for Aβ and p3 ending in residues 40 or 42. Con, control; cells transfected with vector pCEP4 without an insert. Vertical black bars within the pictures indicate that the separated lanes were from the same gel but were not directly next to each other.
To confirm that the C99-mutants were processed by the same presenilin-dependent γ-secretase that cleaves C99-wt, a well characterized potent and specific inhibitor of γ-secretase Merck A (25) was shown to almost completely abolish the generation of Aβ from wt and mutant C99 (Fig. 2B).
Ratio of Aβ42/Aβ40 Depends on the Length of the TMD of C99.
To determine whether the C99-mutants were processed to the same Aβ species as C99-wt (mainly to Aβ40 and some Aβ42), the COS7 cells were metabolically labeled with [35S]methionine. Aβ40 and Aβ42 were then specifically immunoprecipitated from the conditioned medium and separated by gel electrophoresis; their amounts were quantified by PhosphorImaging, and the relative ratios of Aβ42/Aβ40 were determined. An alteration of the Aβ42/Aβ40 ratio compared with C99-wt is a good indication for an altered γ-cleavage of the corresponding mutant C99-protein. Aβ40 and Aβ42 denote the different peptides ending in residues 40 and 42 of wild-type Aβ, but may be longer or shorter than wild-type Aβ if they carry the indicated insertions or deletions. Both antibodies used for the immunoprecipitation also precipitate the p3 peptides ending in the same residues (p340 and p342).
As with C99-wt, most C99-mutants were processed to Aβ40 and p340 as well as to Aβ42 and p342 (Fig. 2C, data shown for the mutants with an altered length of the TMD). However, C99-ΔSN and C99-NTI were merely processed to Aβ40 and p340 and not to Aβ42 and p342. Because both mutants were processed to Aβ (Fig. 2A), this result shows that both mutants must mainly have been processed to Aβ peptides with a C terminus different from Aβ40 and Aβ42. In COS7 cells that were transfected with the vector alone (control), Aβ was not detected, and p3 only in low amounts (Fig. 2C). This p3 must be derived from the endogenous APP of COS7 cells.
Next, we determined the Aβ42/Aβ40 ratios for C99-wt and the C99 mutants in four to six experiments (Fig. 3). The ratios of Aβ42/Aβ40, as determined here, are expressed as percentages and represent relative but not absolute ratios, because the antibodies have different affinities for the corresponding peptides. For C99-wt, the Aβ42/Aβ40 ratio is 4.7%. The mutants with mutations outside of the TMD (C99-Δ1725, K28E, and Δcyto) did not alter significantly the Aβ42/Aβ40 ratios (Δ1725, 5.5 ± 0.9%; Δcyto, 4.1 ± 0.6%; and K28E, 5.4 ± 0.9%), demonstrating that the ectodomain and the cytoplasmic domain of C99 do not contain specific sequence elements that influence the cleavage site of γ-secretase.
Figure 3.
Relative ratios of Aβ42/Aβ40 in the conditioned medium of COS7 cells expressing C99. The amounts of Aβ40 and Aβ42 in Fig. 2 were quantified by PhosphorImaging in four to six independent experiments. Columns represent the mean values. Black error bars give the standard deviation. The asterisks indicate the significance (Student's t test) relative to C99-wt (**, P < 0.01, ***, P < 0.001). a, the calculated ratios are 0% because no Aβ42 was detected.
In contrast, most mutants with an altered length of the TMD led to a changed ratio of Aβ42/Aβ40 and thus an altered γ-cleavage site. Both the C-terminal insertion (CTI) and the N-terminal deletion (ΔNT) of two residues led to a similar increase of the Aβ42/Aβ40-ratio (increase: 4.1-fold (CTI), 4.3 fold (ΔNT); Aβ42/Aβ40: 19.6 ± 2.2% (CTI), 20.5 ± 4.7% (ΔNT); P < 0.001 (CTI), P < 0.01 (ΔNT)). This increase corresponds to a shift of the γ-cleavage site in the C-terminal direction. In contrast, the C-terminal deletion of two residues (ΔCT) did not alter the Aβ42/Aβ40-ratio (4.6 ± 1.5%). For C99-NTI and C99-ΔSN, no Aβ42 could be detected so that the calculated ratio Aβ42/Aβ40 was 0%.
Both antibodies used for the immunoprecipitation of Aβ40 (G2-10) and Aβ42 (G2-11) also immunoprecipitated p340 and p342 (Fig. 2). A comparison with C99-wt revealed that the analyzed mutations altered the ratios p342/p340 to a similar extent as the ratios Aβ42/Aβ40 (data not shown).
Mass Spectrometric Analysis of Aβ.
To determine exactly how the mutations in the TMD of C99 affect the γ-cleavage site, Aβ was immunocaptured by using monoclonal antibody 6E10, which binds to the N terminus of Aβ. Subsequently, the immunocaptured Aβ was analyzed by SELDI-TOF mass spectrometry (Fig. 4). C99-wt gave a prominent signal for Aβ40 (Fig. 4), confirming previous results by us and others (17, 28). The mutants Δcyto and ΔCT showed spectra identical to C99-wt (not shown), and thus no altered γ-cleavage. Similarly, the spectrum for C99-Δ1725 showed that it was mainly processed after residue 40 (not shown). Taken together, the deletions outside of the TMD (Δ1725, Δcyto) did not affect the γ-cleavage site, confirming the results obtained by immunoprecipitation.
Figure 4.
Mass spectrometric determination of the C terminus of the mutant Aβ peptides. Aβ was immunocaptured from the conditioned medium of COS7 cells expressing the indicated C99 mutants. The peaks in the spectra are numbered according to the wild-type C99-sequence (1 to 99). Additionally, all peptides contain the two residues Leu and Glu at the N terminus (see Fig. 1). All spectra were calibrated internally by using the single and double positively charged species of bovine insulin. The error of masses of all identified peptides was ≤ 400 ppm compared with the calculated average masses. Asterisks indicate Aβ peptides oxidized at methionine 35. Masses of these peptides are increased by 16 mass units as expected for methionine sulfoxide derivatives.
In contrast, most mutations within the TMD altered the γ-cleavage site by leading to extended or shortened C termini of Aβ (Fig. 4), demonstrating that the length of the whole TMD of C99 is an important factor in determining the γ-cleavage site. C99-CTI yielded peaks for Aβ40 and also for Aβ42. Because Aβ42 had hardly been detected in the mass spectrum for C99-wt, this result indicates that C99-CTI was cleaved to a larger extent after residue 42 than C99-wt. Additionally, strong peaks were observed for Aβ39 and Aβ38. C99-ΔNT was processed to Aβ40 but also gave a clear signal for Aβ42 and even Aβ43. This finding confirms the result of the immunoprecipitation, which had shown that, for C99-ΔNT, the γ-cleavage site was shifted in the C-terminal direction. C99-NTI, for which hardly any Aβ40 and no Aβ42 had been detected by immunoprecipitation, was processed to Aβ37, but also to smaller Aβ peptides with a C terminus after residues 32, 33, and 34, corresponding to a shift of the γ-cleavage site in the N-terminal direction.
In general, the results of the mass spectrometric analysis are in good agreement with the results obtained by immunoprecipitation (Fig. 2C). However, some of the peptides with longer C termini, which were clearly detected by immunoprecipitation (Fig. 2C), were underrepresented or not detected in the mass spectra (Aβ42 for C99-wt and ΔCT; Aβ40 for NTI and ΔSN). This result may be due to their lower detection efficiency, as noted in a previous study (28).
The control mutant C99-ΔSN, which had no altered length of the TMD but a deletion of two residues just outside of the TMD, revealed an altered γ-cleavage site relative to C99-wt (Fig. 4). C99-ΔSN was mainly processed to Aβ peptides with a shortened C terminus (Aβ32, Aβ33, Aβ34, Aβ37, and Aβ38) and, hence, was cleaved at similar sites as C99-NTI. Thus, the deletion of two residues outside the TMD had a similar effect on γ-cleavage as the insertion of two residues into the TMD. This unexpected result may be explained as follows. In contrast to all other mutants, the deletion of the two polar residues Ser and Asn (ΔSN) just outside the TMD alters the N-terminal border and thus the length of the TMD (Fig. 5), as determined with the algorithm of Kyte and Doolittle (29). In this case, Lys-28 is flanked on both sides by hydrophobic residues and is part of the TMD although, in C99-wt, it is located just outside of the TMD. Therefore, C99-ΔSN may not be used as a mutant without an altered length of the TMD but, instead, as an additional C99-mutant with an increased length of the TMD.
Figure 5.
Major intramembrane γ-cleavage sites in the C99-mutants with an altered length of the TMD. Amino acids are shown in the one letter code and are numbered according to the C99-sequence (1 to 99). The black bar below the sequence shows the length of the TMD for the individual C99-mutant with a vertical bar at the middle of the TMD. The black arrows indicate the major γ-cleavage sites (Aβ peptides) as determined by mass spectrometry (Fig. 4). Open arrows indicate the additional cleavage sites that were detected only by immunoprecipitation (Fig. 2C) but not by mass spectrometry (see text for details).
Discussion
The type I membrane proteins Notch and C99—derived from APP by β-secretase cleavage—both undergo a similar proteolytic cleavage within their TMDs, which is termed regulated intramembrane proteolysis (1). This proteolytic cleavage reaction is poorly understood, and it remains unclear whether the cleavage of Notch and C99 takes place mechanistically in the same way and whether it is carried out by the same protease. This concern stems from the fact that Notch seems to be cleaved at the C-terminal end of its TMD, whereas the γ-cleavage of C99 occurs exactly in the middle of the TMD. By using a systematic series of C99-mutants with an altered length of the TMD or with deletions covering the whole ectodomain and cytoplasmic domain of C99, we analyzed which sequence elements of C99 promote the γ-cleavage to occur mainly in the middle of the TMD. Additionally, we investigated whether the introduced mutations affected the amount of Aβ generated from C99 (cleavage efficiency).
Concerning the cleavage efficiency, we and others have shown previously that the amino acid sequence within the TMD of C99 has little influence on the amount of secreted Aβ (15, 17–21), suggesting a broad substrate specificity of γ-secretase. Here, we extend this analysis further to the ectodomain and the cytoplasmic domain of C99. Our results show that neither domain contains sequence elements that are required for the intramembrane cleavage of C99. Interestingly, this broad substrate specificity is shared with the γ-secretase-like cleavage of Notch (30). Given this result, it is possible that γ-cleavage occurs for more type I proteins than just APP/C99 and Notch. Similar to Notch and APP, such additional proteins may first need to undergo cleavage in their ectodomain to present a short ectodomain stub, which seems to be a requirement for intramembrane proteolysis (30). In support of this idea, the transmembrane receptor tyrosine kinase ErbB4 has just been shown to also undergo a γ-secretase-like cleavage, but only after an initial cleavage in the ectodomain (31).
The broad substrate specificity clearly distinguishes the γ-secretase cleavage of C99 from the similar intramembrane-cleavage of the sterol-regulatory element binding protein (SREBP), which is involved in the cellular cholesterol homeostasis (for an overview, see ref. 1). SREBP is cleaved by the metalloprotease S2P, which requires two amino acid motifs within the SREBP sequence: first, the cytoplasmic residues DRSR, which are located several residues away from the actual cleavage site (32), and, second, the two residues asparagine-proline around the middle of the TMD of SREBP (33). The second motif is also required for the cleavage of ATF6, which is a second substrate of S2P (34).
A major challenge in understanding the mechanism of γ-cleavage results from the fact that the γ-cleavage occurs within the TMD of C99 and that it is difficult to conceive how a protease may cleave within the lipid bilayer. Presenilin 1, which seems to be γ-secretase or at least part of a γ-secretase complex, has two aspartyl residues that are critical for its function located within its TMDs so that γ-cleavage could occur within the membrane (8). However, because no protease has been proven to cleave within the membrane, alternative cleavage mechanisms have been proposed for C99 that invoke the TMD to be C-terminally shorter, such that a cytosolic or membrane-associated protease could cleave C99 (18, 19, 35). If the alternative models are true, we expect that deletions and insertions in the C-terminal half of the TMD have a weak or no effect on the γ-cleavage site. In contrast, if γ-secretase is a membrane protein that cleaves C99 within the membrane, we expect that deletions and insertions at both ends of the TMD affect the γ-cleavage site.
Our results identify the length of the whole TMD as an important factor in determining the γ-cleavage site: most C99-mutants with an altered length of the TMD changed the γ-cleavage site compared with C99-wt, whereas the C99-mutants with mutations outside of the TMD did not. Importantly, mutations at both ends of the TMD and not only in the N-terminal half affected the γ-cleavage site, speaking against a mechanism of γ-cleavage outside of the membrane or at the membrane-boundary. This notion is further reinforced by previous experiments that showed that mutations at every single residue within the C-terminal half of the TMD of C99 altered the γ-cleavage site (17). Our results are consistent with a model in which γ-secretase is a membrane protein and cleaves C99 within the membrane.
In contrast to the role of the TMD of C99 in determining the γ-cleavage site, our study shows that the ectodomain and the cytoplasmic domain do not contain such signals. This result is in good agreement with a previous study (36), which analyzed the proteolytic processing of a non-natural probe for γ-secretase. The authors showed that a peptide consisting of the TMD of APP flanked on both sides by unique epitope tags is cleaved by γ-secretase after residues 40 and 42 (as C99-wt) but also at additional peptide bonds between residues 35 and 42. Because that study did not determine which peptide bond was the main cleavage site, it remains unclear whether that probe is cleaved in exactly the same way as the natural substrate C99, for which the main γ-cleavage occurs after residue 40.
The γ-cleavage of C99-wt mainly occurs at one peptide bond (after residue 40), which is exactly the middle of the putative 24-residue-long TMD. In contrast, most of the mutants with an altered length and thus an altered middle of the TMD were cleaved at two sites within the TMD. One of the sites was located close to the new middle of the TMD, whereas the other one was not. The C-terminal insertion (CTI) as well as the N-terminal deletion (ΔNT), which both have the middle of their TMD more C-terminal than C99-wt, shifted the γ-cleavage site in the C-terminal direction toward less Aβ40 but more Aβ42 and even Aβ43 (Fig. 5). In addition, a second, more N-terminal cleavage site after Aβ38 was detected for C99-CTI. The two mutants NTI and ΔSN, which have the middle of their TMD more N-terminal than C99-wt, shifted the γ-cleavage site in the N-terminal direction. As a result, less Aβ42 and Aβ40 were generated and more Aβ37 and Aβ38 but also shorter peptides with a C terminus after residues 32, 33, and 34. The only mutant that did not show a second major cleavage site is C99-ΔCT with a deletion at the C-terminal end of the TMD. Like C99-wt, this mutant was mainly cleaved after residue 40, although the middle of its TMD is located after residue 39.
Particularly surprising is the result that the mutants NTI and ΔSN were not only processed around the middle of the TMD (Aβ37 and Aβ38) but also much more N-terminal (Aβ32, Aβ33 and Aβ34). Because a potent and specific inhibitor of γ-secretase suppressed the generation not only of the long but also of the short Aβ peptides, it is clear that the short Aβ peptides must also have been generated in a γ-secretase-dependent fashion. However, it remains unclear whether the short Aβ peptides were generated by direct γ-cleavage or whether the initial γ-cleavage occurred in the middle of the TMD and was followed by a secondary proteolytic event, e.g., an additional γ-cleavage or trimming by an exopeptidase. In this latter scenario, the C terminus of Aβ would pretend a γ-cleavage in the N-terminal half of the TMD although the cleavage had occurred in the middle of the TMD. Interestingly, a similar observation has recently been made concerning the C-terminal fragment of C99 that arises through γ-cleavage (CTFγ) and that had not been identified so far. Three studies reported the identification of a CTFγ-like fragment (p6.5) in mouse brain and cultured cells that starts close to the C terminus of the TMD (predominantly residue 50 of C99; refs. 37–39). However, neither group found the slightly longer CTFγ (p7) that would correspond to the γ-cleavage after residues 40 and 42, which led to the conclusion that γ-cleavage may occur simultaneously at different sites within the TMD (after residues 40/42 as well as after residue 50).
Interestingly, the apparent N-terminal γ-cleavage after Aβ33 and Aβ34 of C99-NTI and ΔSN is the opposite side of the cleavage site of Notch, which seems to occur close to the C terminus of its TMD (22). Similar to C99, only one of the proteolytic cleavage products of Notch (the C-terminal fragment) has been identified, so that we cannot rule out that the initial γ-like cleavage of Notch occurs in the middle of the TMD and is followed by a secondary proteolytic event. In this case, the intramembrane cleavage could be similar for both proteins, but the identified cleavage products would pretend a different cleavage mechanism. Determining the C terminus of the N-terminal cleavage fragment of Notch should answer this question.
Our analysis shows that some of the mutations in the N-terminal half of the TMD of C99 (NTI, ΔSN) have a stronger effect on the cleavage site than some of the mutations in the C-terminal half (ΔCT), which is in agreement with a recent report that analyzed the processing of full-length APP with deletions, insertions, and point mutations within the TMD (18). The previous study, however, took this result as evidence for the γ-cleavage site being determined by the length of the N-terminal half but not by the C-terminal half of the TMD of C99. Because our results show that the length of the whole TMD is important for determining the γ-cleavage site, we favor a different explanation. The N-terminal and the C-terminal half of the TMD of C99 have very different amino acid compositions. The C-terminal half consists mainly of large residues (Met, Ile, Leu, and Thr) that are evenly distributed around the axis of the helical TMD. In contrast, the N-terminal half consists of small (Gly and Ala) as well as of large residues (Met, Ile, and Leu), with the small residues on one side of the helix and the large residues on the other side (not shown). One of these two helical sides could be an interface for a functional interaction of C99 with γ-secretase or other proteins. Insertion or deletion of two residues in the N-terminal half of the TMD—as in our C99-mutants—abolishes the amino acid arrangement of one helical side with large residues and one helical side with small residues. Thus, mutations in the N-terminal half of the TMD of C99 may have a much more pronounced effect on the cleavage site of γ-secretase than mutations in the C-terminal half.
In summary, our study shows that γ-secretase has a very loose substrate specificity, which distinguishes γ-secretase from S2P, another protease involved in regulated intramembrane proteolysis. Moreover, our experiments identify the length of the whole TMD of C99 as a major determinant of the γ-cleavage site and suggest that γ-cleavage takes place within the membrane. The γ-cleavage site is located in the middle of the TMD, but an additional cleavage may occur within the N-terminal half of the TMD and thus at the opposite side of the cleavage site of Notch.
Acknowledgments
This work was supported by the Deutsche Forschungsgemeinschaft (through SFB 317 and a postdoctoral Emmy Noether fellowship to S.F.L.) and the Fonds der Chemischen Industrie of Germany.
Abbreviations
- APP
amyloid precursor protein
- Aβ40 and Aβ42
Aβ peptides with different N termini ending at the C terminus with residue 40 or 42 of C99
- C99
C-terminal 99 residues of APP
- TMD
transmembrane domain
- SELDI-TOF
surface-enhanced laser desorption/ionization time-of-flight mass spectrometry
- wt
wild type
- SP
signal peptide
- CTF
C-terminal fragment
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Brown M S, Ye J, Rawson R B, Goldstein J L. Cell. 2000;100:391–398. doi: 10.1016/s0092-8674(00)80675-3. [DOI] [PubMed] [Google Scholar]
- 2.Selkoe D J. Physiol Rev. 2001;81:741–766. doi: 10.1152/physrev.2001.81.2.741. [DOI] [PubMed] [Google Scholar]
- 3.De Strooper B, Annaert W. J Cell Sci. 2000;113:1857–1870. doi: 10.1242/jcs.113.11.1857. [DOI] [PubMed] [Google Scholar]
- 4.Lammich S, Kojro E, Postina R, Gilbert S, Pfeiffer R, Jasionowski M, Haass C, Fahrenholz F. Proc Natl Acad Sci USA. 1999;96:3922–3927. doi: 10.1073/pnas.96.7.3922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buxbaum J D, Liu K N, Luo Y, Slack J L, Stocking K L, Peschon J J, Johnson R S, Castner B J, Cerretti D P, Black R A. J Biol Chem. 1998;273:27765–27767. doi: 10.1074/jbc.273.43.27765. [DOI] [PubMed] [Google Scholar]
- 6.Koike H, Tomioka S, Sorimachi H, Saido T C, Maruyama K, Okuyama A, Fujisawa-Sehara A, Ohno S, Suzuki K, Ishiura S. Biochem J. 1999;343:371–375. [PMC free article] [PubMed] [Google Scholar]
- 7.Vassar R, Citron M. Neuron. 2000;27:419–422. doi: 10.1016/s0896-6273(00)00051-9. [DOI] [PubMed] [Google Scholar]
- 8.Wolfe M S, Haass C. J Biol Chem. 2001;276:5413–5416. doi: 10.1074/jbc.R000026200. [DOI] [PubMed] [Google Scholar]
- 9.Steiner H, Haass C. Nat Rev Mol Cell Biol. 2000;1:217–224. doi: 10.1038/35043065. [DOI] [PubMed] [Google Scholar]
- 10.Yu G, Nishimura M, Arawaka S, Levitan D, Zhang L, Tandon A, Song Y Q, Rogaeva E, Chen F, Kawarai T, et al. Nature (London) 2000;407:48–54. doi: 10.1038/35024009. [DOI] [PubMed] [Google Scholar]
- 11.Seubert P, Vigo Pelfrey C, Esch F, Lee M, Dovey H, Davis D, Sinha S, Schlossmacher M, Whaley J, Swindlehurst C, et al. Nature (London) 1992;359:325–327. doi: 10.1038/359325a0. [DOI] [PubMed] [Google Scholar]
- 12.Shoji M, Golde T E, Ghiso J, Cheung T T, Estus S, Shaffer L M, Cai X D, McKay D M, Tintner R, Frangione B, et al. Science. 1992;258:126–129. doi: 10.1126/science.1439760. [DOI] [PubMed] [Google Scholar]
- 13.Haass C, Schlossmacher M G, Hung A Y, Vigo Pelfrey C, Mellon A, Ostaszewski B L, Lieberburg I, Koo E H, Schenk D, Teplow D B, et al. Nature (London) 1992;359:322–325. doi: 10.1038/359322a0. [DOI] [PubMed] [Google Scholar]
- 14.Cao X, Sudhof T C. Science. 2001;293:115–120. doi: 10.1126/science.1058783. [DOI] [PubMed] [Google Scholar]
- 15.Lichtenthaler S F, Ida N, Multhaup G, Masters C L, Beyreuther K. Biochemistry. 1997;36:15396–15403. doi: 10.1021/bi971071m. [DOI] [PubMed] [Google Scholar]
- 16.Citron M, Diehl T S, Gordon G, Biere A L, Seubert P, Selkoe D J. Proc Natl Acad Sci USA. 1996;93:13170–13175. doi: 10.1073/pnas.93.23.13170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lichtenthaler S F, Wang R, Grimm H, Uljon S N, Masters C L, Beyreuther K. Proc Natl Acad Sci USA. 1999;96:3053–3058. doi: 10.1073/pnas.96.6.3053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murphy M P, Hickman L J, Eckman C B, Uljon S N, Wang R, Golde T E. J Biol Chem. 1999;274:11914–11923. doi: 10.1074/jbc.274.17.11914. [DOI] [PubMed] [Google Scholar]
- 19.Tischer E, Cordell B (1919) J Biol Chem. 1996;271:21914–21912. doi: 10.1074/jbc.271.36.21914. [DOI] [PubMed] [Google Scholar]
- 20.Maruyama K, Tomita T, Shinozaki K, Kume H, Asada H, Saido T C, Ishiura S, Iwatsubo T, Obata K. Biochem Biophys Res Commun. 1996;227:730–735. doi: 10.1006/bbrc.1996.1577. [DOI] [PubMed] [Google Scholar]
- 21.Suzuki N, Cheung T T, Cai X D, Odaka A, Otvos L, Jr, Eckman C, Golde T E, Younkin S G. Science. 1994;264:1336–1340. doi: 10.1126/science.8191290. [DOI] [PubMed] [Google Scholar]
- 22.Schroeter E H, Kisslinger J A, Kopan R. Nature (London) 1998;393:382–386. doi: 10.1038/30756. [DOI] [PubMed] [Google Scholar]
- 23.Ida N, Hartmann T, Pantel J, Schröder J, Zerfass R, Förstl H, Sandbrink R, Masters C L, Beyreuther K. J Biol Chem. 1996;271:22908–22914. doi: 10.1074/jbc.271.37.22908. [DOI] [PubMed] [Google Scholar]
- 24.Schägger H, von Jagow G. Anal Biochem. 1987;166:368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- 25.Shearman M S, Beher D, Clarke E E, Lewis H D, Harrison T, Hunt P, Nadin A, Smith A L, Stevenson G, Castro J L. Biochemistry. 2000;39:8698–8704. doi: 10.1021/bi0005456. [DOI] [PubMed] [Google Scholar]
- 26.Dyrks T, Dyrks E, Masters C, Beyreuther K. FEBS Lett. 1992;309:20–24. doi: 10.1016/0014-5793(92)80730-5. [DOI] [PubMed] [Google Scholar]
- 27.Munro S. EMBO J. 1995;14:4695–4704. doi: 10.1002/j.1460-2075.1995.tb00151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang R, Sweeney D, Gandy S E, Sisodia S S. J Biol Chem. 1996;271:31894–31902. doi: 10.1074/jbc.271.50.31894. [DOI] [PubMed] [Google Scholar]
- 29.Kyte J, Doolittle R F. J Mol Biol. 1982;157:105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- 30.Struhl G, Adachi A. Mol Cell. 2000;6:625–636. doi: 10.1016/s1097-2765(00)00061-7. [DOI] [PubMed] [Google Scholar]
- 31.Ni C Y, Murphy M P, Golde T E, Carpenter G. Science. 2001;294:2179–2181. doi: 10.1126/science.1065412. [DOI] [PubMed] [Google Scholar]
- 32.Duncan E A, Dave U P, Sakai J, Goldstein J L, Brown M S. J Biol Chem. 1998;273:17801–17809. doi: 10.1074/jbc.273.28.17801. [DOI] [PubMed] [Google Scholar]
- 33.Ye J, Dave U P, Grishin N V, Goldstein J L, Brown M S. Proc Natl Acad Sci USA. 2000;97:5123–5128. doi: 10.1073/pnas.97.10.5123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ye J, Rawson R B, Komuro R, Chen X, Dave U P, Prywes R, Brown M S, Goldstein J L. Mol Cell. 2000;6:1355–1364. doi: 10.1016/s1097-2765(00)00133-7. [DOI] [PubMed] [Google Scholar]
- 35.Coles M, Bicknell W, Watson A A, Fairlie D P, Craik D J. Biochemistry. 1998;37:11064–11077. doi: 10.1021/bi972979f. [DOI] [PubMed] [Google Scholar]
- 36.Bunnell W L, Pham H V, Glabe C G. J Biol Chem. 1998;273:31947–31955. doi: 10.1074/jbc.273.48.31947. [DOI] [PubMed] [Google Scholar]
- 37.Sastre M, Steiner H, Fuchs K, Capell A, Multhaup G, Condron M M, Teplow D B, Haass C. EMBO Rep. 2001;2:835–841. doi: 10.1093/embo-reports/kve180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gu Y, Misonou H, Sato T, Dohmae N, Takio K, Ihara Y. J Biol Chem. 2001;276:35235–35238. doi: 10.1074/jbc.C100357200. [DOI] [PubMed] [Google Scholar]
- 39.Yu C, Kim S H, Ikeuchi T, Xu H, Gasparini L, Wang R, Sisodia S S. J Biol Chem. 2001;276:43756–43760. doi: 10.1074/jbc.C100410200. [DOI] [PubMed] [Google Scholar]