Abstract
N6-methyladenosine (m6A), the most abundant internal modification in eukaryotic messenger RNA (mRNA) and long noncoding RNA (lncRNA), is dynamically modulated by methyltransferases (“writers”), demethylases (“erasers”), and binding proteins (“readers”). As a central epitranscriptomic regulator, m6A governs RNA stability, splicing, translation, and degradation, thereby orchestrating a wide range of physiological and pathological pathways. Accumulating evidence has underscored its pivotal involvement in the pathogenesis of kidney disorders. This review delineates the regulatory landscape of m6A methylation across various kidney diseases, with emphasis on diabetic nephropathy (DN), acute kidney injury (AKI), chronic kidney disease (CKD), focal segmental glomerulosclerosis (FSGS), lupus nephritis (LN), hyperuricemic nephropathy (HN), autosomal dominant polycystic kidney disease (ADPKD), and clear cell renal cell carcinoma (ccRCC). Disease-specific alterations in m6A levels and the expression patterns of core regulators, including METTL3, METTL14, FTO, ALKBH5, YTH domain proteins, and IGF2BPs, are systematically summarized. By elucidating their roles in inflammation, fibrosis, apoptosis, and metabolic imbalance, this review highlights the translational potential of m6A-centric interventions and offers novel insights into epitranscriptomic regulation within renal pathophysiology.
Keywords: m6A modification, Kidney diseases, Expression profiles, Regulatory mechanisms, Clinical significance
Introduction
RNA modifications have recently gained prominence as a vital layer of post-transcriptional gene regulation [1, 2]. Among over 170 identified modifications, N6-methyladenosine (m6A) is the most prevalent internal modification in eukaryotic messenger RNAs (mRNAs) and long noncoding RNAs (lncRNAs) [3, 4]. This modification modulates RNA metabolism through a reversible and tightly regulated mechanism involving three classes of proteins: methyltransferases (“writers”), demethylases (“erasers”), and RNA-binding proteins (“readers”) [5–7]. The methyltransferase complex, comprising METTL3, METTL14, and auxiliary components such as WTAP, RBM15/15B, and VIRMA (KIAA1429), catalyzes the deposition of m6A marks on target transcripts. Conversely, demethylases such as fat mass and obesity-associated protein (FTO) and alkB homolog 5 (ALKBH5) remove these modifications, enabling dynamic regulation of RNA methylation. m6A-modified transcripts are subsequently recognized by reader proteins, primarily the YTH domain-containing family (YTHDF1/2/3, YTHDC1/2) and IGF2 mRNA-binding proteins (IGF2BP1/2/3), which govern RNA splicing, stability, localization, and translational efficiency [4, 8]. Advances in high-throughput techniques, including methylated RNA immunoprecipitation sequencing (MeRIP-seq) and crosslinking immunoprecipitation sequencing (CLIP-seq), have facilitated transcriptome-wide and site-specific mapping of m6A, unveiling its dynamic regulatory functions under both physiological and pathological conditions [9, 10]. Functionally, m6A methylation plays indispensable roles in embryogenesis, immune homeostasis, neural development, stem cell fate determination, and metabolic regulation [11, 12]. Emerging evidence has implicated aberrant m6A modification in the etiology of various human diseases, including malignancies, metabolic disorders, neurodegenerative conditions, and notably, kidney diseases [13–15].
The kidney plays a central role in maintaining systemic homeostasis by regulating fluid and electrolyte balance, blood pressure, acid-base status, and the excretion of metabolic waste [16, 17]. Its anatomical and cellular complexity, encompassing glomerular podocytes, tubular epithelial cells (TECs), endothelial cells, and interstitial immune cells, renders it highly vulnerable to a broad spectrum of pathological insults, including metabolic dysregulation, ischemia-reperfusion injury, genetic abnormalities, autoimmune reactions, and neoplastic transformation [18–20]. These diverse etiologies contribute to a wide array of kidney disorders characterized by marked clinical and molecular heterogeneity, including diabetic nephropathy (DN), acute kidney injury (AKI), focal segmental glomerulosclerosis (FSGS), chronic kidney disease (CKD), lupus nephritis (LN), hyperuricemic nephropathy (HN), autosomal dominant polycystic kidney disease (ADPKD), and clear cell renal cell carcinoma (ccRCC) [21–23]. Despite advancements in diagnostic modalities and supportive care, many renal conditions progress inexorably toward fibrosis and ultimately culminate in end-stage renal disease (ESRD), highlighting the critical need to elucidate underlying molecular mechanisms and identify novel therapeutic targets [24, 25].
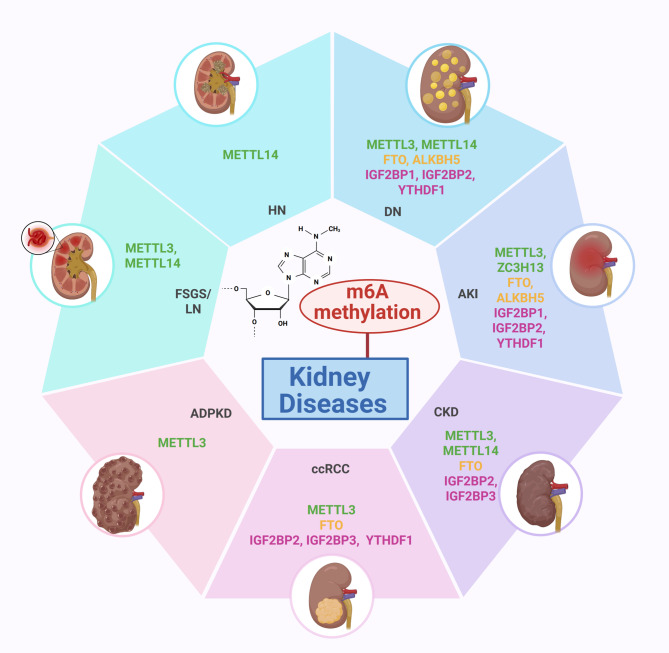
Emerging evidence implicates dysregulated m6A modification as a key contributor to renal pathogenesis through the modulation of essential cellular processes, including inflammation, epithelial-mesenchymal transition (EMT), apoptosis, pyroptosis, metabolic reprogramming, and autophagy [26–28]. These mechanisms are associated with hallmark pathological events such as podocyte damage and glomerulosclerosis in DN, tubular cell injury in AKI, cyst formation in polycystic kidney disease, and tumorigenesis in ccRCC (Fig. 1) [29–31]. Deciphering the m6A epitranscriptomic landscape and its regulatory components in renal pathology offers novel insights into disease progression and therapeutic intervention [32, 33]. This review presents a comprehensive synthesis of current knowledge on m6A RNA methylation in kidney disease, with focused analyses of DN, AKI, CKD, FSGS, LN, HN, ADPKD, and ccRCC. The mechanistic roles of m6A writers, erasers, and readers in modulating gene expression and cellular behavior during renal injury and repair are systematically examined. Additionally, recent advances in m6A-based biomarkers and targeted therapeutic strategies are discussed, emphasizing the translational potential of m6A regulation in the field of kidney diseases.
Fig. 1.
Dysregulated m6 A RNA methylation in kidney diseases. In kidney diseases, alterations in m6A methylation and its regulatory machinery play pivotal roles in disease progression via multiple mechanisms. Overexpression of METTL3 and IGF2BP3, along with reduced activity of METTL14 or FTO, have been identified in conditions such as DN, AKI, CKD, FSGS, LN, HN, ADPKD, and ccRCC. These disruptions result in abnormal m6A modification patterns, affecting critical pathways like inflammation, fibrosis, EMT, autophagy dysregulation, ferroptosis, and mitochondrial dysfunction. Targeting the m6A modification system presents a promising therapeutic avenue for mitigating renal damage and halting disease progression
Molecular mechanisms of m6A methylation in diverse kidney diseases
m6A methylation in diabetic nephropathy (DN)
RNA methylation regulators have emerged as pivotal modulators of DN, orchestrating critical pathological events including podocyte injury, autophagy impairment, tubular dysfunction, inflammation, and fibrosis (Fig. 2). Transcriptomic profiling has identified 24 differentially expressed RNA methylation regulators in glomerular tissue from patients with DN compared to healthy controls, indicating extensive epitranscriptomic dysregulation [34].
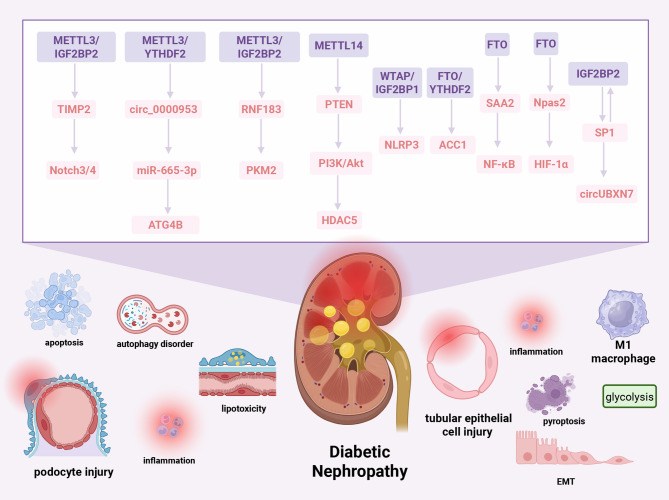
Fig. 2.
Regulatory mechanisms of m6A methylation dysregulation in DN. In DN, dysregulated m6A methylation accelerates disease progression through interconnected pathways that mediate podocyte injury, autophagy dysfunction, tubular impairment, immune activation, and fibrotic remodeling. In podocytes, METTL3 upregulates m6A methylation of TIMP2 mRNA via IGF2BP2, activating Notch3/4 signaling. Concurrently, METTL3 enhances YTHDF2-mediated degradation of circ_0000953, impairing miR-665-3p-ATG4B-dependent autophagy. Hyperglycemic conditions suppress METTL3 and IGF2BP2, leading to downregulation of RNF183 and disruption of PKM2 ubiquitination. FTO overexpression stabilizes ACC1 mRNA through YTHDF2 while destabilizing SAA2 to activate NF-κB signaling. In TECs, METTL14 downregulation inhibits PTEN, activating the PI3K/Akt-HDAC5 axis. Additionally, WTAP facilitates m6A modification and IGF2BP1-mediated stabilization of NLRP3 mRNA, promoting pyroptosis and inflammation. In the interstitial compartment, SP1 transcriptionally activates circUBXN7, which forms a positive feedback loop with IGF2BP2 to drive macrophage infiltration and fibrosis. FTO also regulates Npas2 mRNA methylation and stability via Prrc2a, enhancing HIF-1α signaling and mitigating M1 macrophage-driven inflammation and glycolysis. SP1: specificity protein 1, HDAC5: Histone Deacetylase 5, TIMP2: Tissue Inhibitor of Metalloproteinases 2, RNF183: Ring Finger Protein 183, PTEN: Phosphatase and Tensin Homolog, NLRP3: NOD-like receptor family pyrin domain containing 3, ACC1: Acetyl-CoA Carboxylase 1, PKM2: Pyruvate Kinase M2, ATG4B: Autophagy Related 4B Cysteine Peptidase
Podocyte injury, a defining feature of early-stage DN, is strongly associated with autophagy suppression [35, 36]. In renal biopsies from patients with DN, METTL3 expression in podocytes shows a positive correlation with 24-hour urinary microalbumin and creatinine levels and a negative correlation with estimated glomerular filtration rate (eGFR). METTL3 is markedly upregulated in both type 1 and type 2 diabetic mouse models and functions as a central driver of podocyte damage. It enhances m6A methylation and stabilizes tissue inhibitor of metalloproteinase 2 (TIMP2) mRNA in an IGF2BP2-dependent manner. TIMP2 overexpression activates the Notch3/4 signaling axis, thereby exacerbating inflammation and apoptosis in podocytes. Rescue experiments demonstrated that overexpression of TIMP2 in high-glucose (HG)-stimulated METTL3 knockdown podocytes abrogated the renoprotective effects conferred by METTL3 silencing, reinstating inflammatory responses, podocyte apoptosis, and cytoskeletal injury [37]. METTL3 silencing in db/db mice ameliorates albuminuria and podocyte injury, reinforcing its therapeutic relevance. Concurrently, METTL3 mediates m6A modification and YTHDF2-mediated degradation of circ_0000953, a circular RNA significantly downregulated in HG-stimulated podocytes and DN kidney tissues [38]. Functionally, circ_0000953 serves as a molecular sponge for miR-665-3p, thereby regulating autophagy-related gene 4B (ATG4B) expression to restore autophagic flux, attenuate podocyte injury, and reduce proteinuria. In HG-treated human podocyte (HPC) cells, both METTL3 and IGF2BP2 are significantly downregulated after 48 h. These two factors coordinately regulate ring finger protein 183 (RNF183), an endoplasmic reticulum-associated E3 ubiquitin ligase. RNF183 downregulation under HG conditions impairs pyruvate kinase M2 (PKM2) ubiquitination, contributing to metabolic dysregulation and podocyte dysfunction [39]. Beyond podocytes, m6A modification exerts regulatory control over renal TEC function under diabetic conditions, driving EMT and interstitial fibrosis. High-glucose exposure reduces global m6A levels and suppresses METTL14 expression, resulting in downregulation of phosphatase and tensin homolog (PTEN) and subsequent activation of the PI3K/Akt signaling pathway. This cascade leads to histone deacetylase 5 (HDAC5) upregulation, facilitating EMT and fibrotic remodeling [40]. Conversely, both global m6A abundance and WTAP expression are elevated in DN kidneys, correlating positively with clinical indices including eGFR, blood urea nitrogen (BUN), serum creatinine, and albuminuria. In HG-treated HK-2 cells, WTAP promotes m6A modification of NLR family pyrin domain containing 3 (NLRP3) mRNA, which is stabilized by IGF2BP1, thereby enhancing pyroptotic cell death and inflammation. WTAP knockdown reverses these molecular events and alleviates renal injury and fibrosis in diabetic mouse models [41].
FTO exhibits a dual, context-specific function in DN, with distinct functional outputs in podocytes and immune cells. In lipid-overloaded podocytes, FTO is upregulated and exerts pathogenic effects by erasing m⁶A modifications from key metabolic transcripts. Specifically, FTO demethylates acetyl-CoA carboxylase 1 (ACC1) mRNA, enhancing its stability through a YTHDF2-dependent mechanism. This stabilization drives excessive fatty acid synthesis and lipotoxic accumulation, contributing to podocyte injury and functional decline [42]. Additionally, FTO-mediated demethylation of serum amyloid A2 (SAA2) mRNA increases its decay, since lower SAA2 levels attenuate NF-κB activation and reduce podocyte stress [43]. Pharmacological inhibition of FTO using agents such as meclofenamic acid (MA) or diacerein (DIA) has been shown to restore lipid homeostasis, reduce podocyte damage, and improve renal function in db/db mice, highlighting the translational relevance of targeting this epitranscriptomic node [42]. Beyond its role in podocytes, FTO exhibits a contrasting protective function in immune compartments. In macrophages derived from DN patients, reduced FTO expression correlates with impaired renal function and heightened inflammation. Mechanistically, FTO sustains the stability of neuronal PAS domain protein 2 (Npas2) mRNA through a proline-rich coiled-coil 2 A (Prrc2a)-dependent pathway. Stabilized Npas2 activates hypoxia-inducible factor-1α (HIF-1α) signaling, thereby suppressing inflammation and glycolytic flux in M1 macrophages [44]. These findings suggest an anti-inflammatory, immunomodulatory role of FTO in the macrophage, forming a regulatory axis distinct from its function in podocytes.
Moreover, under high-glucose conditions, the transcription factor specificity protein 1 (SP1) transcriptionally activates circUBXN7, which in turn forms a positive feedback loop with IGF2BP2 and SP1 by enhancing SP1 mRNA stability. This IGF2BP2–SP1 circuit facilitates macrophage infiltration and renal fibrosis, further amplifying DN pathogenesis [45].
Collectively, these findings delineate the multifaceted roles of m6A regulators, including writers (METTL3, METTL14, WTAP), erasers (FTO), and readers (IGF2BPs, YTHDF2), in orchestrating key pathological events such as podocyte injury, autophagy dysfunction, immune cell infiltration, inflammatory signaling, EMT, and fibrosis in DN. The complex regulatory landscape of m6A modifications underscores their potential as epitranscriptomic biomarkers and therapeutic targets in the clinical management of DN.
Acute Kidney Injury (AKI)
AKI entails a rapid decline in renal function initiated by ischemic, toxic, or infectious insults, with mounting evidence identifying m6A RNA methylation as a key modulator of epithelial cell injury, inflammatory signaling, and tissue repair in the kidney (Fig. 3).
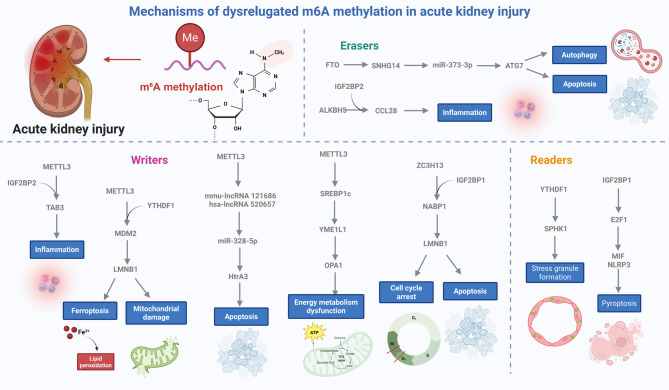
Fig. 3.
Regulatory roles of m6A RNA methylation in AKI. In AKI, METTL3 is consistently upregulated across various models, promoting m6A modification and IGF2BP2-mediated stabilization of Table 3 mRNA, thereby activating TGF-β-driven inflammation. METTL3 also enhances MDM2 mRNA methylation, which is translated via YTHDF1, leading to p53 degradation and subsequent activation of LMNB1. Additionally, METTL3 stabilizes mmu-lncRNA 121,686 and hsa-lncRNA 520,657, which act as sponges for miR-328-5p, derepressing HtrA3 expression and triggering tubular cell apoptosis. METTL3 further facilitates the AKI-CKD transition by stabilizing SREBP1c mRNA, which represses YME1L1, impairing OPA1-mediated mitochondrial dynamics. ZC3H13, another m6A writer, is upregulated in TECs during AKI and promotes NABP1 mRNA methylation and stabilization via IGF2BP1. Among the erasers, FTO is downregulated in sepsis-induced AKI, resulting in increased SNHG14 stability and activation of miR-373-3p-ATG7-mediated autophagy and apoptosis. ALKBH5 demethylates CCL28 mRNA in I/R injury, limiting Treg recruitment via IGF2BP2-dependent recognition; inhibiting ALKBH5 enhances CCL28 stability and attenuates inflammation. As a reader, IGF2BP1 stabilizes E2F1 mRNA, promoting MIF expression and NLRP3 inflammasome activation, leading to pyroptosis. YTHDF1, elevated in TECs under stress, facilitates stress granule formation and protects survival-related transcripts such as SPHK1, mitigating tubular injury associated with AKI
Among m6A methyltransferases, METTL3 has emerged as a critical effector in AKI pathogenesis. Across various AKI models, including those induced by cisplatin, ischemia/reperfusion (I/R), lipopolysaccharide (LPS), and cecal ligation and puncture (CLP), both METTL3 expression and global m6A levels are consistently elevated in renal tissues, especially within TECs. METTL3 facilitates m6A modification of Table 3 mRNA, which is stabilized through IGF2BP2 interaction, subsequently activating TGF-β-dependent inflammatory cascades. Pharmacologic blockade of METTL3 via cpd-564 confers renal protection in cisplatin- and I/R-induced AKI, underscoring the therapeutic potential of the METTL3–Table 3–IGF2BP2 signaling axis [46]. METTL3 also enhances m6A methylation of MDM2 mRNA, which is recognized by YTHDF1, promoting p53 ubiquitination and downstream LMNB1 activation. This cascade facilitates ferroptosis and mitochondrial injury in TECs. Silencing METTL3 attenuates oxidative stress and tissue damage in vitro and in CLP-induced sepsis models [47]. Additionally, METTL3 regulates long non-coding RNAs (lncRNAs) such as mmu-lncRNA 121,686 (mouse) and hsa-lncRNA 520,657 (human) by increasing their m6A-mediated stability, enabling their function as competing endogenous RNAs (ceRNAs) that sponge miR-328-5p. This derepression of HtrA3 HtrA serine peptidase 3 (HtrA3) contributes to apoptotic and morphological damage in tubular cells [48]. Further, METTL3 plays a pivotal role in the AKI-to-CKD transition by disrupting mitochondrial metabolism. Through m6A modification, METTL3 stabilizes sterol regulatory element-binding protein 1c (SREBP1c) mRNA, which represses YME1-like 1 (YME1L1) transcription, impairing optic atrophy 1 (OPA1)-dependent mitochondrial dynamics. Restoration of YME1L1 reverses mitochondrial dysfunction and injury, highlighting the pathogenic significance of the METTL3–SREBP1c–YME1L1 axis in AKI progression toward CKD [49]. ZC3H13, another m6A writer, is markedly upregulated in cisplatin-induced and human AKI tissues, with predominant expression in TECs. It enhances m6A methylation of nucleic acid binding protein 1 (NABP1) mRNA, increasing its stability via IGF2BP1 binding. Elevated NABP1 levels induce G2/M cell cycle arrest and apoptosis. Suppression of ZC3H13 alleviates renal injury, positioning the ZC3H13–NABP1–IGF2BP1 pathway as an additional epitranscriptomic mechanism contributing to AKI pathogenesis [50].
In sepsis-associated AKI, the m6A demethylase FTO is markedly downregulated in human renal tissues. Mechanistically, FTO destabilizes small nucleolar RNA host gene 14 (SNHG14) in an m6A-dependent manner, thereby limiting its expression in LPS-stimulated HK-2 cells. Functionally, SNHG14 operates as a ceRNA by sequestering miR-373-3p, leading to autophagy-related gene 7 (ATG7) upregulation and consequent promotion of autophagy and apoptosis under septic stress [51]. ALKBH5 serves a distinct role in modulating inflammation and tissue injury during renal ischemia–reperfusion injury (IRI). Predominantly expressed in mTECs, ALKBH5 exhibits dynamic expression patterns post-IRI, with early upregulation followed by decline at the injury peak, mirroring fluctuations in global m6A levels. ALKBH5 directly demethylates and destabilizes CCL28 mRNA, a chemokine essential for regulatory T cell (Treg) recruitment, through an IGF2BP2-dependent pathway. Loss of ALKBH5 function, either genetically in mTECs or pharmacologically via IOX1, enhances CCL28 stability, increases Treg infiltration, and suppresses innate immune activation, thereby conferring protection against both acute and chronic renal injury [52].
Among m6A readers, IGF2BP1 is upregulated in pyroptosis-associated AKI and functions as a potent driver of inflammatory cell death. Through m6A-mediated stabilization of E2F Transcription Factor 1 (E2F1) mRNA, IGF2BP1 amplifies macrophage migration inhibitory factor (MIF) expression and activates the NLRP3 inflammasome, culminating in pyroptotic damage. IGF2BP1 inhibition dampens renal inflammation and tubular apoptosis during infectious AKI, identifying it as a promising therapeutic target in infection-driven kidney injury [53]. YTHDF1 has been recognized as a critical m6A reader that confers cytoprotection during AKI by regulating mRNA fate within stress granules (SGs). In both human and murine AKI models, YTHDF1 is upregulated in TECs. Under stress conditions, it facilitates SG assembly by recruiting m6A-modified transcripts, safeguarding the translation of genes essential for cellular survival and regeneration. Among these, sphingosine kinase 1 (SPHK1) mRNA, a key enzyme in sphingosine-1-phosphate (S1P) biosynthesis, is selectively preserved, underscoring YTHDF1’s pivotal role in m6A-dependent stress adaptation and its capacity to attenuate tubular injury during AKI [54].
Chronic Kidney Disease (CKD)
Kidney fibrosis represents a common pathological endpoint across various CKDs, including obstructive nephropathy, transplant rejection, and age-related renal decline. Emerging evidence implicates m6A RNA methylation as a pivotal regulator of fibrotic progression through modulation of EMT, inflammatory pathways, autophagy, and mitochondrial function (Fig. 4).
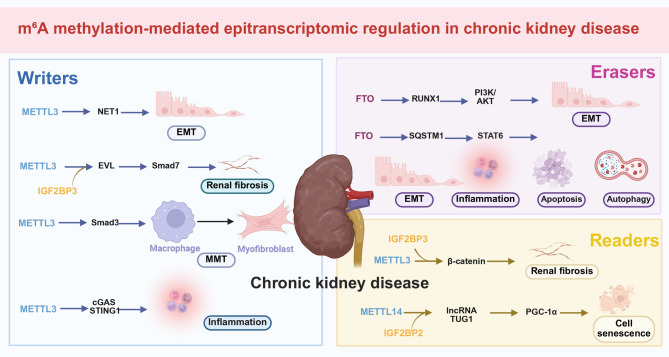
Fig. 4.
m6A methylation-mediated epitranscriptomic regulation of CKD and renal fibrosis. In CKD, m6A dysregulation promotes fibrogenesis through modulation of EMT, immune signaling, autophagy, and mitochondrial homeostasis. METTL3 is upregulated in TGF-β-stimulated HK-2 cells, UUO and UIRI mouse models, and IgA nephropathy biopsies, correlating with proteinuria and tubulointerstitial fibrosis. Mechanistically, METTL3 enhances m6A modification of NET1 and β-catenin mRNAs, stabilizing these transcripts via IGF2BP3 and activating profibrotic signaling. METTL3 also stabilizes EVL mRNA through IGF2BP2, impairing Smad7-mediated inhibition of TGF-β/Smad3 signaling. In CAR, METTL3 promotes M2 macrophage-driven mesenchymal transition (MMT) via stabilization of Smad3 mRNA; METTL3 knockout or STM2457 treatment reverses fibrosis. METTL3 additionally enhances m6A methylation of cGAS and STING1, amplifying inflammatory signaling through the cGAS-STING pathway. FTO, upregulated in UUO and TGF-β-treated TECs, stabilizes RUNX1 mRNA, activating the PI3K/AKT pathway to promote EMT. In contrast, Cana suppresses FTO, enhancing SQSTM1 mRNA stability, restoring FAO through degradation of STAT6, and reducing fibrosis. In aging kidneys, reduced METTL14 levels decrease m6A modification of TUG1, destabilizing the lncRNA and impairing PGC-1α-mediated mitochondrial quality control via IGF2BP2
Among m6A methyltransferases, METTL3 has been identified as a key epigenetic driver of renal fibrosis. Its expression and global m6A levels are consistently elevated in TGF-β-stimulated HK-2 cells, as well as in in vivo models of unilateral ischemia-reperfusion injury (UIRI) and unilateral ureteral obstruction (UUO), and in kidney biopsies from patients with CKD. Immunohistochemical analyses reveal robust nuclear METTL3 staining in TECs, particularly in IgA nephropathy [55]. METTL3 expression shows a positive correlation with proteinuria, tubulointerstitial fibrosis, serum creatinine, and BUN, and an inverse correlation with eGFR, underscoring its value as both a prognostic marker and therapeutic target [56]. Mechanistically, METTL3 facilitates EMT by catalyzing m6A modification of neuroepithelial cell transforming 1 (NET1) mRNA and enhances β-catenin mRNA stability through IGF2BP3-mediated binding, thereby sustaining β-catenin signaling and activating profibrotic transcriptional programs [55]. Suppression of METTL3, via siRNA, proximal tubule-specific knockout, or the selective inhibitor STM2457, leads to significant downregulation of fibrosis markers such as α-SMA and collagen I, mitigating renal fibrotic remodeling in vivo [56]. Additionally, METTL3 promotes m6A methylation of Ena/VASP-like (EVL) mRNA, stabilizing its expression through IGF2BP2. Upregulated EVL binds to Smad7, disrupting its repressive function on the TGF-β1/Smad3 axis and further accelerating fibrosis. Structure-based compound screening has identified Isoliquiritin, a natural monomer, as a potent METTL3 inhibitor that disrupts the METTL3–EVL–m6A circuit and exerts robust anti-fibrotic activity [57]. In chronic active renal allograft rejection (CAR), METTL3 is likewise upregulated and facilitates macrophage-to-myofibroblast transition (MMT) via amplification of TGF-β1/Smad3 signaling in M2 macrophages. Paradoxically, METTL3 knockout (KO) results in increased m6A modification but decreased expression of Smad3, thereby suppressing MMT and fibrotic progression. Pharmacological inhibition of METTL3 using STM2457 reverses M2-induced MMT and alleviates fibrotic injury in CAR, emphasizing the therapeutic relevance of the METTL3–m6A–Smad3 regulatory axis in transplant-associated fibrosis [58]. Moreover, METTL3-driven m6A hypermethylation contributes to the activation of the cyclic guanosine monophosphate-AMP synthase (cGAS)-stimulator of IFN genes (STING) signaling pathway in CKD. Elevated m6A levels stabilize cGAS and STING1 mRNAs, resulting in increased expression of downstream effectors and amplification of immune inflammation in CKD [59].
Recent studies indicate that FTO is upregulated in both UUO mouse models and TGF-β1-treated TECs, exacerbating inflammation, apoptosis, and autophagy inhibition. Mechanistically, FTO demethylates Runt-related transcription factor 1 (RUNX1) mRNA, enhancing its stability and expression. RUNX1, in turn, activates the PI3K/AKT pathway, promoting EMT and extracellular matrix deposition [60]. Additionally, Canagliflozin (Cana), a sodium-glucose cotransporter 2 (SGLT2) inhibitor, mitigates renal fibrosis and lipid metabolic disorders induced by UUO and I/R in an autophagy-dependent manner. Cana reduces FTO expression, leading to increased global m6A levels and enhanced SQSTM1 mRNA stability, a key autophagy receptor. This process facilitates the autophagic degradation of STAT6, promoting fatty acid oxidation (FAO) and mitigating fibrosis. The protective effects of Cana are abolished in the absence of SQSTM1, emphasizing the anti-fibrotic and metabolic regulatory role of the FTO-m6A-SQSTM1-autophagy-STAT6 axis in renal fibrosis [61].
Emerging evidence underscores the critical role of m6A RNA methylation in age-related kidney dysfunction, particularly through its regulation of mitochondrial homeostasis. Aged kidneys exhibit increased fibrosis and reduced global m6A levels compared to their younger counterparts. Decreased expression of METTL14 leads to reduced m6A modification of lncRNA TUG1, a transcript crucial for mitochondrial health, impairing its stability and expression via the m6A reader IGF2BP2 [62]. Functionally, TUG1 upregulates PGC-1α, a master regulator of mitochondrial biogenesis, thereby promoting mitochondrial biogenesis and maintaining mitochondrial quality control (MQC), which in turn alleviates cellular senescence and renal fibrosis. These findings, validated in accelerated aging mouse models and senescent HK-2 cells, highlight the METTL14/IGF2BP2-TUG1-PGC1α axis as a promising therapeutic target for age-related kidney injury.
In conclusion, renal fibrosis is tightly controlled by a dynamic epitranscriptomic landscape involving writers (METTL3, METTL14), erasers (FTO), and readers (IGF2BP2, IGF2BP3). These factors work synergistically to regulate critical pathways such as TGF-β/Smad, PI3K/AKT, β-catenin, cGAS-STING, and autophagy, thus shaping fibrotic responses in CKD, CAR, aging, and obstructive nephropathy.
Focal Segmental Glomerulosclerosis (FSGS)
Recent studies suggest that elevated mRNA m6A methylation mediated by METTL14 plays a pivotal role in the progression of podocytopathies. In both adriamycin (ADR)-induced nephropathy and DN mouse models, kidneys show significantly increased global m6A RNA levels and METTL14 expression. This upregulation of METTL14 was also observed in renal biopsy samples from patients with FSGS and DN, as well as in cultured human podocytes exposed to ADR or advanced glycation end product (AGE) stimulation in vitro. Mechanistically, METTL14 enhances the m6A methylation of Sirtuin 1 (SIRT1) mRNA, a key deacetylase involved in maintaining podocyte homeostasis. This modification accelerates SIRT1 mRNA degradation, leading to reduced SIRT1 expression and exacerbating podocyte injury in proteinuric conditions. These findings identify the METTL14-m6A-SIRT1 axis as a key epitranscriptomic pathway that contributes to glomerular damage and proteinuria in diseases like FSGS and DN [63].
Lupus Nephritis (LN)
m6A methylation has emerged as a novel epigenetic mechanism in the pathogenesis of LN. The expression patterns of m6A regulators exhibit greater variation in the glomeruli than in the tubulointerstitium or whole kidney tissue in patients with LN [64]. Using least absolute shrinkage and selection operator (LASSO) logistic regression, a robust m6A regulator gene signature, including METTL3, WTAP, YTHDC2, YTHDF1, and fragile X mental retardation 1 (FMR1), was identified, effectively distinguishing patients with LN from healthy controls. This highlights the substantial differences in m6A modification patterns between LN and non-diseased kidneys. Furthermore, m6A regulators are strongly linked to immune features in LN, particularly with major histocompatibility complex class I (MHC-I)-mediated antigen presentation, cytokinesis, inflammatory responses, and interferon (IFN)-mediated signaling pathways, emphasizing their critical role in modulating immune dysregulation in LN. Aberrant m6A methylation patterns have also been detected in peripheral blood mononuclear cells (PBMCs) and kidney tissues from patients with systemic lupus erythematosus (SLE). In the kidneys of MRL/lpr mice, a model of SLE, METTL3 expression is markedly increased in glomerular inflammatory cells, renal TECs, and infiltrating plasma cells. Mechanistically, METTL3 promotes m6A-dependent upregulation of interferon regulatory factor 4 (IRF4), driving plasma cell infiltration and contributing to SLE-related kidney damage [65]. These findings highlight the METTL3-IRF4 axis as a potential epigenetic mechanism linking autoimmunity and renal injury in LN.
Hyperuricemic Nephropathy (HN)
In HN mice, renal m6A methylation levels are significantly reduced, along with decreased METTL14 expression. In vitro, overexpression of METTL14 enhances m6A modification and degradation of GLUT9 mRNA, a key urate transporter in TECs, thus reducing HN-related reabsorption and mitigating renal fibrosis. Treatment with the urate-lowering agent benzbromarone restores m6A levels, upregulates METTL14, and alleviates kidney injury, reinforcing the protective role of the METTL14-GLUT9 axis in hyperuricemic renal damage [66].
Autosomal Dominant Polycystic Kidney Disease (ADPKD)
Recent studies highlight METTL3’s pivotal role in ADPKD by linking methionine metabolism to the epitranscriptomic activation of cyst growth. Both METTL3 expression and m6A levels are elevated in the kidneys of ADPKD mice and humans, with kidney-specific overexpression of METTL3 sufficient to induce tubular cyst formation. This is accompanied by increased methionine and its methyl donor S-adenosylmethionine (SAM) levels. Functionally, both methionine and SAM further stimulate METTL3 expression and promote ex vivo cyst expansion, whereas dietary methionine restriction significantly reduces cyst growth in vivo. Mechanistically, METTL3 drives cystogenesis by enhancing m6A methylation and translation of c-Myc and arginine vasopressin receptor 2 (Avpr2) mRNAs, activating c-Myc and cyclic AMP (cAMP) signaling pathways. Genetic deletion of METTL3 significantly reduces cyst growth across multiple ADPKD models. Moreover, dietary methionine restriction, including plant-based or vegan diets, lowers methionine and SAM levels, downregulates METTL3 expression, and effectively slows cyst expansion [67].
Clear Cell Renal Cell Carcinoma (ccRCC)
ccRCC exhibits significant disruption in epitranscriptomic homeostasis, with dysregulated m⁶A RNA methylation playing a pivotal role. However, global m6A methylation levels in ccRCC do not follow a uniform pattern but instead display stage- and regulator-specific heterogeneity. Among the m6A “writers,” METTL3 is notably upregulated in ccRCC tissues compared to adjacent normal kidney tissue, and elevated METTL3 expression correlates with poor clinical outcomes. Functionally, METTL3 promotes m6A modification of human endogenous retrovirus-H long terminal repeat-associating 2 (HHLA2) mRNA, enhancing its translation and driving ccRCC cell proliferation, migration, and invasion [68]. In advanced RCC, prolonged hypoxia induces HIF-1α-mediated transcriptional activation of METTL3, which further increases m6A modification and translation of procollagen-lysine, 2-oxoglutarate 5-dioxygenase 2 (PLOD2) mRNA, thereby promoting hypoxia-driven tumor cell invasiveness [69]. Notably, inhibition of METTL3 using the selective inhibitor STM2457 significantly reduces tumor burden in xenograft models, suggesting that the METTL3-HHLA2 and METTL3-PLOD2 axes represent promising therapeutic targets in ccRCC. While the majority of high-impact studies have reported that METTL3 acts as an oncogenic driver in ccRCC, accumulating evidence from other malignancies suggests that its functional role may be context-dependent.
In contrast, the m6A “eraser” FTO is paradoxically upregulated in ccRCC, as indicated by TCGA and GEO datasets (GSE53757 and GSE66271), despite the global reduction in m6A. High FTO expression correlates with poor 10-year overall survival and is recognized as an independent prognostic factor. Mechanistically, FTO promotes ccRCC progression by destabilizing salt-inducible kinase 2 (SIK2) mRNA in an m6A-IGF2BP2-dependent manner, inhibiting autophagy and enhancing tumorigenesis. Pharmacological inhibition of FTO with FB23-2 markedly reduces tumor growth and extends survival in patient-derived xenograft (PDX) models, underscoring the therapeutic relevance of the FTO-SIK2-autophagy axis [70].
Among the m6A “readers,” IGF2BPs play pivotal roles in tumor progression. IGF2BP3, in particular, is significantly upregulated in ccRCC and correlates with poor prognosis. It binds to m6A-modified circular RNA circRARS (hsa_circ_0001550), enhancing its stability and translation of oncogenic targets, thereby amplifying tumor-promoting pathways [71]. Similarly, IGF2BP2 and YTHDF1 synergistically regulate zinc finger protein 677 (ZNF677), a tumor suppressor that is markedly downregulated in RCC. IGF2BP2 stabilizes ZNF677 mRNA, while YTHDF1 promotes its translation by recognizing m6A-modified coding sequences [72]. ZNF677 exerts tumor-suppressive effects by transcriptionally repressing cyclin-dependent kinase inhibitor 3 (CDKN3). Restoration of ZNF677 inhibits cell proliferation, induces apoptosis, and restores the anti-tumor phenotype, emphasizing the importance of the IGF2BP2/YTHDF1-ZNF677-CDKN3 axis in RCC suppression. These findings suggest that global m6A dynamics in ccRCC are influenced by the balance between the activities of writers and erasers, as well as the functional presence of readers. Rather than simply increasing or decreasing, m6A remodeling in ccRCC represents a finely tuned epitranscriptomic reprogramming, selectively enhancing or repressing specific transcripts that drive tumor progression.
Although accumulating evidence underscores the regulatory significance of m⁶A RNA methylation in several prevalent kidney diseases such as DN, AKI, CKD, and ccRCC, its role in less-studied renal disorders like FSGS and HN remains largely elusive. Preliminary transcriptomic data and limited mechanistic studies suggest the possible involvement of specific m⁶A regulators in these diseases, while direct experimental validation is still lacking. Elucidating epitranscriptomic investigations into these underrepresented kidney diseases may uncover novel molecular mechanisms and pave the way for m⁶A-based clinical strategies across a broader spectrum of kidney diseases.
Potential clinical prospects of m6A methylation in kidney diseases
With the increasing recognition of m6A methylation as a key regulator in renal pathophysiology, it has emerged as a promising therapeutic and prognostic target in kidney diseases [29, 73, 74]. Growing preclinical evidence indicates that pharmacological modulation of m6A regulators, including writers and erasers, can effectively mitigate key pathological processes such as inflammation, fibrosis, ferroptosis, and tumorigenesis in various kidney disease models [27, 32, 75]. For example, selective METTL3 inhibitors like STM2457 and cpd-564 have proven effective in reducing renal fibrosis, inflammation, and ferroptosis in AKI and CKD models [46]. STM2457 has also demonstrated significant anti-tumor effects in ccRCC [69]. Conversely, FTO inhibitors such as FB23-2, MA, and DIA have shown protective effects in DN and ccRCC, primarily through restoring lipid homeostasis, enhancing autophagy, and suppressing inflammation and tumor progression [42, 55, 70]. Additionally, inhibition of ALKBH5 with IOX1 has been found to promote Treg infiltration, thereby reducing sterile inflammation and fibrosis in IRI models [52]. Furthermore, isoforsythiaside, a natural compound identified through virtual screening, acts as a direct METTL3 inhibitor with potent anti-fibrotic properties [57]. Benzbromarone, a urate-lowering agent, has also been shown to restore m6A levels and METTL14 expression, thereby alleviating hyperuricemia-induced renal injury [66]. These findings provide strong preclinical evidence supporting the potential of pharmacologically manipulating m6A methylation to mitigate kidney damage. Genetic approaches further support the therapeutic relevance of m6A modulation. METTL3 knockdown significantly reduces cyst formation in multiple ADPKD models and alleviates kidney fibrosis in both in vivo and in vitro settings [55, 67]. Tubule-specific deletion of METTL3 or antisense oligonucleotide-based silencing offers protection against inflammation and tissue remodeling [48, 59]. In parallel, silencing METTL14 or IGF2BP2 in renal TECs disrupts mitochondrial homeostasis, while IGF2BP3 knockdown inhibits cancer stemness and xenograft growth, underscoring the multifaceted role of m6A readers in ccRCC [62, 71].
However, these therapeutic strategies are complicated by the contextual plasticity of m⁶A regulators. Their expression and function vary not only across different kidney disease types but also within distinct models of the same disease. For instance, FTO is upregulated in lipid-overloaded podocytes, promoting injury via stabilization of ACC1 and lipotoxic stress, but is downregulated in DN-associated macrophages, where its restoration suppresses inflammation via the Npas2-HIF-1α axis. In ccRCC, FTO acts oncogenically, enhancing stemness and survival pathways. These divergent effects likely reflect differences in cell-type-specific transcriptomes, differential expression of m⁶A readers, or post-translational modifications of m⁶A regulators that influence substrate specificity, and highlight the necessity of tailoring therapeutic interventions to specific cellular contexts and disease stages to prevent unintended consequences.
Beyond therapeutic applications, m6A regulators also show substantial potential as biomarkers for early diagnosis and risk stratification in kidney diseases. Aberrant expression of METTL3, METTL14, FTO, WTAP, and IGF2BP3, along with altered global m6A levels, correlates with clinical parameters such as eGFR, BUN, proteinuria, disease stage, and the extent of fibrotic lesions [41, 42, 55, 56, 71]. Integrating m6A profiling with clinical parameters could enable the development of predictive models for disease progression and therapeutic responsiveness, particularly in DN, CKD, and ccRCC [37, 44, 58, 69, 70]. While current studies have primarily focused on cross-sectional associations between m⁶A regulator expression and clinical parameters such as eGFR, proteinuria, and fibrotic lesions, there remains a critical gap in longitudinal validation. To establish the prognostic relevance of m⁶A dysregulation, future studies should incorporate time-course analyses in patient cohorts, correlating temporal shifts in m⁶A profiles with disease progression and therapeutic response.
Despite the promising therapeutic potential of targeting m⁶A regulators, several translational barriers remain unresolved. First, small-molecule inhibitors such as STM2457 and FB23-2 may exert off-target effects due to the pleiotropic functions of m⁶A enzymes across tissues. Second, effective and cell-type-specific delivery of m⁶A-targeted agents to glomerular podocytes or tubular epithelial cells, remains a formidable challenge. Additionally, interspecies differences in m⁶A epitranscriptomic regulation may complicate the extrapolation of findings from rodent models to human disease. These limitations highlight the need for refined preclinical models, precision-targeting strategies, and pharmacokinetic profiling to ensure the safe and effective clinical translation of m⁶A-based therapies in kidney diseases. Innovative tools from synthetic biology further expand the therapeutic potential of m6A methylation [76–78]. Engineered RNA-binding proteins and programmable m6A editors offer potential tools for cell-type-specific, temporally controlled regulation of pathogenic gene expression [79–81]. When coupled with advanced delivery systems such as lipid nanoparticles or exosome-based vectors, these strategies have the potential to overcome existing barriers in renal-targeted therapies [82–84]. As our understanding of m6A mechanisms deepens, the translation of m6A-targeted approaches into clinical practice holds great promise for enhancing the diagnosis, monitoring, and treatment of a wide range of kidney diseases.
In summary, while substantial progress has been made in preclinical exploitation of m⁶A regulators for kidney disease intervention, future research must address translational hurdles. Nonetheless, the convergence of m⁶A epitranscriptomics, precision medicine, and RNA-based technologies provides an exciting frontier for advancing clinical management of kidney diseases.
Conclusions
In recent years, m6A RNA methylation has emerged as a critical epitranscriptomic mechanism in the regulation of kidney disease progression. Dysregulation of m6A methylation and its associated regulators, including writers, erasers, and readers, has been mechanistically linked to a variety of key pathological processes in the kidney, such as inflammation, apoptosis, autophagy, EMT, and metabolic imbalance. These insights have significantly advanced our understanding of the molecular landscape underlying renal disorders, ranging from DN, AKI, CKD, and ADPKD, to ccRCC. As mechanistic links between m6A regulation and renal pathophysiology continue to emerge, m6A regulators are being actively investigated as both prognostic biomarkers and therapeutic targets. Preclinical studies across various disease models support the use of small-molecule modulators of METTL3 and FTO, demonstrating promising renoprotective and anti-fibrotic effects. Furthermore, advances in RNA editing and synthetic biology provide innovative precision strategies for manipulating m6A methylation in a cell-specific and disease-contextual manner. In summary, integrating m6A epitranscriptomics into nephrology research not only uncovers new layers of post-transcriptional regulation but also opens promising avenues for the development of targeted therapies and personalized medicine in kidney diseases.
Table 1.
Levels and roles of m6A methylation and its regulators in kidney diseases
| Kidney disease | Disease models | Injured Cell/Location | Methylation levels | M6A regulators | Expression | Biological processes | Year | References |
|---|---|---|---|---|---|---|---|---|
| DN | STZ-induced type 1 diabetic mice and HFD-induced type 2 diabetic mice; Mouse podocyte clone 5 (MPC5); Kidney biopsy samples of patients with DN and paracancerous (healthy) samples of patients with renal cancer | mouse podocyte clone 5 (MPC5) | / | METTL3/YTHDF2 | / | exacerbate podocyte injury and autophagy disorder | 2024 | [38] |
| DN | STZ-induced type 1 diabetic mice and type 2 diabetic db/db mice; high glucose-cultured HK2 cells; kidney biopsy specimens with DN | HK-2 cells | ↑ | METTL3/IGF2BP2 | ↑ | promote diabetic podocyte inflammation and apoptosis | 2022 | [37] |
| DN | diabetic mice; HPCs; kidney biopsy tissues of patients with DN | HPCs | ↓ | METTL3/IGF2BP2 | ↓ | decrease autophagy and promote apoptosis | 2025 | [39] |
| DN | diabetic mice and UUO mice; high glucose-cultured HK2 cells | HK-2 cells | ↓ | METTL14 | ↓ | induce EMT and renal interstitial fibrosis | 2021 | [40] |
| DN | diabetic mice; high glucose-cultured HK2 cells; kidney biopsy tissues of patients with DN (n = 63) | HK-2 cells | ↑ | WTAP/IGF2BP1 | ↑ | induce tubular pyroptosis and inflammation | 2022 | [41] |
| DN | diabetic mice; HPCs; kidney biopsy tissues of patients with DN | HPCs | ↑ | FTO/YTHDF2 | ↑ | contribute to lipotoxicity-mediated injury of DN podocytes | 2025 | [42] |
| DN | diabetic mice; human podocyte cells (HPCs); kidney biopsy tissues of patients with DN | HPCs | ↑ | FTO | ↑ | promote podocyte injury | 2024 | [43] |
| DN | diabetic mice; HG-induced BMDMs and primary kidney macrophages; kidney biopsy tissues of patients with DN | HG-induced BMDMs and primary kidney macrophages | ↓ | FTO | ↓ | promote M1 macrophage inflammation and glycolysis | 2025 | [44] |
| DN | diabetic mice; high glucose-cultured HK2 cells; plasma of DKD patients (n = 49) and healthy people (n = 54) | HK-2 cells | / | IGF2BP2 | / | promote macrophage activation, inflammation, EMT, and fibrosis | 2023 | [45] |
| AKI | cisplatin- and LPS-induced AKI mouse models; TNF-a–, cisplatin-, and LPS-treated HK2 cells or mouse TECs; kidney biopsies from patients with AKI | HK-2 cells and mouse TECs | ↑ | METTL3/IGF2BP2 | ↑ | promote renal inflammation and injury | 2022 | [46] |
| AKI | CLP-induced AKI mouse model; LPS-induced HK2 cells; | HK-2 cells | / | METTL3/YTHDF1 | / | promote mitochondrial damage and ferroptosis of kidney tubular epithelial cells | 2023 | [47] |
| AKI | cisplatin-induced and I/R AKI mouse model; HK-2 cells and mouse TECs; kidney biopsies from patients with AKI | HK-2 cells and mouse TECs | ↑ | METTL3 | ↑ | induce mitochondrial energy metabolism dysfunction | 2025 | [49] |
| AKI | I/R-, VAN-, and CLP-induced AKI mouse model; HK-2 cells and mouse TECs | HK-2 cells and mouse TECs | ↑ | METTL3 | ↑ | promote cell apoptosis | 2022 | [48] |
| AKI | cisplatin-induced AKI mouse model; cisplatin-treated HK2 cells; kidney biopsies from patients with AKI | HK-2 cells | ↑ | ZC3H13/IGF2BP1 | ↑ | promote the G2/M cell cycle arrest and apoptosis | 2025 | [50] |
| AKI | septic AKI mouse model; LPS-induced HK-2 cells; kidney biopsies from patients with S-AKI | HK2 cells | ↓ | FTO/IGF2BP2 | ↓ | induce cell autophagy and apoptosis | 2024 | [51] |
| AKI | I/R–induced AKI mouse model; mouse TECs | mouse TECs | ↓ initially at 12 h post-IRI, but sharply ↑ at 24 h and 48 h post-IRI, and then returned close to normal levels at 120 h post-IRI | ALKBH5 | ↑ at 12 h, then ↓ at 24 h and 48 h, and began to recover at 120 h after IRI | inhibit the recruitment of Tregs and induce inflammatory response | 2023 | [52] |
| AKI | septic AKI mouse model; LPS-treated HK2 cells; AKI patients and controls | HK-2 cells | ↑ | IGF2BP1 | ↑ | induce pyroptosis | 2023 | [53] |
| AKI | cisplatin or renal I/R-induced AKI mouse models; HK2 cells and cisplatin-induced mouse TECs; Kidney tissues were obtained from AKI patients | HK2 cells | ↑ | YTHDF1 | ↑ | favor cell survival | 2023 | [54] |
| CKD | UUO mouse model and TGF-β-treated HK-2 cells; Kidney tissue was collected from 16 CKD patients with diabetic nephropathy (n = 8) or IgA nephropathy (n = 8) and control subjects (n = 11) | HK-2 cells | ↑ | METTL3 | ↑ | induce EMT and inflammation | 2024 | [55] |
| CKD | UIRI and UUO mouse models and TGF-β-treated HK-2 cells; Kidney tissue was collected from 25 CKD patients and 5 control subjects. | HK-2 cells | ↑ | METTL3/IGF2BP3 | ↑ | promote fibrotic lesions | 2024 | [56] |
| CKD | UUO and I/R mouse model; HK-2 cells; human fibrotic kidney specimens | HK-2 cells | ↑ | METTL3/IGF2BP2 | ↑ | exacerbate fibrotic responses | 2023 | [57] |
| CKD | UUO mouse model; cisplatin-treated HK-2 cells; human CKD samples | HK-2 cells | ↑ | METTL3 | ↑ | enhance sterile inflammation and fibrosis | 2024 | [59] |
| CKD | CAR mouse model; macrophage; human fibrotic kidney specimens | Macrophage | ↑ | METTL3 | ↑ | drive MMT and renal fibrosis | 2025 | [58] |
| CKD | accelerated aged mouse model and accelerated aged HK-2 cells; 20 kidney tissues were collected from participants who were diagnosed with renal tumor | HK-2 cells | ↓ | METTL14/IGF2BP2 | ↓ | maintain the homeostasis of MQC | 2024 | [62] |
| CKD | UUO and I/R mouse model; TGF-β1-induced HK2 cells | / | FTO | / | inhibit autophagy and cause lipid metabolic disorders | 2023 | [61] | |
| CKD | UUO mouse model; TGF-β1-induced TECs | TECs | ↑ | FTO | ↑ | induce inflammation, apoptosis, EMT, and inhibition of autophagy | 2024 | [60] |
| FSGS | ADR and DN mouse models; ADR or AGE-treated human podocytes; renal biopsy samples included eight cases of DN, six FSGS, six minimal change disease (MCD) and four normal control tissues | ADR or AGE-treated human podocytes | ↑ | METTL14 | ↑ | inhibit autophagy and promote apoptosis and inflammation | 2021 | [63] |
| LN | MRL/lpr mice; Romas cells; PBMCs and kidney tissues from patients with LN | Romas cells | ↑ | METTL3 | ↑ | promote plasma cell infiltration and kidney damage | 2024 | [65] |
| HN | HUA mouse model; TCMK-1 cells | mouse kidney epithelial cell line (TCMK-1) | ↓ | METTL14 | ↓ | increase uric acid reabsorption and renal interstitial fibrosis | 2024 | [66] |
| ADPKD | ADPKD mouse models; Pkd1 mutant cells; kidney tissues from patients with ADPKD | Pkd1 mutant cells | ↑ | METTL3 | ↑ | promote cyst growth | 2021 | [67] |
| ccRCC | tumor xenograft models; human ccRCC cell lines (ACHN and 786-O); ccRCC tissue array | human ccRCC cell lines (ACHN and 786-O) | ↑ | METTL3 | ↑ | promote cell viability, migration, and invasion abilities | 2022 | [68] |
| ccRCC | orthotopic tumor xenograft model; human ccRCC cell lines (Caki-1, ACHN, 786-o, and 769-p); 39 paired RCC specimens and adjacent normal renal epithelial tissues | human RCC cell lines (Caki-1, ACHN, 786-o, and 769-p) | ↑ | METTL3 | ↑ | facilitate cell proliferation, migration, and invasion | 2024 | [69] |
| ccRCC | orthotopic tumor xenograft and PDX models; human ccRCC cell lines (OSRC-2, 786-O, Caki-1, 769-P, ACHN, and A498); 99 pairs of adjacent non-tumor and ccRCC tissue samples | human ccRCC cell lines (OSRC-2, 786-O, Caki-1, 769-P, ACHN, and A498) | ↑ | FTO/IGF2BP2 | ↓ | suppress autophagy and enhance ccRCC proliferation and metastasis | 2022 | [70] |
| ccRCC | orthotopic tumor xenograft and metastasis models; human RCC cell lines (ACHN, A498, Caki-1, OS‐RC‐2, 786‐O) | human RCC cell lines (ACHN, A498, Caki-1, OS‐RC‐2, and 786‐O) | ↑ | IGF2BP3 | ↑ | promote proliferation of RCC cells, pulmonary metastasis, | 2023 | [71] |
| ccRCC | tumor xenograft models; human ccRCC cell lines (786-O, 769‐P, CAKI1, CAKI2 and OSRC); paired RCC specimens and adjacent normal renal epithelial tissues | human RCC cell lines (786-O, 769‐P, CAKI1, CAKI2 and OSRC) | ↓ | IGF2BP2/YTHDF1 | ↓ | inhibit RCC cells proliferation and promotes cell apoptosis | 2022 | [72] |
Table 2.
Regulatory mechanisms and clinical potential of m6A methylation in kidney diseases
| Kidney disease | M6A regulators | Methylation levels | Target | Diagnostic implications | Intervention + Effects | Year | References |
|---|---|---|---|---|---|---|---|
| DN | METTL3/YTHDF2 | / | circ_0000953 | / | / | 2024 | [38] |
| DN | METTL3/IGF2BP2 | ↑ | TIMP2 | Podocyte METTL3 expression positively correlates with 24-h urinary microalbumin and creatinine levels, and negatively correlates with eGFR. | / | 2022 | [37] |
| DN | METTL3/IGF2BP2 | ↓ | RNF183 | / | / | 2025 | [39] |
| DN | METTL14 | ↓ | PTEN | / | / | 2021 | [40] |
| DN | WTAP/IGF2BP1 | ↑ | NLRP3 | WTAP levels are associated with clinical indicators such as eGFR, BUN, serum creatinine, and albuminuria. | WTAP knockdown suppresses pyroptosis and pro-inflammatory cytokine release, alleviating renal injury and fibrosis in diabetic mice. | 2022 | [41] |
| DN | FTO/YTHDF2 | ↑ | ACC1 | FTO expression is upregulated gradually with DN progression (stage I-III). | Inhibition of FTO with MA and DIA restores lipid homeostasis, alleviates glomerular injury, and improves renal function in db/db diabetic mice | 2025 | [42] |
| DN | FTO | ↑ | SAA2 | / | / | 2024 | [43] |
| DN | FTO | ↓ | Npas2 | FTO expression in macrophages negatively correlates with blood creatinine, urinary albumin, and positively with eGFR. | / | 2025 | [44] |
| DN | IGF2BP2 | / | SP1 | / | / | 2023 | [45] |
| CKD | METTL3 | ↑ | NET1 | METTL3 protein expression is ↑ in the tissues of CKD patients with diabetic or IgA nephropathy | METTL3 siRNA in vitro or the METTL3-specific inhibitor STM2457 attenuates the degree of kidney fibrosis. | 2024 | [55] |
| CKD | METTL3/IGF2BP3 | ↑ | β-catenin | METTL3 levels has a correlation with the extent of fibrotic lesions in CKD patients, serum creatinine, BUN, and eGFR. | Proximal tubule-specific ablation of METTL3 protects kidney against fibrosis. | 2024 | [56] |
| CKD | METTL3 | ↑ | EVL | / | Isoforsythiaside is identified as a direct METTL3 inhibitor that has a good effect in preventing renal fibrosis and kidney damage both in vitro and in vivo. | 2023 | [57] |
| CKD | METTL3 | ↑ | cGAS and STING1 | m6A modification levels has a close correlation with the fibrosis extent in CKD patients. | Both the tubule-specific deletion of METTL3 and the use of antisense oligonucleotides to inhibit METTL3 expression protect mice from sterile inflammation and fibrosis. | 2024 | [59] |
| CKD | METTL3 | ↑ | Smad3 | ↑ m6A methylation and METTL3 expression are associated with aggravated fibrosis. | / | 2025 | [58] |
| CKD | METTL14/IGF2BP2 | ↓ | lncRNA TUG1/PGC1-α | / | siMETTL14 or siIGF2BP2 in HK-2 cells exhibits lower mtDNA and ATP contents, along with abnormal mitochondrial morphology. | 2024 | [62] |
| CKD | FTO | / | SQSTM1 | / | Cana suppresses FTO, thereby increasing autophagy, restoring FAO and attenuating fibrosis. | 2023 | [61] |
| CKD | FTO | ↑ | RUNX1 | / | FTO deficiency attenuates renal fibrosis in UUO-induced mice. | 2024 | [60] |
| AKI | METTL3/IGF2BP2 | ↑ | Table 3 | / | Pharmacologic inhibition of METTL3 with cpd-564 exerts renoprotective effects in both cisplatin- and I/R-induced AKI models. | 2022 | [46] |
| AKI | METTL3/YTHDF1 | / | MDM2 | / | METTL3 knockdown suppresses mitochondrial damage and ferroptosis during AKI in CLP mice. | 2023 | [47] |
| AKI | METTL3 | ↑ | SREBP1c | / | / | 2025 | [49] |
| AKI | METTL3 | ↑ | mmu-lncRNA 121,686 and hsa-lncRNA 520,657 | / | PT-METTL3-KO or METTL3 siRNA suppresses ischemic, septic, and vancomycin-induced AKI. | 2022 | [48] |
| AKI | ZC3H13/IGF2BP1 | ↑ | NABP1 | / | ZC3H13 inhibition attenuates G2/M cell cycle arrest, apoptosis, and kidney injury in mice treated with cisplatin. | 2025 | [50] |
| AKI | FTO/IGF2BP2 | ↓ | SNHG14 | / | / | 2024 | [51] |
| AKI | ALKBH5 | ↓ initially at 12 h post-IRI, but sharply ↑ at 24 h and 48 h post-IRI, and then returned close to normal levels at 120 h post-IRI | CCL28 | / | Inhibition of ALKBH5 with IOX1 promotes Treg infiltration, thereby protecting against both AKI and chronic fibrosis post-IRI. | 2023 | [52] |
| AKI | IGF2BP1 | ↑ | E2F1/MIF | / | Inhibiting IGF2BP1 is a potential strategy to protect against renal septic injury. | 2023 | [53] |
| AKI | YTHDF1 | ↑ | SHPK1 | / | YTHDF1 mitigates acute renal tubular injury by protecting m6A-methylated mRNAs within SGs. | 2023 | [54] |
| FSGS | METTL14 | ↑ | Sirt1 | / | / | 2021 | [63] |
| LN | METTL3 | ↑ | IRF4 | / | / | 2024 | [65] |
| HN | METTL14 | ↓ | GLUT9 | / | Benzbromarone treatment restores m6A methylation levels and METTL14 expression and alleviates renal injury. | 2024 | [66] |
| ADPKD | METTL3 | ↑ | c-Myc and Avpr2 | / | Genetic deletion of METTL3 attenuates cyst growth across multiple ADPKD models. | 2021 | [67] |
| ccRCC | METTL3 | ↑ | HHLA2 | METTL3 serves as an important prognostic predictor for ccRCC patients. | / | 2022 | [68] |
| ccRCC | METTL3 | ↑ | PLOD2 | Upregulated METTL3 predicts poor survival in RCC patients. | Selective METTL3 inhibitor STM2457 shows anti-tumor effects. | 2024 | [69] |
| ccRCC | FTO/IGF2BP2 | ↑ | SIK2 | High FTO expression correlates with poorer 10-year overall survival and serves as an independent prognostic factor for patient outcomes. | FB23-2 targeting FTO inhibits tumor growth and prolongs survival in the PDX model mice. | 2022 | [70] |
| ccRCC | IGF2BP3 | ↑ | circRARS | IGF2BP3 is a biomarker for prognosis and diagnosis of RCC and its mRNA level is associated with clinical stage and pathological grade | IGF2BP3 knockdown attenuates the RCC cancer stemness and inhibits orthotopic xenografts growth and diminishes pulmonary metastasis. | 2023 | [71] |
| ccRCC | IGF2BP2/YTHDF1 | ↓ | ZNF677 | / | / | 2022 | [72] |
Acknowledgements
Not applicable.
Abbreviations
- m⁶A
N⁶-methyladenosine
- mRNA
messenger RNA
- lncRNA
Long noncoding RNA
- DN
Diabetic nephropathy
- AKI
Acute kidney injury
- CKD
Chronic kidney disease
- FSGS
Focal segmental glomerulosclerosis
- LN
Lupus nephritis
- HN
Hyperuricemic nephropathy
- ADPKD
Autosomal dominant polycystic kidney disease
- ccRCC
Clear cell renal cell carcinoma
- METTL3
Methyltransferase-like 3
- FTO
Fat mass and obesity-associated protein
- ALKBH5
alkB homolog 5
- MeRIP-seq
Methylated RNA immunoprecipitation sequencing
- CLIP-seq
Crosslinking immunoprecipitation sequencing
- ESRD
End-stage renal disease
- EMT
Epithelial-mesenchymal transition
- eGFR
Estimated glomerular filtration rate
- HG
High-glucose
- HIF-1α
Hypoxia-inducible factor-1α
- ceRNAs
Competing endogenous RNAs
- Treg
Regulatory T cell
- SGs
Stress granules
- UUO
Unilateral ureteral obstruction
- CAR
Chronic active renal allograft rejection
- MMT
Macrophage-to-myofibroblast transition
- KO
Knockout
- cGAS
Cyclic guanosine monophosphate-AMP synthase
- PBMCs
Peripheral blood mononuclear cells
- SLE
Systemic lupus erythematosus
Author contributions
Conceptualization, Qinfan Yao, Dajin Chen; Investigation and Writing Original Draft, Qinfan Yao, Yitong Chen, Xinyi Zhang; Writing- Review & Editing, Lefeng Wang, Jingyi Li; Visualization, Junhao Lv. Funding Acquisition, Dajin Chen; Supervision, Jianghua Chen and Dajin Chen.
Funding
This research was funded by the National Natural Science Foundation of China (82360157 and U21A20350), the Key Research and Development Program of Science and Technology Department of Zhejiang Province (2023C04011), the Key Research and Development Project of Xinjiang Production and Construction Corps Science and Technology Bureau (2023AB018-03), and Scientific Research Fund of Zhejiang Provincial Education Department (Y202147593).
Data availability
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
All the authors have read and approved the final manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Jianghua Chen, Email: chenjianghua@zju.edu.cn.
Dajin Chen, Email: zju2001@zju.edu.cn.
References
- 1.Li B, Qu L, Yang J, RNA-Guided RNA, Modifications. Biogenesis, functions, and applications. Acc Chem Res. 2023;56:3198–210. 10.1021/acs.accounts.3c00474. [DOI] [PubMed] [Google Scholar]
- 2.Frye M, Jaffrey SR, Pan T, Rechavi G, Suzuki T. RNA modifications: what have we learned and where are we headed? Nat Rev Genet. 2016;17:365–72. 10.1038/nrg.2016.47. [DOI] [PubMed] [Google Scholar]
- 3.Zhao LY, Song J, Liu Y, Song CX, Yi C. Mapping the epigenetic modifications of DNA and RNA. Protein Cell. 2020;11:792–808. 10.1007/s13238-020-00733-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roundtree IA, Evans ME, Pan T, He C. Dynamic RNA modifications in gene expression regulation. Cell. 2017;169:1187–200. 10.1016/j.cell.2017.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen D, Gu X, Nurzat Y, Xu L, Li X, Wu L, et al. Writers, readers, and erasers RNA modifications and drug resistance in cancer. Mol Cancer. 2024;23:178. 10.1186/s12943-024-02089-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang W, Zhao Y, Yang Y. Dynamic RNA methylation modifications and their regulatory role in mammalian development and diseases. Sci China Life Sci. 2024;67:2084–104. 10.1007/s11427-023-2526-2. [DOI] [PubMed] [Google Scholar]
- 7.Dimitrakov D. Electrocardiographic changes in patients with chronic renal failure. Folia Med (Plovdiv). 1985;27:16–21. [PubMed] [Google Scholar]
- 8.Delaunay S, Helm M, Frye M. RNA modifications in physiology and disease: towards clinical applications. Nat Rev Genet. 2024;25:104–22. 10.1038/s41576-023-00645-2. [DOI] [PubMed] [Google Scholar]
- 9.Zhang Y, Lu L, Li X. Detection technologies for RNA modifications. Exp Mol Med. 2022;54:1601–16. 10.1038/s12276-022-00821-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang G, Ding Q, Xie D, Cai Z, Zhao Z. Technical challenges in defining RNA modifications. Semin Cell Dev Biol. 2022;127:155–65. 10.1016/j.semcdb.2021.11.009. [DOI] [PubMed] [Google Scholar]
- 11.Shi H, Wei J, He C. Where, when, and how: Context-Dependent functions of RNA methylation writers, readers, and erasers. Mol Cell. 2019;74:640–50. 10.1016/j.molcel.2019.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Song J, Yi C. Chemical modifications to RNA: A new layer of gene expression regulation. ACS Chem Biol. 2017;12:316–25. 10.1021/acschembio.6b00960. [DOI] [PubMed] [Google Scholar]
- 13.Barbieri I, Kouzarides T. Role of RNA modifications in cancer. Nat Rev Cancer. 2020;20:303–22. 10.1038/s41568-020-0253-2. [DOI] [PubMed] [Google Scholar]
- 14.Nian Z, Deng M, Ye L, Tong X, Xu Y, Xu Y, et al. RNA epigenetic modifications in digestive tract cancers: friends or foes. Pharmacol Res. 2024;206:107280. 10.1016/j.phrs.2024.107280. [DOI] [PubMed] [Google Scholar]
- 15.Wang C, Hou X, Guan Q, Zhou H, Zhou L, Liu L, et al. RNA modification in cardiovascular disease: implications for therapeutic interventions. Signal Transduct Target Ther. 2023;8:412. 10.1038/s41392-023-01638-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eknoyan G, Lameire N, Barsoum R, Eckardt KU, Levin A, Levin N, et al. The burden of kidney disease: improving global outcomes. Kidney Int. 2004;66:1310–4. 10.1111/j.1523-1755.2004.00894.x. [DOI] [PubMed] [Google Scholar]
- 17.Bao YW, Yuan Y, Chen JH, Lin WQ. Kidney disease models: tools to identify mechanisms and potential therapeutic targets. Zool Res. 2018;39:72–86. 10.24272/j.issn.2095-8137.2017.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stenvinkel P, Shiels PG, Painer J, Miranda JJ, Natterson-Horowitz B, Johnson RJ. A planetary health perspective for kidney disease. Kidney Int. 2020;98:261–5. 10.1016/j.kint.2020.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pollak MR, Friedman DJ. The genetic architecture of kidney disease. Clin J Am Soc Nephrol. 2020;15:268–75. 10.2215/cjn.09340819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nair D, Liu CK, Raslan R, McAdams-DeMarco M, Hall RK. Frailty in kidney disease: A comprehensive review to advance its clinical and research applications. Am J Kidney Dis. 2025;85:89–103. 10.1053/j.ajkd.2024.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Câmara NO, Iseki K, Kramer H, Liu ZH, Sharma K. Kidney disease and obesity: epidemiology, mechanisms and treatment. Nat Rev Nephrol. 2017;13:181–90. 10.1038/nrneph.2016.191. [DOI] [PubMed] [Google Scholar]
- 22.Levey AS, Coresh J. Chronic kidney disease. Lancet. 2012;379:165–80. 10.1016/s0140-6736(11)60178-5. [DOI] [PubMed] [Google Scholar]
- 23.Kidney disease. A global health priority. Nat Rev Nephrol. 2024;20:421–3. 10.1038/s41581-024-00829-x. [DOI] [PubMed] [Google Scholar]
- 24.Schaub JA, Hamidi H, Subramanian L, Kretzler M. Systems biology and kidney disease. Clin J Am Soc Nephrol. 2020;15:695–703. 10.2215/cjn.09990819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nogrady B. The genetic revolution transforming kidney disease. Nature. 2023;615:S14–5. 10.1038/d41586-023-00654-5. [DOI] [PubMed] [Google Scholar]
- 26.Song B, Zeng Y, Cao Y, Zhang J, Xu C, Pan Y, et al. Emerging role of METTL3 in inflammatory diseases: mechanisms and therapeutic applications. Front Immunol. 2023;14:1221609. 10.3389/fimmu.2023.1221609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang J, Yang F, Liu Y, Wang Y. N6-methyladenosine RNA methylation in diabetic kidney disease. Biomed Pharmacother. 2024;171:116185. 10.1016/j.biopha.2024.116185. [DOI] [PubMed] [Google Scholar]
- 28.Wang Q, Fan X, Sheng Q, Yang M, Zhou P, Lu S, et al. N6-methyladenosine methylation in kidney injury. Clin Epigenetics. 2023;15:170. 10.1186/s13148-023-01586-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ni WJ, Lu H, Ma NN, Hou BB, Zeng J, Zhou H, et al. RNA N(6) -methyladenosine modifications and potential targeted therapeutic strategies in kidney disease. Br J Pharmacol. 2023;180:5–24. 10.1111/bph.15968. [DOI] [PubMed] [Google Scholar]
- 30.Lee H, Zhuang L, Gan B. VHL governs m6A modification and PIK3R3 mRNA stability in clear cell renal cell carcinomas. J Clin Invest. 2024;134. 10.1172/jci179560. [DOI] [PMC free article] [PubMed]
- 31.Qi S, Song J, Chen L, Weng H. The role of N-methyladenosine modification in acute and chronic kidney diseases. Mol Med. 2023;29:166. 10.1186/s10020-023-00764-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ye W, Lv X, Gao S, Li Y, Luan J, Wang S. Emerging role of m6A modification in fibrotic diseases and its potential therapeutic effect. Biochem Pharmacol. 2023;218:115873. 10.1016/j.bcp.2023.115873. [DOI] [PubMed] [Google Scholar]
- 33.Zhong J, Liu Z, Cai C, Duan X, Deng T, Zeng G. m(6)A modification patterns and tumor immune landscape in clear cell renal carcinoma. J Immunother Cancer. 2021;9. 10.1136/jitc-2020-001646. [DOI] [PMC free article] [PubMed]
- 34.Li J, Liu D, Ren J, Li G, Zhao Z, Zhao H, et al. Integrated analysis of RNA methylation regulators crosstalk and immune infiltration for predictive and personalized therapy of diabetic nephropathy. Hum Genomics. 2023;17:6. 10.1186/s40246-023-00457-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barutta F, Bellini S, Kimura S, Hase K, Corbetta B, Corbelli A, et al. Protective effect of the tunneling nanotube-TNFAIP2/M-sec system on podocyte autophagy in diabetic nephropathy. Autophagy. 2023;19:505–24. 10.1080/15548627.2022.2080382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jin J, Shi Y, Gong J, Zhao L, Li Y, He Q, et al. Exosome secreted from adipose-derived stem cells attenuates diabetic nephropathy by promoting autophagy flux and inhibiting apoptosis in podocyte. Stem Cell Res Ther. 2019;10:95. 10.1186/s13287-019-1177-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jiang L, Liu X, Hu X, Gao L, Zeng H, Wang X, et al. METTL3-mediated m(6)A modification of TIMP2 mRNA promotes podocyte injury in diabetic nephropathy. Mol Ther. 2022;30:1721–40. 10.1016/j.ymthe.2022.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu X, Jiang L, Zeng H, Gao L, Guo S, Chen C, et al. Circ-0000953 deficiency exacerbates podocyte injury and autophagy disorder by targeting Mir665-3p-Atg4b in diabetic nephropathy. Autophagy. 2024;20:1072–97. 10.1080/15548627.2023.2286128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guo D, Pang Y, Wang W, Feng Y, Wang L, Sun Y, et al. Modification of RNF183 via m6A methylation mediates podocyte dysfunction in diabetic nephropathy by regulating PKM2 ubiquitination and degradation. Cells. 2025;14. 10.3390/cells14050365. [DOI] [PMC free article] [PubMed]
- 40.Xu Z, Jia K, Wang H, Gao F, Zhao S, Li F, et al. METTL14-regulated PI3K/Akt signaling pathway via PTEN affects HDAC5-mediated epithelial-mesenchymal transition of renal tubular cells in diabetic kidney disease. Cell Death Dis. 2021;12:32. 10.1038/s41419-020-03312-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lan J, Xu B, Shi X, Pan Q, Tao Q. WTAP-mediated N(6)-methyladenosine modification of NLRP3 mRNA in kidney injury of diabetic nephropathy. Cell Mol Biol Lett. 2022;27:51. 10.1186/s11658-022-00350-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chang K, Hong F, Liu H, Fang Y, Wang H, Song N, et al. FTO aggravates podocyte injury and diabetic nephropathy progression via m6A-dependent stabilization of ACC1 mRNA and promoting fatty acid metabolism. Biochem Pharmacol. 2025;235:116819. 10.1016/j.bcp.2025.116819. [DOI] [PubMed] [Google Scholar]
- 43.Lang Y, Wang Q, Sheng Q, Lu S, Yang M, Kong Z, et al. FTO-mediated m6A modification of serum amyloid A2 mRNA promotes podocyte injury and inflammation by activating the NF-κB signaling pathway. Faseb J. 2024;38:e23409. 10.1096/fj.202301419RR. [DOI] [PubMed] [Google Scholar]
- 44.Zhu S, Jiang L, Liu X, Chen C, Luo X, Jiang S, et al. m6A demethylase Fto inhibited macrophage activation and Glycolysis in diabetic nephropathy via m6A/Npas2/Hif-1α axis. Faseb J. 2025;39:e70332. 10.1096/fj.202403014R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lin Z, Lv D, Liao X, Peng R, Liu H, Wu T, et al. CircUBXN7 promotes macrophage infiltration and renal fibrosis associated with the IGF2BP2-dependent SP1 mRNA stability in diabetic kidney disease. Front Immunol. 2023;14:1226962. 10.3389/fimmu.2023.1226962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang JN, Wang F, Ke J, Li Z, Xu CH, Yang Q, et al. Inhibition of METTL3 attenuates renal injury and inflammation by alleviating table 3 m6A modifications via IGF2BP2-dependent mechanisms. Sci Transl Med. 2022;14:eabk2709. 10.1126/scitranslmed.abk2709. [DOI] [PubMed] [Google Scholar]
- 47.Hu C, Zhang B, Zhao S. METTL3-mediated N6-methyladenosine modification stimulates mitochondrial damage and ferroptosis of kidney tubular epithelial cells following acute kidney injury by modulating the stabilization of MDM2-p53-LMNB1 axis. Eur J Med Chem. 2023;259:115677. 10.1016/j.ejmech.2023.115677. [DOI] [PubMed] [Google Scholar]
- 48.Pan J, Xie Y, Li H, Li X, Chen J, Liu X, et al. mmu-lncRNA 121686/hsa-lncRNA 520657 induced by METTL3 drive the progression of AKI by targeting miR-328-5p/HtrA3 signaling axis. Mol Ther. 2022;30:3694–713. 10.1016/j.ymthe.2022.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xin W, Zhou J, Peng Y, Gong S, Liao W, Wang Y, et al. SREBP1c-Mediated transcriptional repression of YME1L1 contributes to acute kidney injury by inducing mitochondrial dysfunction in tubular epithelial cells. Adv Sci (Weinh). 2025;12:e2412233. 10.1002/advs.202412233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sheng Q, Yu Q, Lu S, Yang M, Fan X, Su H, et al. The Inhibition of ZC3H13 attenuates G2/M arrest and apoptosis by alleviating NABP1 m6A modification in cisplatin-induced acute kidney injury. Cell Mol Life Sci. 2025;82:86. 10.1007/s00018-025-05596-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yang N, Yan N, Bai Z, Du S, Zhang J, Zhang L, et al. FTO attenuates LPS-induced acute kidney injury by inhibiting autophagy via regulating SNHG14/miR-373-3p/ATG7 axis. Int Immunopharmacol. 2024;128:111483. 10.1016/j.intimp.2023.111483. [DOI] [PubMed] [Google Scholar]
- 52.Chen J, Xu C, Yang K, Gao R, Cao Y, Liang L, et al. Inhibition of ALKBH5 attenuates I/R-induced renal injury in male mice by promoting Ccl28 m6A modification and increasing Treg recruitment. Nat Commun. 2023;14:1161. 10.1038/s41467-023-36747-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mao Y, Jiang F, Xu XJ, Zhou LB, Jin R, Zhuang LL, et al. Inhibition of IGF2BP1 attenuates renal injury and inflammation by alleviating m6A modifications and E2F1/MIF pathway. Int J Biol Sci. 2023;19:593–609. 10.7150/ijbs.78348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yang W, Zhang M, Li J, Qu S, Zhou F, Liu M, et al. YTHDF1 mitigates acute kidney injury via safeguarding m(6)A-methylated mRNAs in stress granules of renal tubules. Redox Biol. 2023;67:102921. 10.1016/j.redox.2023.102921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jung HR, Lee J, Hong SP, Shin N, Cho A, Shin DJ, et al. Targeting the m(6)A RNA methyltransferase METTL3 attenuates the development of kidney fibrosis. Exp Mol Med. 2024;56:355–69. 10.1038/s12276-024-01159-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Long Y, Song D, Xiao L, Xiang Y, Li D, Sun X, et al. m(6)A RNA methylation drives kidney fibrosis by upregulating β-catenin signaling. Int J Biol Sci. 2024;20:3185–200. 10.7150/ijbs.96233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ni WJ, Zhou H, Lu H, Ma NN, Hou BB, Li W, et al. Genetic and Pharmacological Inhibition of METTL3 alleviates renal fibrosis by reducing EVL m6A modification through an IGF2BP2-dependent mechanism. Clin Transl Med. 2023;13:e1359. 10.1002/ctm2.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yao Q, Zheng X, Zhang X, Wang Y, Zhou Q, Lv J, et al. METTL3 potentiates M2 Macrophage-Driven MMT to aggravate renal allograft fibrosis via the TGF-β1/Smad3 pathway. Adv Sci (Weinh). 2025;12:e2412123. 10.1002/advs.202412123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tsai YC, Hsieh TH, Liao YR, Tsai MT, Lin TP, Lee DY, et al. METTL3-Mediated N 6 -Methyladenosine mRNA modification and cGAS-STING pathway activity in kidney fibrosis. J Am Soc Nephrol. 2024;35:1312–29. 10.1681/asn.0000000000000428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang DX, Bao SY, Song NN, Chen WZ, Ding XQ, Walker RJ, et al. FTO-mediated m6A mRNA demethylation aggravates renal fibrosis by targeting RUNX1 and further enhancing PI3K/AKT pathway. Faseb J. 2024;38:e23436. 10.1096/fj.202302041R. [DOI] [PubMed] [Google Scholar]
- 61.Yang Y, Li Q, Ling Y, Leng L, Ma Y, Xue L, et al. m6A eraser FTO modulates autophagy by targeting SQSTM1/P62 in the prevention of Canagliflozin against renal fibrosis. Front Immunol. 2022;13:1094556. 10.3389/fimmu.2022.1094556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhu Y, Yang B, Chen S, Chen G, Zeng X, Min H, et al. M6A RNA Methylation-Mediated TUG1 stability maintains mitochondrial homeostasis during kidney aging by epigenetically regulating PGC1-α expression. Antioxid Redox Signal. 2024;41:993–1013. 10.1089/ars.2024.0631. [DOI] [PubMed] [Google Scholar]
- 63.Lu Z, Liu H, Song N, Liang Y, Zhu J, Chen J, et al. METTL14 aggravates podocyte injury and glomerulopathy progression through N(6)-methyladenosine-dependent downregulating of Sirt1. Cell Death Dis. 2021;12:881. 10.1038/s41419-021-04156-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhao H, Pan S, Duan J, Liu F, Li G, Liu D, et al. Integrative analysis of m(6)A Regulator-Mediated RNA methylation modification patterns and immune characteristics in lupus nephritis. Front Cell Dev Biol. 2021;9:724837. 10.3389/fcell.2021.724837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liu Y, Wang X, Huang M, Luo A, Liu S, Cai M, et al. METTL3 facilitates kidney injury through promoting IRF4-mediated plasma cell infiltration via an m6A-dependent manner in systemic lupus erythematosus. BMC Med. 2024;22:511. 10.1186/s12916-024-03735-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yang J, Jiang T, Lu X, Li X, Zhou X, Guo X, et al. METTL14 downregulates GLUT9 through m6A methylation and attenuates hyperuricemia-induced fibrosis in mouse renal tubular epithelial cells. Int Immunopharmacol. 2024;143:113308. 10.1016/j.intimp.2024.113308. [DOI] [PubMed] [Google Scholar]
- 67.Ramalingam H, Kashyap S, Cobo-Stark P, Flaten A, Chang CM, Hajarnis S, et al. A methionine-METTL3-N(6)-methyladenosine axis promotes polycystic kidney disease. Cell Metab. 2021;33:1234–47..e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhu D, Liu Y, Chen J, Wang Q, Li Y, Zhu Y, et al. The methyltransferase METTL3 promotes tumorigenesis via mediating HHLA2 mRNA m6A modification in human renal cell carcinoma. J Transl Med. 2022;20:298. 10.1186/s12967-022-03496-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chen Y, He Y, Li Z, Zhang N, Zhou C, He X, et al. METTL3 facilitates renal cell carcinoma progression by PLOD2 m(6)A-methylation under prolonged hypoxia. Cell Death Dis. 2024;15:62. 10.1038/s41419-023-06411-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Xu Y, Zhou J, Li L, Yang W, Zhang Z, Zhang K, et al. FTO-mediated autophagy promotes progression of clear cell renal cell carcinoma via regulating SIK2 mRNA stability. Int J Biol Sci. 2022;18:5943–62. 10.7150/ijbs.77774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liu Y, Chen K, Shou Y, Li S, Wang J, Zhang Q, et al. CircRARS synergises with IGF2BP3 to regulate RNA methylation recognition to promote tumour progression in renal cell carcinoma. Clin Transl Med. 2023;13:e1512. 10.1002/ctm2.1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Li A, Cao C, Gan Y, Wang X, Wu T, Zhang Q, et al. ZNF677 suppresses renal cell carcinoma progression through N6-methyladenosine and transcriptional repression of CDKN3. Clin Transl Med. 2022;12:e906. 10.1002/ctm2.906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Xue T, Qiu X, Liu H, Gan C, Tan Z, Xie Y, et al. Epigenetic regulation in fibrosis progress. Pharmacol Res. 2021;173:105910. 10.1016/j.phrs.2021.105910. [DOI] [PubMed] [Google Scholar]
- 74.Sakashita M, Inagi R. mRNA modification and mechanisms of kidney fibrosis. J Am Soc Nephrol. 2024;35:1297–9. 10.1681/asn.0000000000000474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Alhammadi MA, Bajbouj K, Talaat IM, Hamoudi R. The role of RNA-modifying proteins in renal cell carcinoma. Cell Death Dis. 2024;15:227. 10.1038/s41419-024-06479-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Spåhr H, Chia T, Lingford JP, Siira SJ, Cohen SB, Filipovska A, et al. Modular SsDNA binding and Inhibition of telomerase activity by designer PPR proteins. Nat Commun. 2018;9:2212. 10.1038/s41467-018-04388-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Huang Y, Xue Q, Chang J, Wang Y, Cheng C, Xu S, et al. M6A methylation modification in autoimmune diseases, a promising treatment strategy based on epigenetics. Arthritis Res Ther. 2023;25:189. 10.1186/s13075-023-03149-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Du W, Huang Y, Chen X, Deng Y, Sun Y, Yang H et al. Discovery of a PROTAC degrader for METTL3-METTL14 complex. Cell Chem Biol. 2024;31:177– 83.e17. 10.1016/j.chembiol.2023.12.009 [DOI] [PubMed]
- 79.Rauch S, Jones KA, Dickinson BC. Small Molecule-Inducible RNA-Targeting systems for Temporal control of RNA regulation. ACS Cent Sci. 2020;6:1987–96. 10.1021/acscentsci.0c00537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mo J, Weng X, Zhou X, Detection. Clinical application, and manipulation of RNA modifications. Acc Chem Res. 2023;56:2788–800. 10.1021/acs.accounts.3c00395. [DOI] [PubMed] [Google Scholar]
- 81.Chen Q, Zhang Y, Yin H. Recent advances in chemical modifications of guide RNA, mRNA and donor template for CRISPR-mediated genome editing. Adv Drug Deliv Rev. 2021;168:246–58. 10.1016/j.addr.2020.10.014. [DOI] [PubMed] [Google Scholar]
- 82.Vader P, Mol EA, Pasterkamp G, Schiffelers RM. Extracellular vesicles for drug delivery. Adv Drug Deliv Rev. 2016;106:148–56. 10.1016/j.addr.2016.02.006. [DOI] [PubMed] [Google Scholar]
- 83.Srinivasarao M, Low PS. Ligand-Targeted drug delivery. Chem Rev. 2017;117:12133–64. 10.1021/acs.chemrev.7b00013. [DOI] [PubMed] [Google Scholar]
- 84.Huang X, Ma Y, Li Y, Han F, Lin W. Targeted drug delivery systems for kidney diseases. Front Bioeng Biotechnol. 2021;9:683247. 10.3389/fbioe.2021.683247. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.