Abstract
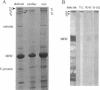
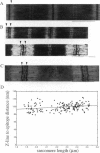
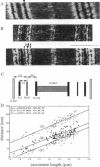
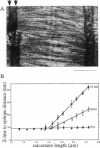
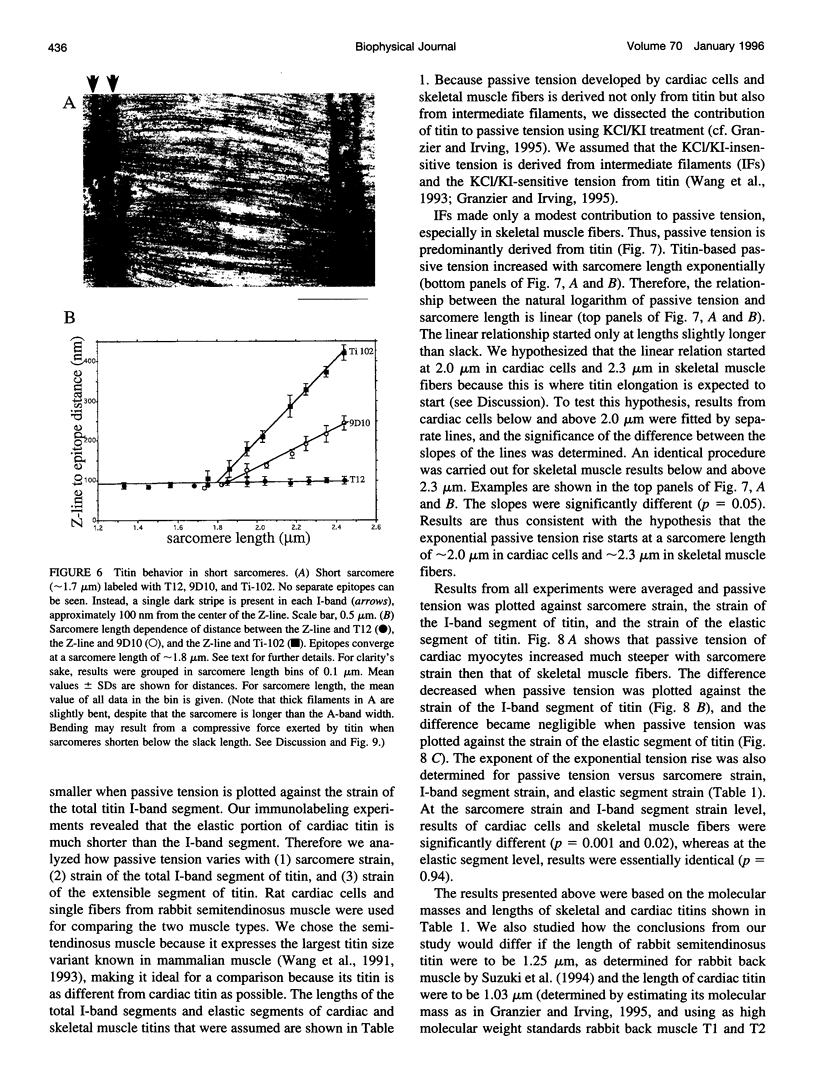
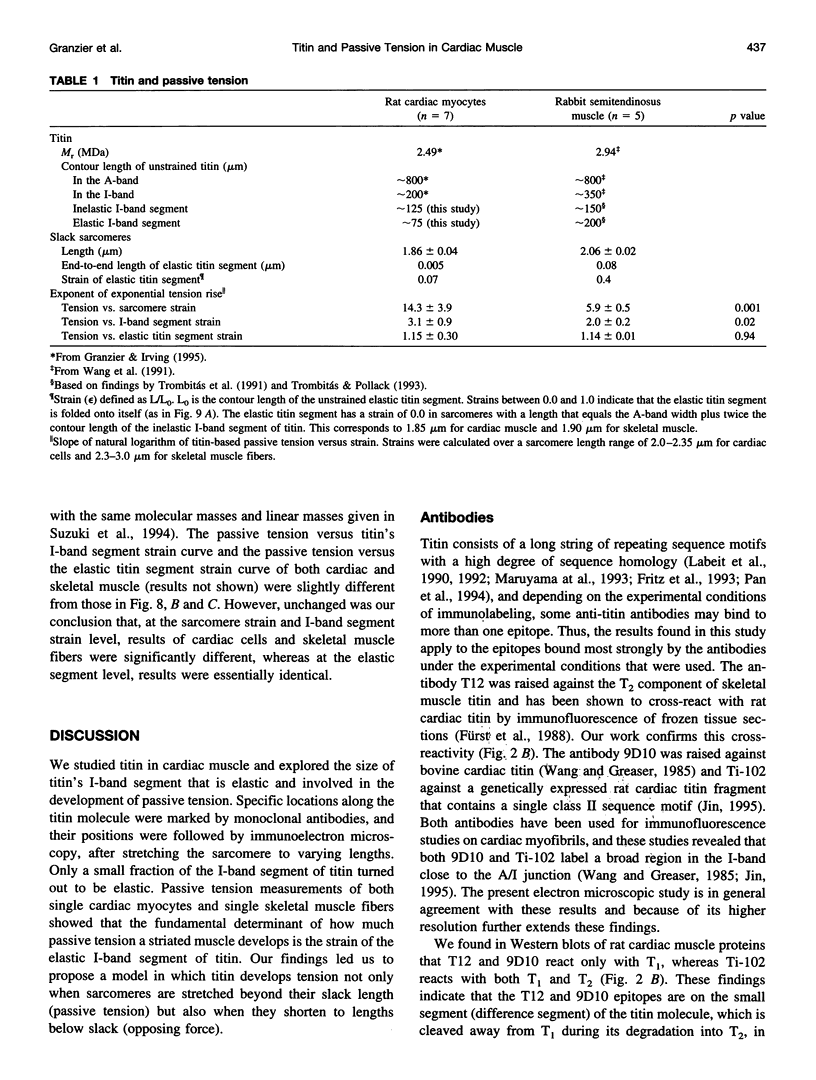
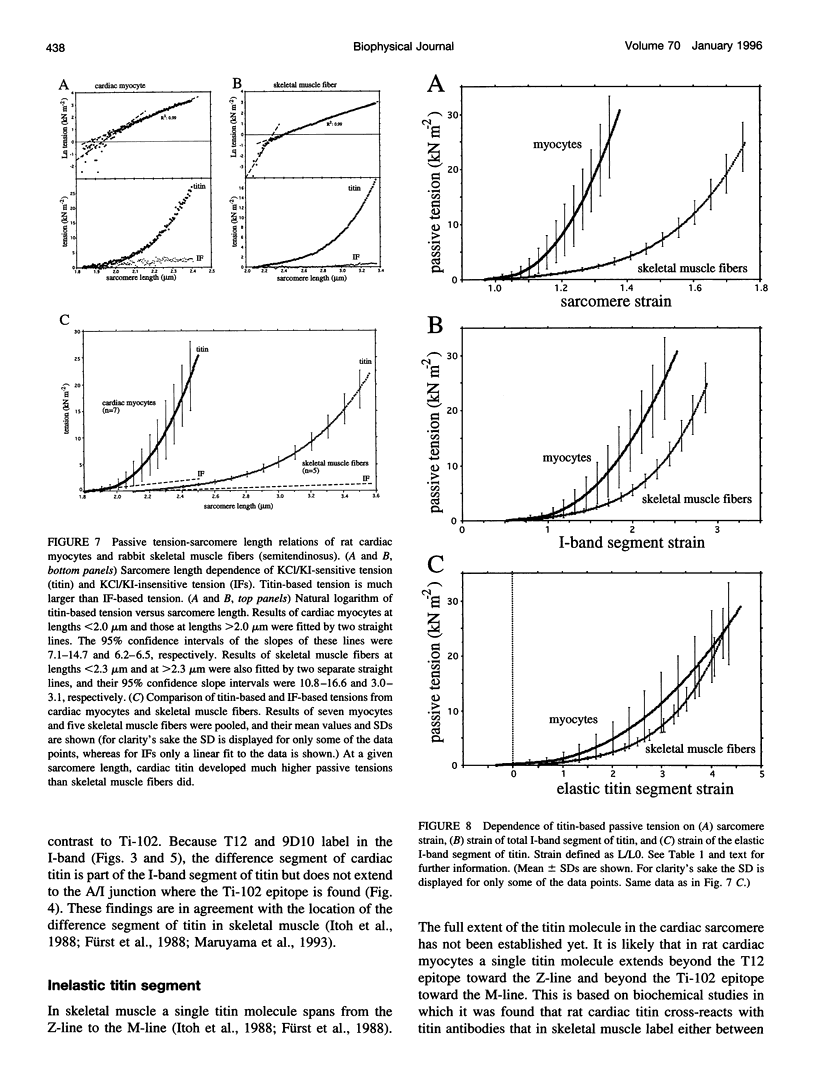
Titin (also known as connectin) is a muscle-specific giant protein found inside the sarcomere, spanning from the Z-line to the M-line. The I-band segment of titin is considered to function as a molecular spring that develops tension when sarcomeres are stretched (passive tension). Recent studies on skeletal muscle indicate that it is not the entire I-band segment of titin that behaves as a spring; some sections are inelastic and do not take part in the development of passive tension. To better understand the mechanism of passive tension development in the heart, where passive tension plays an essential role in the pumping function, we investigated titin's elastic segment in cardiac myocytes using structural and mechanical techniques. Single cardiac myocytes were stretched by various amounts and then immunolabeled and processed for electron microscopy in the stretched state. Monoclonal antibodies that recognize different titin epitopes were used, and the locations of the titin epitopes in the sarcomere were studied as a function of sarcomere length. We found that only a small region of the I-band segment of titin is elastic; its contour length is estimated at approximately 75 nm, which is only approximately 40% of the total I-band segment of titin. Passive tension measurements indicated that the fundamental determinant of how much passive tension the heart develops is the strain of titin's elastic segment. Furthermore, we found evidence that in sarcomeres that are slack (length, approximately 1.85 microns) the elastic titin segment is highly folded on top of itself. Based on the data, we propose a two-stage mechanism of passive tension development in the heart, in which, between sarcomere lengths of approximately 1.85 microns and approximately 2.0 microns, titin's elastic segment straightens and, at lengths longer than approximately 2.0 microns, the molecular domains that make up titin's elastic segment unravel. Sarcomere shortening to lengths below slack (approximately 1.85 microns) also results in straightening of the elastic titin segment, giving rise to a force that opposes shortening and that tends to bring sarcomeres back to their slack length.
Full text
PDF

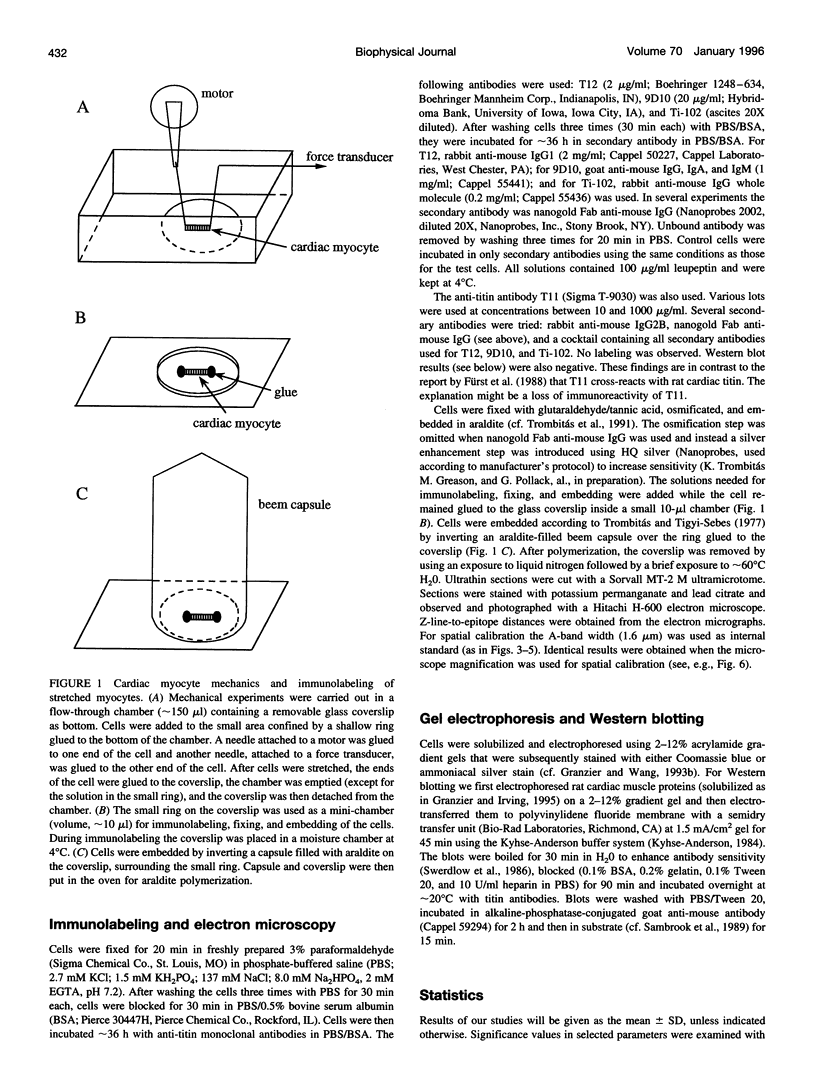
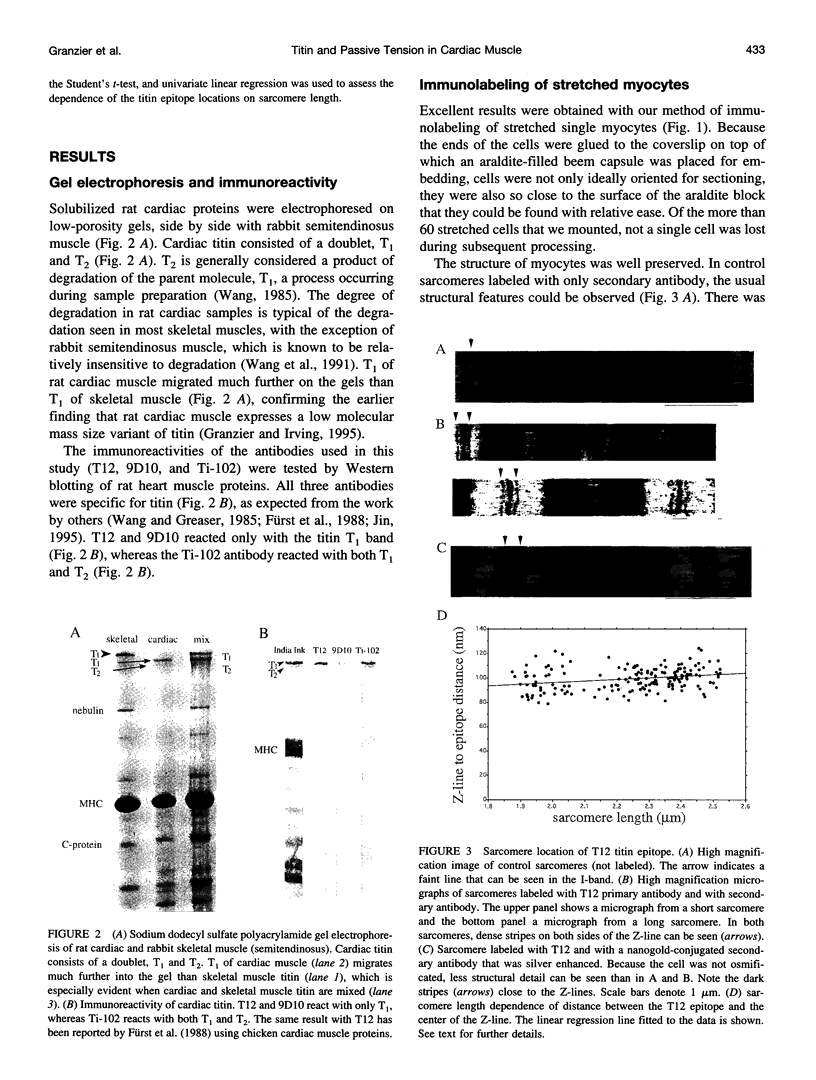
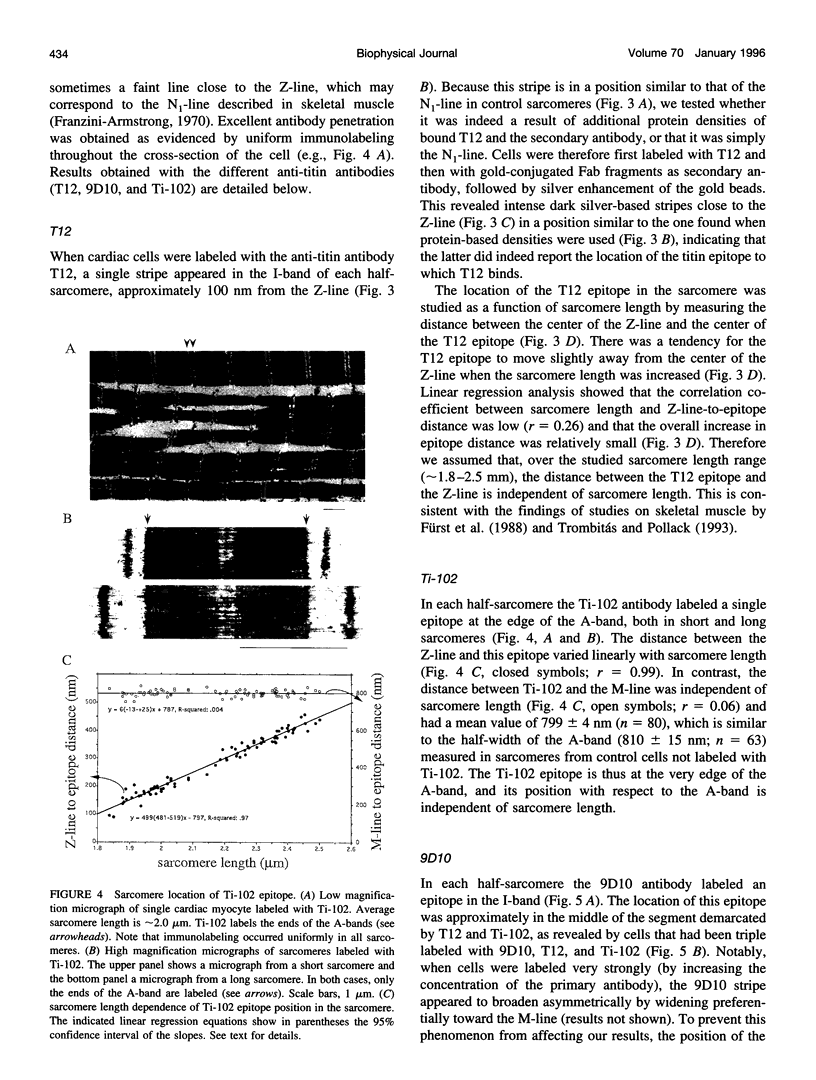
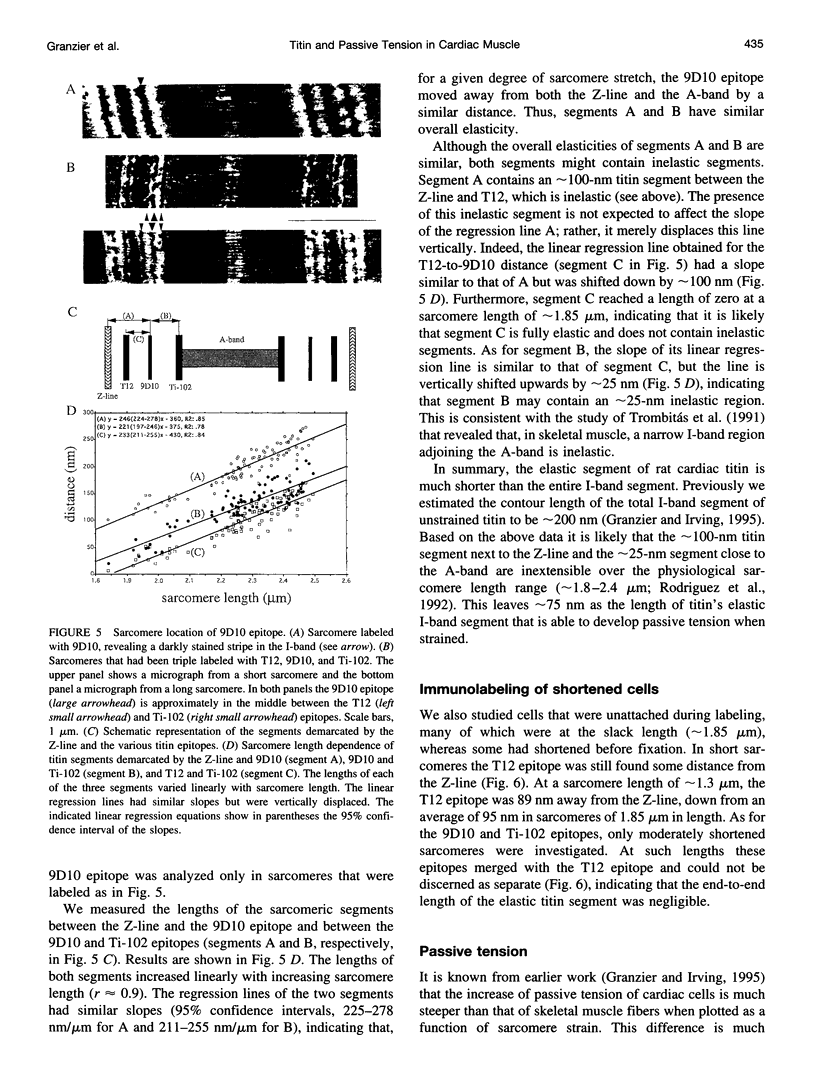




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brady A. J. Mechanical properties of isolated cardiac myocytes. Physiol Rev. 1991 Apr;71(2):413–428. doi: 10.1152/physrev.1991.71.2.413. [DOI] [PubMed] [Google Scholar]
- Erickson H. P. Reversible unfolding of fibronectin type III and immunoglobulin domains provides the structural basis for stretch and elasticity of titin and fibronectin. Proc Natl Acad Sci U S A. 1994 Oct 11;91(21):10114–10118. doi: 10.1073/pnas.91.21.10114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritz J. D., Wolff J. A., Greaser M. L. Characterization of a partial cDNA clone encoding porcine skeletal muscle titin: comparison with rabbit and mouse skeletal muscle titin sequences. Comp Biochem Physiol B. 1993 Jun;105(2):357–360. doi: 10.1016/0305-0491(93)90241-v. [DOI] [PubMed] [Google Scholar]
- Fulton A. B., Isaacs W. B. Titin, a huge, elastic sarcomeric protein with a probable role in morphogenesis. Bioessays. 1991 Apr;13(4):157–161. doi: 10.1002/bies.950130403. [DOI] [PubMed] [Google Scholar]
- Funatsu T., Kono E., Higuchi H., Kimura S., Ishiwata S., Yoshioka T., Maruyama K., Tsukita S. Elastic filaments in situ in cardiac muscle: deep-etch replica analysis in combination with selective removal of actin and myosin filaments. J Cell Biol. 1993 Feb;120(3):711–724. doi: 10.1083/jcb.120.3.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fürst D. O., Osborn M., Nave R., Weber K. The organization of titin filaments in the half-sarcomere revealed by monoclonal antibodies in immunoelectron microscopy: a map of ten nonrepetitive epitopes starting at the Z line extends close to the M line. J Cell Biol. 1988 May;106(5):1563–1572. doi: 10.1083/jcb.106.5.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granzier H. L., Irving T. C. Passive tension in cardiac muscle: contribution of collagen, titin, microtubules, and intermediate filaments. Biophys J. 1995 Mar;68(3):1027–1044. doi: 10.1016/S0006-3495(95)80278-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granzier H. L., Wang K. Gel electrophoresis of giant proteins: solubilization and silver-staining of titin and nebulin from single muscle fiber segments. Electrophoresis. 1993 Jan-Feb;14(1-2):56–64. doi: 10.1002/elps.1150140110. [DOI] [PubMed] [Google Scholar]
- Granzier H. L., Wang K. Interplay between passive tension and strong and weak binding cross-bridges in insect indirect flight muscle. A functional dissection by gelsolin-mediated thin filament removal. J Gen Physiol. 1993 Feb;101(2):235–270. doi: 10.1085/jgp.101.2.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granzier H. L., Wang K. Passive tension and stiffness of vertebrate skeletal and insect flight muscles: the contribution of weak cross-bridges and elastic filaments. Biophys J. 1993 Nov;65(5):2141–2159. doi: 10.1016/S0006-3495(93)81262-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuchi H. Changes in contractile properties with selective digestion of connectin (titin) in skinned fibers of frog skeletal muscle. J Biochem. 1992 Mar;111(3):291–295. doi: 10.1093/oxfordjournals.jbchem.a123752. [DOI] [PubMed] [Google Scholar]
- Higuchi H., Nakauchi Y., Maruyama K., Fujime S. Characterization of beta-connectin (titin 2) from striated muscle by dynamic light scattering. Biophys J. 1993 Nov;65(5):1906–1915. doi: 10.1016/S0006-3495(93)81261-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuchi H., Suzuki T., Kimura S., Yoshioka T., Maruyama K., Umazume Y. Localization and elasticity of connectin (titin) filaments in skinned frog muscle fibres subjected to partial depolymerization of thick filaments. J Muscle Res Cell Motil. 1992 Jun;13(3):285–294. doi: 10.1007/BF01766456. [DOI] [PubMed] [Google Scholar]
- Hill C., Weber K. Monoclonal antibodies distinguish titins from heart and skeletal muscle. J Cell Biol. 1986 Mar;102(3):1099–1108. doi: 10.1083/jcb.102.3.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowits R., Kempner E. S., Bisher M. E., Podolsky R. J. A physiological role for titin and nebulin in skeletal muscle. Nature. 1986 Sep 11;323(6084):160–164. doi: 10.1038/323160a0. [DOI] [PubMed] [Google Scholar]
- Horowits R., Maruyama K., Podolsky R. J. Elastic behavior of connectin filaments during thick filament movement in activated skeletal muscle. J Cell Biol. 1989 Nov;109(5):2169–2176. doi: 10.1083/jcb.109.5.2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowits R. Passive force generation and titin isoforms in mammalian skeletal muscle. Biophys J. 1992 Feb;61(2):392–398. doi: 10.1016/S0006-3495(92)81845-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowits R., Podolsky R. J. The positional stability of thick filaments in activated skeletal muscle depends on sarcomere length: evidence for the role of titin filaments. J Cell Biol. 1987 Nov;105(5):2217–2223. doi: 10.1083/jcb.105.5.2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu D. H., Kimura S., Maruyama K. Sodium dodecyl sulfate gel electrophoresis studies of connectin-like high molecular weight proteins of various types of vertebrate and invertebrate muscles. J Biochem. 1986 May;99(5):1485–1492. doi: 10.1093/oxfordjournals.jbchem.a135618. [DOI] [PubMed] [Google Scholar]
- Itoh Y., Suzuki T., Kimura S., Ohashi K., Higuchi H., Sawada H., Shimizu T., Shibata M., Maruyama K. Extensible and less-extensible domains of connectin filaments in stretched vertebrate skeletal muscle sarcomeres as detected by immunofluorescence and immunoelectron microscopy using monoclonal antibodies. J Biochem. 1988 Oct;104(4):504–508. doi: 10.1093/oxfordjournals.jbchem.a122499. [DOI] [PubMed] [Google Scholar]
- Jin J. P. Cloned rat cardiac titin class I and class II motifs. Expression, purification, characterization, and interaction with F-actin. J Biol Chem. 1995 Mar 24;270(12):6908–6916. [PubMed] [Google Scholar]
- Kruger M., Wright J., Wang K. Nebulin as a length regulator of thin filaments of vertebrate skeletal muscles: correlation of thin filament length, nebulin size, and epitope profile. J Cell Biol. 1991 Oct;115(1):97–107. doi: 10.1083/jcb.115.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurzban G. P., Wang K. Giant polypeptides of skeletal muscle titin: sedimentation equilibrium in guanidine hydrochloride. Biochem Biophys Res Commun. 1988 Feb 15;150(3):1155–1161. doi: 10.1016/0006-291x(88)90750-4. [DOI] [PubMed] [Google Scholar]
- Kyhse-Andersen J. Electroblotting of multiple gels: a simple apparatus without buffer tank for rapid transfer of proteins from polyacrylamide to nitrocellulose. J Biochem Biophys Methods. 1984 Dec;10(3-4):203–209. doi: 10.1016/0165-022x(84)90040-x. [DOI] [PubMed] [Google Scholar]
- Labeit S., Barlow D. P., Gautel M., Gibson T., Holt J., Hsieh C. L., Francke U., Leonard K., Wardale J., Whiting A. A regular pattern of two types of 100-residue motif in the sequence of titin. Nature. 1990 May 17;345(6272):273–276. doi: 10.1038/345273a0. [DOI] [PubMed] [Google Scholar]
- Labeit S., Gautel M., Lakey A., Trinick J. Towards a molecular understanding of titin. EMBO J. 1992 May;11(5):1711–1716. doi: 10.1002/j.1460-2075.1992.tb05222.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linke W. A., Popov V. I., Pollack G. H. Passive and active tension in single cardiac myofibrils. Biophys J. 1994 Aug;67(2):782–792. doi: 10.1016/S0006-3495(94)80538-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruyama K. Connectin, an elastic filamentous protein of striated muscle. Int Rev Cytol. 1986;104:81–114. doi: 10.1016/s0074-7696(08)61924-5. [DOI] [PubMed] [Google Scholar]
- Maruyama K. Connectin, an elastic protein of striated muscle. Biophys Chem. 1994 May;50(1-2):73–85. doi: 10.1016/0301-4622(94)85021-6. [DOI] [PubMed] [Google Scholar]
- Maruyama K., Endo T., Kume H., Kawamura Y., Kanzawa N., Nakauchi Y., Kimura S., Kawashima S., Maruyama K. A novel domain sequence of connectin localized at the I band of skeletal muscle sarcomeres: homology to neurofilament subunits. Biochem Biophys Res Commun. 1993 Aug 16;194(3):1288–1291. doi: 10.1006/bbrc.1993.1963. [DOI] [PubMed] [Google Scholar]
- Maruyama K., Kimura S., Yoshidomi H., Sawada H., Kikuchi M. Molecular size and shape of beta-connectin, an elastic protein of striated muscle. J Biochem. 1984 May;95(5):1423–1433. doi: 10.1093/oxfordjournals.jbchem.a134750. [DOI] [PubMed] [Google Scholar]
- Page E., McCallister L. P., Power B. Sterological measurements of cardiac ultrastructures implicated in excitation-contraction coupling. Proc Natl Acad Sci U S A. 1971 Jul;68(7):1465–1466. doi: 10.1073/pnas.68.7.1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan K. M., Damodaran S., Greaser M. L. Isolation and characterization of titin T1 from bovine cardiac muscle. Biochemistry. 1994 Jul 12;33(27):8255–8261. doi: 10.1021/bi00193a012. [DOI] [PubMed] [Google Scholar]
- Pierobon-Bormioli S., Betto R., Salviati G. The organization of titin (connectin) and nebulin in the sarcomeres: an immunocytolocalization study. J Muscle Res Cell Motil. 1989 Dec;10(6):446–456. doi: 10.1007/BF01771820. [DOI] [PubMed] [Google Scholar]
- Rodriguez E. K., Hunter W. C., Royce M. J., Leppo M. K., Douglas A. S., Weisman H. F. A method to reconstruct myocardial sarcomere lengths and orientations at transmural sites in beating canine hearts. Am J Physiol. 1992 Jul;263(1 Pt 2):H293–H306. doi: 10.1152/ajpheart.1992.263.1.H293. [DOI] [PubMed] [Google Scholar]
- Roos K. P., Brady A. J. Stiffness and shortening changes in myofilament-extracted rat cardiac myocytes. Am J Physiol. 1989 Feb;256(2 Pt 2):H539–H551. doi: 10.1152/ajpheart.1989.256.2.H539. [DOI] [PubMed] [Google Scholar]
- Suzuki J., Kimura S., Maruyama K. Connectin content in rabbit cardiac and skeletal muscle. Int J Biochem. 1993 Dec;25(12):1853–1858. [PubMed] [Google Scholar]
- Suzuki J., Kimura S., Maruyama K. Electron microscopic filament lengths of connection and its fragments. J Biochem. 1994 Aug;116(2):406–410. doi: 10.1093/oxfordjournals.jbchem.a124539. [DOI] [PubMed] [Google Scholar]
- Swerdlow P. S., Finley D., Varshavsky A. Enhancement of immunoblot sensitivity by heating of hydrated filters. Anal Biochem. 1986 Jul;156(1):147–153. doi: 10.1016/0003-2697(86)90166-1. [DOI] [PubMed] [Google Scholar]
- Trinick J. Elastic filaments and giant proteins in muscle. Curr Opin Cell Biol. 1991 Feb;3(1):112–119. doi: 10.1016/0955-0674(91)90173-v. [DOI] [PubMed] [Google Scholar]
- Trinick J., Knight P., Whiting A. Purification and properties of native titin. J Mol Biol. 1984 Dec 5;180(2):331–356. doi: 10.1016/s0022-2836(84)80007-8. [DOI] [PubMed] [Google Scholar]
- Trombitás K., Baatsen P. H., Kellermayer M. S., Pollack G. H. Nature and origin of gap filaments in striated muscle. J Cell Sci. 1991 Dec;100(Pt 4):809–814. doi: 10.1242/jcs.100.4.809. [DOI] [PubMed] [Google Scholar]
- Trombitás K., Pollack G. H. Elastic properties of the titin filament in the Z-line region of vertebrate striated muscle. J Muscle Res Cell Motil. 1993 Aug;14(4):416–422. doi: 10.1007/BF00121293. [DOI] [PubMed] [Google Scholar]
- Trombitás K., Pollack G. H., Wright J., Wang K. Elastic properties of titin filaments demonstrated using a "freeze-break" technique. Cell Motil Cytoskeleton. 1993;24(4):274–283. doi: 10.1002/cm.970240408. [DOI] [PubMed] [Google Scholar]
- Wang K., McCarter R., Wright J., Beverly J., Ramirez-Mitchell R. Regulation of skeletal muscle stiffness and elasticity by titin isoforms: a test of the segmental extension model of resting tension. Proc Natl Acad Sci U S A. 1991 Aug 15;88(16):7101–7105. doi: 10.1073/pnas.88.16.7101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K., McCarter R., Wright J., Beverly J., Ramirez-Mitchell R. Viscoelasticity of the sarcomere matrix of skeletal muscles. The titin-myosin composite filament is a dual-stage molecular spring. Biophys J. 1993 Apr;64(4):1161–1177. doi: 10.1016/S0006-3495(93)81482-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K., Ramirez-Mitchell R., Palter D. Titin is an extraordinarily long, flexible, and slender myofibrillar protein. Proc Natl Acad Sci U S A. 1984 Jun;81(12):3685–3689. doi: 10.1073/pnas.81.12.3685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K. Sarcomere-associated cytoskeletal lattices in striated muscle. Review and hypothesis. Cell Muscle Motil. 1985;6:315–369. doi: 10.1007/978-1-4757-4723-2_10. [DOI] [PubMed] [Google Scholar]
- Wang S. M., Greaser M. L. Immunocytochemical studies using a monoclonal antibody to bovine cardiac titin on intact and extracted myofibrils. J Muscle Res Cell Motil. 1985 Jun;6(3):293–312. doi: 10.1007/BF00713171. [DOI] [PubMed] [Google Scholar]