Abstract
Striking sex difference was detected in the expression of estrogen receptor (ER) β mRNA and protein by nonisotopic in situ hybridization and immunohistochemistry in the anteroventral periventricular nucleus (AVPV) of the rat preoptic area. In females more than in males, a significantly larger number of ERβ mRNA-positive cells were visualized in the medial-most portion of the AVPV within 50 μm from the ependymal lining of the third ventricle. Rats of 7, 14, 21, 35, and 60 days of age (d 1 = day of birth) showed the sex difference. Orchidectomy of male neonates or estrogen treatment of female pups reversed the brain phenotype when examined on d 14. In the AVPV of adult females, ERα immunoreactivity colocalized in 83% of ERβ mRNA-positive cells. Tyrosine hydroxylase immunoreactivity colocalized in 18% of ERβ immunoreactive cells in d 21 females. Infusion of an ERβ antisense oligonucleotide into the third ventricle in the vicinity of the AVPV resulted in significantly longer days of successive estrus and a 50% reduction in the number of ERβ-immunoreactive cells in the AVPV. These findings provide support for the hypothesis that activation of ERβ in the AVPV is an important regulatory event in the female-typical induction of luteinizing hormone surge by estrogen.
Estrogen plays critical roles in sexual differentiation of the developing brain and sex-specific regulation of reproductive neuroendocrinology in adults (1, 2). Cellular estrogen signaling is conveyed by nuclear estrogen receptors (ERs), which include the classical ERα as well as the recently cloned ERβ (3). Both ERs are expressed in the preoptic area (POA), hypothalamus and limbic structures, which have been implicated in the regulation of reproduction (4–6). It is unclear, however, whether ERβ, like ERα (7), is expressed in a sex-specific manner (8, 9). Furthermore, the presence of both ERs in the same neurons could alter the specificity of the transcription by forming heterodimers (10) and might produce different responses to estrogen in different cells, depending on the ratios of ERα and ERβ (11).
Disruption of either ERα or ERβ affects various aspects of reproduction. Female and male ERα knockout mice are infertile (12), and ERβ knockout females have a reduced fecundity (13). Anovulation and polycystic or hemorrhagic ovary are present in the ERα knockouts (14). Reductions of ovulatory capacity and polycystic ovary occur in the ERβ knockouts (13). The syndrome may be caused, at least partially, by the impairment in the central mechanism for the secretion of luteinizing hormone.
The anteroventral periventricular nucleus (AVPV) is sexually dimorphic with over three times as many dopaminergic neurons in the female rat compared with males (15), and this supports the importance of this brain region in the control of estrous cyclicity, which is absent in males. Indeed, small lesions confined to the AVPV block the cyclic release of luteinizing hormone in female rat and results in anovulatory, persistent estrous state (16). Implantation of microcannulae containing antiestrogens into the AVPV suppressed spontaneous luteinizing hormone surges (17).
In the present study, striking sex difference, detected in the ERβ expression in the AVPV, was reversed by altering neonatal steroid environment. ERβ mRNA and ERα immunoreactivity colocalized in many AVPV cells, some of which would be dopaminergic in nature. Infusion of ERβ antisense oligonucleotides prolonged vaginal estrus and was accompanied by a 50% reduction of ERβ immunoreactivity.
Materials and Methods
Subjects.
Female and male Sprague–Dawley rats (Saitama Experimental Animals, Saitama, Japan) were used on postnatal d 7, 14, 21, 35, or 60 (d 1 = the day of birth) for brain morphometry. They were maintained in a controlled environment at 23°C with a 12-hr light/12-h dark cycle (lights on at 11 a.m.). The weaning occurred on d 21. Free access to laboratory chow and water was allowed thereafter. A cohort of animals underwent endocrine manipulations as neonates or pups: males were orchidectomized under hypothermia on d 1; females received daily s.c. injections of 10 μg 17β-estradiol benzoate (Sigma) in oil on d 1 through d 10. All juvenile males and females were used without gonadectomy.
ERβ mRNA expression was determined on d 14 in each sex and in each experimental group (n = 5 in each; total n = 20) by means of in situ hybridization histochemistry. ERβ protein and tyrosine hydroxylase were visualized in d 21 males and females. After the confirmation of the sex difference in the distribution pattern of ERβ in the AVPV at both d 14 and d 21, we also looked at adult animals. The males and females were gonadectomized under i.p. pentobarbital sodium (35 mg/kg body weight) and killed for examination of ERβ mRNA on d 60. Simultaneous visualization of ERβ mRNA and ERα protein was accomplished in ovariectomized adult females. For oligonucleotide infusions, adult intact females were screened for their regular cyclicity before use.
Tissue Preparation.
The animals were anesthetized with an overdose of pentobarbital sodium, and perfused through the heart with 0.1 M PBS, pH 7.4, followed by ice cold 4% (wt/vol) paraformaldehyde fixative in 0.1 M phosphate buffer. The brain was removed and postfixed in the fixative at 4°C overnight and then transferred into 30% sucrose in 0.1 M phosphate buffer until it settled. Serial coronal sections (20-μm thick) that encompassed the organum vasculosum of the lamina terminalis (OVLT) rostrally and the medial preoptic nucleus caudally, were cut by a cryostat and mounted onto silan-coated (Shinetsu Silicon Chemicals, Tokyo) slides. Six series of slides were prepared for each brain, and serial sections were distributed to them in sequence, so that every sixth section, all 120 μm apart, was mounted on each. They were stored at −80°C until further processing.
In Situ Hybridization Histochemistry.
The in situ hybridization method used has been described (18). Briefly, full-length rat ERβ cDNA was ligated into pBluescript KS(+), linearized with NotI, and transcribed by T3 RNA polymerase in the presence of digoxigenin (DIG)-11-UTP (Roche Molecular Biochemicals) to prepare an antisense probe. ERβ cDNA, linearized with ClaI and transcribed by T7 RNA polymerase, was used as a sense probe. Full-length rat ERα cDNA was ligated into pBluescript SK(+), linearized with BamHI, and transcribed by T7 RNA polymerase with DIG-11-UTP to prepare an antisense probe. ERα cDNA, linearized with HindIII and transcribed by T3 RNA polymerase, was used as a sense probe. The labeled probes were precipitated, purified, and then diluted to 20 μg/μl with diethyl pyrocarbonate-treated water.
The sections were passed through a series of 0.1 M PBS, 0.2N HCl, 0.1 M PBS, proteinase K (20 μg/ml, Roche Molecular Biochemicals) in 0.1 M PBS, 0.1 M PBS, and dehydrated and dried. Hybridization with the probe (final concentration, 20 ng/100 μl) occurred overnight at 50°C in a buffer containing 50% formamide, 20 mM Tris⋅HCl, 0.3 M NaCl, 2.5 mM EDTA, 10% dextran sulfate (Sigma), E. coli tRNA (0.5 mg/ml, Roche Molecular Biochemicals) in 1× Denhardt's solution (0.02% polyvinylpyrrolidone/0.02% Ficoll/0.02% BSA) at pH = 8.0. The slides were washed in 2× SSC (1× SSC = 0.15 M sodium chloride/0.015 M sodium citrate, pH 7) in 50% formamide at 50°C for 1 h and treated by RNase A (20 μg/ml in 10 mM Tris⋅HCl/0.5 M NaCl, pH = 8.0; Roche Molecular Biochemicals) at 37°C for 30 min and in 1× SSC in 50% formamide at 50°C for 1 h, then incubated in a 1% blocking reagent in buffer I (0.1 M Tris⋅HCl/0.15 M NaCl, pH 7.5). For visualization, the sections were reacted with alkaline-phosphatase conjugate of anti-DIG-Fab (1:500), washed in buffer I, transferred to buffer III (0.1 M Tris⋅HCl/0.15 M NaCl/0.05 M MgCl2, pH 8.0), and developed by nitroblue tetrazolium and 5-bromo-4-chloro-3-indolyl-phosphate. The reaction was terminated at 4 h by buffer IV (0.1 M Tris⋅HCl/0.15 M NaCl/0.01 M EDTA, pH 8.0). The sections were rinsed in MilliQ water and dried and covered by CrystalMount (Biomedia, Foster City, CA). The signals were visualized in the cytoplasm as blue/purple products.
Throughout the experiment, care was taken to perform all procedures under fixed conditions. Analyses of the sex differences or the effects of neonatal endocrine manipulations were made between sections that underwent similar, simultaneous processing. Otherwise, to allow for comparison, temperature and other hybridization requirements and reaction times for visualization were kept constant for all experiments.
Specificity of the Probe.
ERβ cRNA probe used in the present study hybridized in the paraventricular and supraoptic nuclei, which have been established to express ERβ mRNA across species (4, 9, 19–21) and in the ERα knockout mouse (22). The specificity of the ERβ probe is further supported by the overlap of the hybridization signals and ERβ immunoreactivity in the AVPV in the present study (Fig. 1 and Fig. 2).
Figure 1.
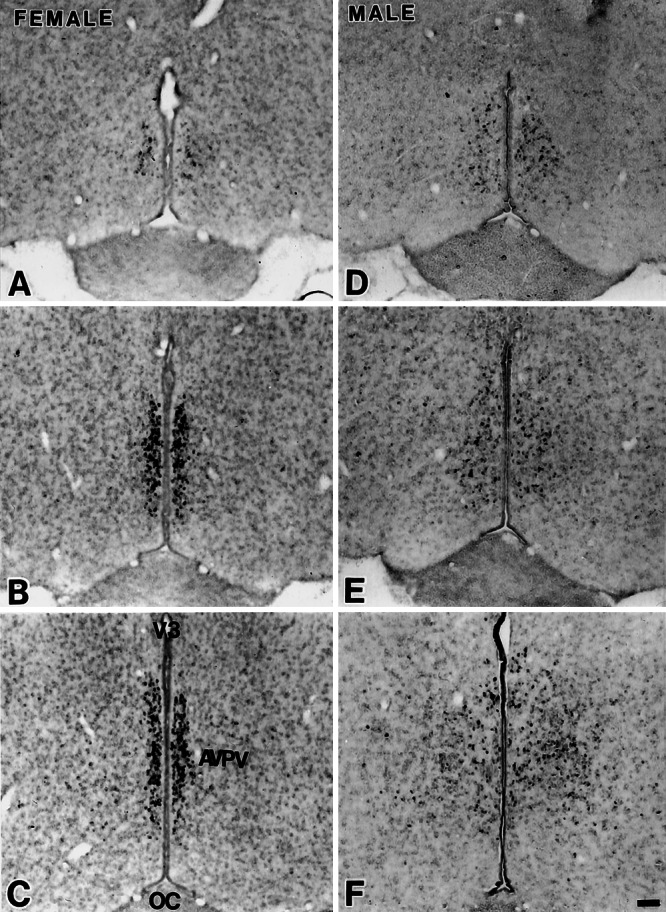
ERβ mRNA signals in the AVPV of the female (A–C) and male (D–F) rats through rostrocaudal axis. ERβ mRNA-positive cells aggregated densely in the medial part of the AVPV in the female but not in the male. OC, optic chiasm; V3, third ventricle. (Scale = 100 μm.)
Figure 2.
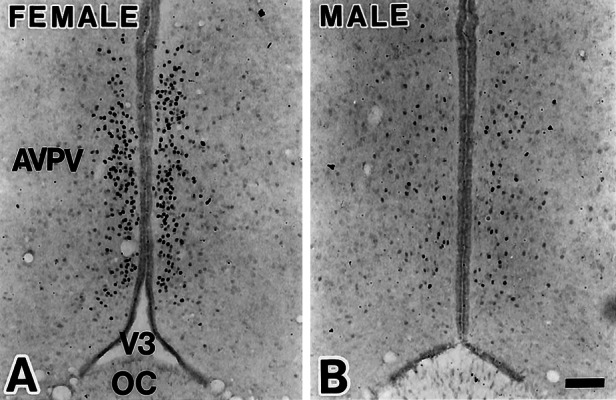
Sexually dimorphic distribution of ERβ protein in the AVPV visualized by immunohistochemistry for female (A) and male (B) rats. (Scale = 100 μm.)
Morphometric Analysis.
The sections were matched on anatomical landmarks that included the OVLT, third ventricle, anterior commissure, and optic chiasm and were compared across animal groups. Photomicrographs were made at 670× at 120-μm intervals. Identification of cells with hybridization signals was based on blue/purple stains in the cytoplasm, with a distinctive boundary toward the background. The nucleus remained clear. The number of labeled cells was tallied by two experimenters who were blind to the treatment. Two groups of data thus produced closely coincided without significant difference in paired t tests. Medial-lateral distribution of the labeled cells in each section was analyzed by dividing the POA into six vertical strips at the level of the AVPV, each 50-μm wide, with the medial-most strip including the ependyma of the third ventricle.
Immunohistochemistry for ERα, ERβ, or Tyrosine Hydroxylase.
The sections were rinsed in PBST (0.1 M PBS/0.5% Triton X-100), treated with 3% (vol/vol) H2O2 in methanol for 15 min, and incubated for 2 h with 5% (vol/vol) normal goat serum in PBST that contained 1% BSA and 0.07% sodium azide. The sections were reacted overnight with either anti-rabbit ERα serum (MC-20, Santa Cruz Biotechnology, 1:4,000 in BSA-PBST), anti-rabbit ERβ serum (Zymed, 1 μg/ml) or anti-rabbit tyrosine hydroxylase serum (Chemicon, 1:1,000 in BSA-PBST). Immunoparticipate was visualized by an ABC Elite kit and 3,3′-diaminobenzidine (DAB) methods (Vector Laboratories). Some sections were hybridized initially for ERβ mRNA and subsequently processed for ERα immunoreactivity in the nucleus. Simultaneous immunohistochemical labels for nuclear ERβ and cytoplasmic tyrosine hydroxylase were performed by a nickel-intensified DAB method for ERβ and DAB for tyrosine hydroxylase.
Effect of ERβ Antisense Oligonucleotide on Vaginal Cyclicity.
Antisense and scramble oligonucleotides were synthesized as phosphorothioated 20-mer. The antisense oligo was targeted as complement to the initiation site of ERβ gene translation (5′TGTCATAGCTGAATACTCAT3′). Scramble oligo was sequenced as 5′TGACGTATCACCGTCATCGT3′. Possible complementarity of the oligos to other eukaryotic gene was precluded on the GenBank database. Oligos were dissolved in 0.9% saline to a final concentration of 840 ng/μl. An Alzet osmotic minipump with a nominal pumping rate of 0.5 μl/h (Model 2002, Durect, Cupertino, CA) was loaded with oligo or saline. Female rats (n = 25) were screened for regular vaginal cyclicity over a 2-week period. They were anesthetized with a combination of pentobarbital sodium (25 mg/kg) and ketamine hydrochloride (25 mg/kg) for stereotaxic placement of the cannula in the third ventricle with its tip next to the AVPV. The cannula was chronically fit to the skull by dental cement. The pump was placed s.c. in the interscapular region and connected to the cannula by polyethylene tubing (PE60). After their recovery, daily examination of vaginal smear was begun and continued for 14 days until the animals were perfused. Mean successive days of vaginal estrus, which would be 1.0 in regular cycling animals, were determined. Cannula placements were confirmed, and the brain was processed for ERβ immunoreactivity in the AVPV.
Statistics.
Analyses of medial-lateral distribution of ERβ-positive cells in the AVPV were accomplished by dividing the nucleus into vertical, 50-μm wide strips in coronal sections. ANOVA and Student–Newman–Keuls test were used to compare corresponding subdivisions of the AVPV between animal groups. Numbers of ERβ immunoreactive cells in the AVPV after the oligonucleotide infusion were analyzed by 2 factorial ANOVA (2 oligos × 4 sections; scramble vs. antisense, 4 sections in repeated measures). The mean length of successive days in estrus was determined in each experimental group (saline, scramble, and antisense), which were analyzed by ANOVA, followed by Student–Newman–Keuls test. The criterion level of significance was set at P < 0.05.
Results
ERβ-Positive Cells.
In the forebrain of both sexes, cells with ERβ mRNA or protein were found rostrally in the AVPV (Figs. 1 and 2) and this pattern was consistent throughout the ages studied (d 7 through d 60). In the more caudal sections, they dispersed caudally into the medial preoptic nucleus and the bed nucleus of the stria terminals (Fig. 3). The overlap of ERβ immunoreactivity and the hybridization signals in the AVPV indicates that the ERβ message was translated into ERβ protein in these structures.
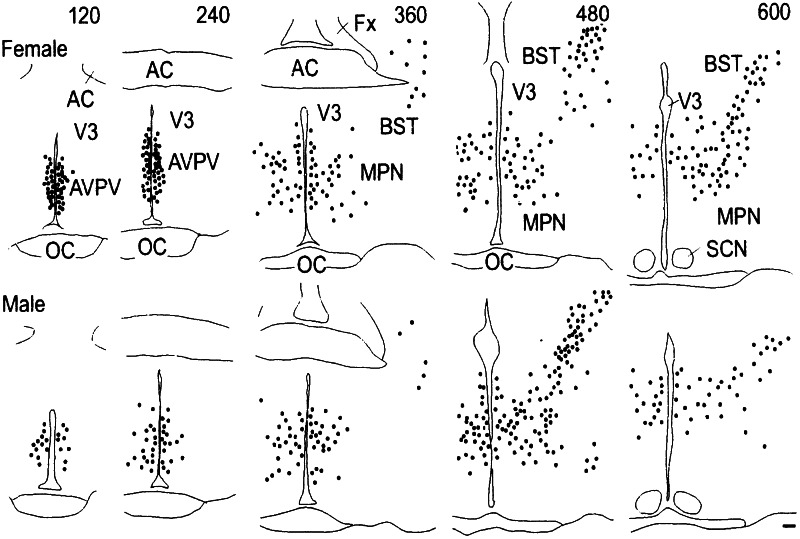
Figure 3.
Schematic representation of the distribution of ERβ mRNA-positive cells in the forebrain of female and male rats through rostrocaudal axis. Each dot represents five labeled cells. Numbers at the top of each panel represent distance from the organum vasculosum of the lamina terminalis in μm. (Scale = 100 μm.) AC, anterior commissure; AVPV, anteroventral periventricular nucleus; BST, bed nucleus of the stria terminals; Fx, fornix; MPN, medial preoptic nucleus; OC, optic chiasm; SCN, suprachiasmatic nucleus; V3, third ventricle.
Sex Difference in ERβ Expression.
Females had a larger number of ERβ mRNA-positive cells in the medial-most portion of the AVPV in a dense aggregate within 50 μm from the ependymal lining of the third ventricle, whereas the labeled cells dispersed laterally in males (Fig. 1). The sexual dimorphism in ERβ mRNA expression was maintained from d 7 through d 60 (data not shown). Immunohistochemistry revealed a similar sexually dimorphic expression of ERβ protein (Fig. 2). The difference was confined to the AVPV. We did not detect any gross sex differences in the pattern of ERβ expression in the medial preoptic nucleus and the bed nucleus of the stria terminals (Fig. 3).
Topographic Analysis of the Sex Difference.
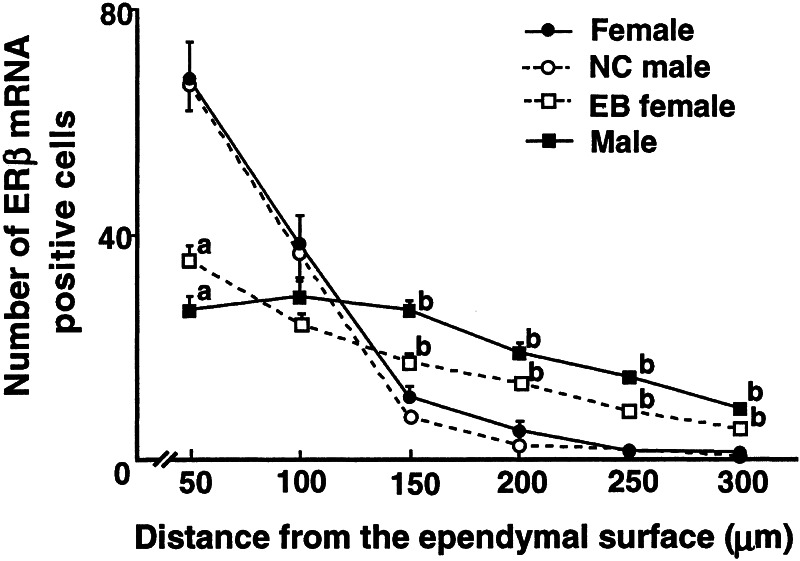
When determined in 50 μm-wide vertical strips in the coronal section of the AVPV, the number of ERβ mRNA-positive cells in the medial-most portion of the AVPV up to 100 μm from the ependyma of the third ventricle was significantly larger in the females (n = 5) than in the males (n = 5; Fig. 4). On the other hand, in the more lateral strips, which extended over 150–300 μm from the ependyma, the males had a larger number of ERβ mRNA-positive neurons than the females. Thus, the distribution pattern, but not the total number of ERβ mRNA-positive neurons (female, 126 ± 9; male, 125 ± 7, mean ± SE), was different between the sexes.
Figure 4.
The AVPV was divided into six vertical strips each with 50-μM width in the coronal plane, starting medially with the ependymal lining of the third ventricle. The number of ERβ mRNA-positive cells in each strip (mean ± SE) was determined for female, male, neonatally estrogenized (EB) female, and neonatal castrated (NC) male rats (n = 5, each). a, significant difference from female and NC male; b, significant difference from all others.
Neonatal Endocrine Manipulations.
Orchidectomy of male neonates or estrogen treatment of female pups reversed the sex difference in ERβ mRNA expression in the AVPV when examined on d 14 (Fig. 5). Statistical analyses detected no difference between the female and the neonatally orchidectomized males on the one hand, and between the male and the estrogenized females on the other (Fig. 4).
Figure 5.
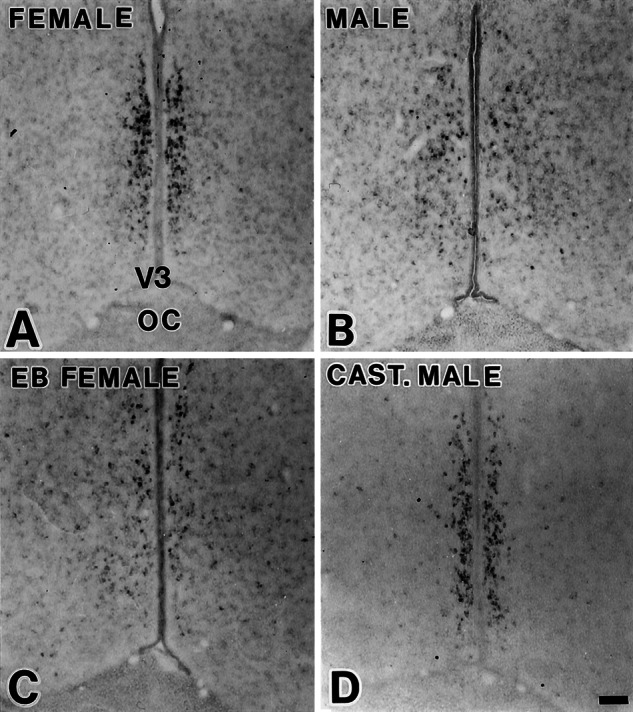
Neonatal endocrine manipulations reversed sexual dimorphism in the expression of ERβ mRNA. (A) Female. (B) Male. (C) Neonatally estrogenized female. (D) Neonatally castrated male. (Scale = 100 μm.)
Colocalization of ERβ and ERα.
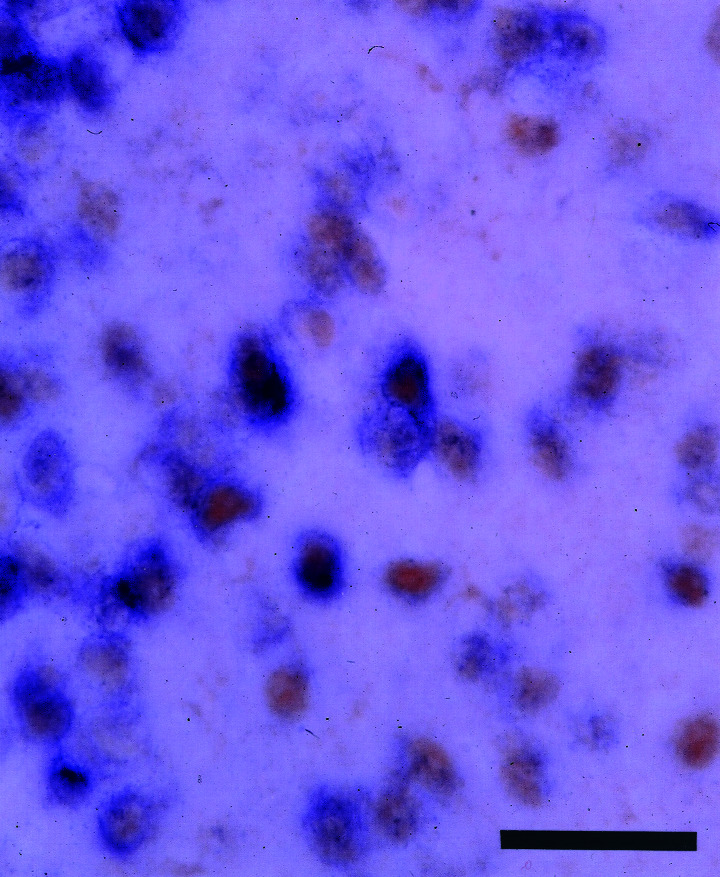
In situ hybridization for the two ERs in alternate POA sections of d 14 females revealed partial overlap in the distribution of ERβ and ERα mRNA-positive cells. Whereas ERβ mRNA signals were highly concentrated in the medial portion of the AVPV, ERα mRNA signals in the AVPV dispersed laterally in a similar pattern in both sexes, albeit with a higher expression in the females than in the males (Fig. 6). Simultaneous visualization of ERβ mRNA and ERα protein in same sections revealed that 83 ± 4% (mean ± SE, n = 3) of ERβ mRNA-positive cells in the female AVPV were immunoreactive to ERα antibody (Fig. 7).
Figure 6.
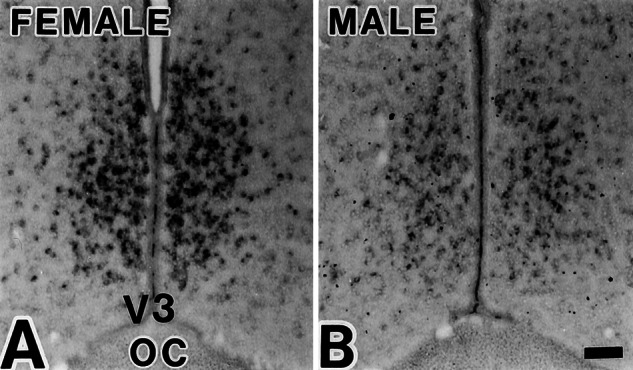
ERα mRNA hybridization signals in d 14 female (A) and male (B) rats, which were without topographic difference; only ERα mRNA-positive cells were more numerous in the female. (Scale = 100 μm.)
Figure 7.
Simultaneous visualization of ERβ mRNA (purple, cytoplasm) and ERα protein (brown, nucleus) in the AVPV in a d 60 ovariectomized rat. (Scale = 25 μm.)
Tyrosine Hydroxylase and ERβ.
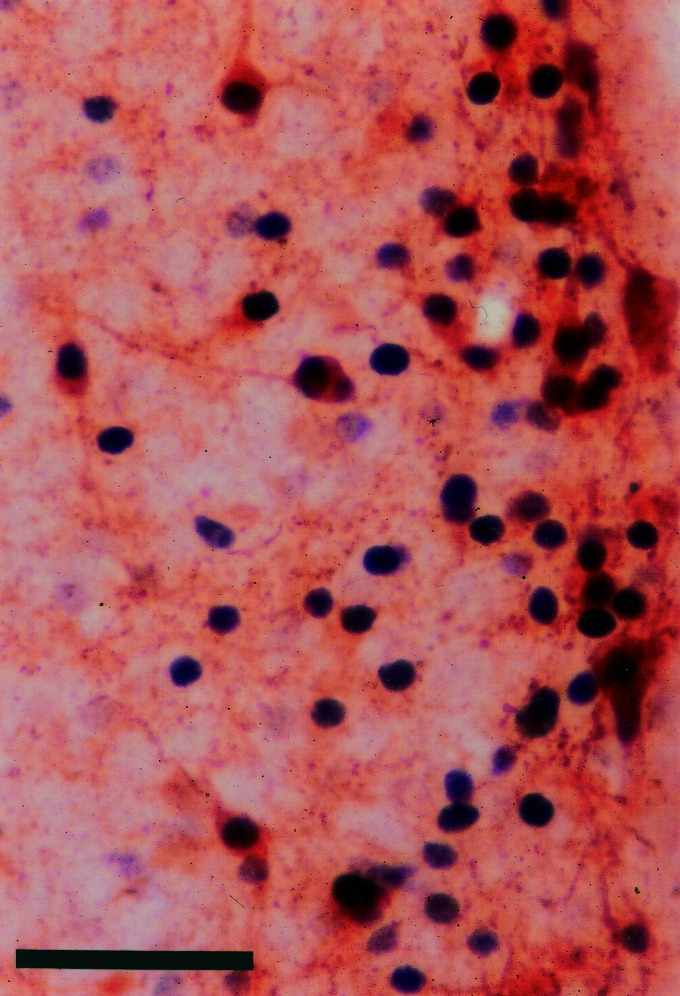
In d 14 females, the distribution of tyrosine hydroxylase immunoreactive neurons in the AVPV overlapped that of ERβ mRNA-positive neurons. Colocalization was not detected in the males. Tyrosine hydroxylase immunoreactive neurons were confined in the female AVPV, in a comparable pattern to that of ERβ mRNA-positive neurons (Fig. 8). In the AVPV of a d 21 female, tyrosine hydroxylase immunoreactivity was visualized in 28 ± 7 (mean ± SE, n = 5) among 154 ± 32 ERβ immunoreactive cells (18 ± 3%) within the 100-μm boundary from the ependymal lining (Fig. 9).
Figure 8.
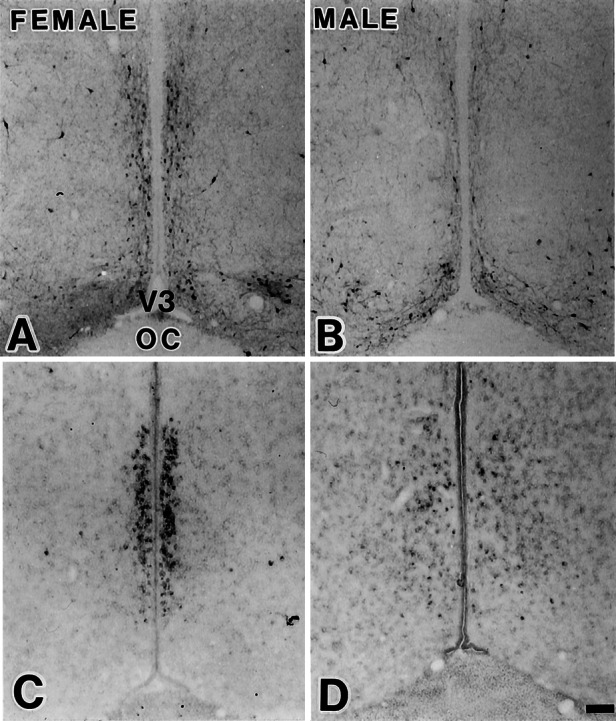
Tyrosine hydroxylase (TH) immunohistochemistry (A and B) and ERβ mRNA hybridization (C and D) in adjacent sections. Note that in females (A and C), but not in males (B and D), TH immunoreactivity had a similar topography as ERβ mRNA. (Scale = 100 μm.)
Figure 9.
Simultaneous visualization of ERβ protein (black, nucleus) and TH (brown, cytoplasm) by immunohistochemistry in the AVPV in a d 21 female rat. TH colocalized in 18% of ERβ immunoreactive cells. (Scale = 50 μm.)
Antisense Oligonucleotide Infusion.
Animals injected with antisense oligonucleotide against ERβ showed a prolonged vaginal estrus of 2.6 ± 0.3 (mean ± SE, n = 10) days in succession, whereas control animals infused with saline (1.2 ± 0.1 days, n = 8) or those that received scrambled oligonucleotide (1.7 ± 0.3 days, n = 7) had significantly fewer successive days of estrus (P < 0.01 by Student–Newman–Keuls test). The number of ERβ immunoreactive cells in the AVPV of animals that received antisense oligonucleotide was 132 ± 11 (mean ± SE, n = 6), which was a 50% reduction when compared with that in animals infused with scrambled oligonucleotide (258 ± 1, n = 3). The reduction was statistically significant (F[1,3] = 493.5, P < 0.001).
Discussion
In the AVPV, females have a greater density and a larger number of ERβ-positive cells than males. The distinctive sex difference was detected in immature rats as early as d 7 and was maintained into adulthood. Orchidectomy of male neonates or estrogen treatment of female pups, which are well established to alter sex-specific reproductive capability, resulted in a complete sex reversal of the sexual phenotype of the AVPV. Indeed, the ERβ in AVPV may mediate, at least in part, the actions of estrogen to promote ovulation, because infusion of antisense oligonucleotides against ERβ mRNA prolonged vaginal estrus while producing a 50% reduction of ERβ immunoreactivity.
In addition to the AVPV, ERβ hybridization signals and immunoreactivity were visualized in the more lateral portion of the POA, the paraventricular, supraoptic, and ventromedial nuclei of the hypothalamus, and the medial amygdala in both sexes. Expression of ERβ in the supraoptic nucleus was less intense, and no signals were found in the arcuate nucleus, in which ERα predominated. This pattern of distribution, including that in the AVPV, agrees well with earlier reports (4–6).
ERs play important roles in the sexual differentiation of the brain (23). The survival of reproductive behavior in ERβ gene-deficient male mice (24) or the lack of territorial aggression in male mice carrying disruption of ERα gene (25) implicates ERα in the brain sexual differentiation toward the male phenotype. Observations in ERα-knockout male mice, in which more tyrosine hydroxylase immunoreactive cells have been visualized in the AVPV, as in wild-type females but different from that in normal males (26), also favors this interpretation. Because 83% of ERβ mRNA-positive cells coexpressed ERα immunoreactivity in the female rat AVPV, it is likely that sexual differentiation of this structure depends on ERα as in dopaminergic cells (15).
In the males, ERβ-positive cells are spread over into the more lateral portions of the AVPV than the females. As a result, the total number of ERβ-positive cells within the AVPV was not different between the sexes. It is possible that the observed sex difference in the distribution pattern of ERβ-positive cells in the AVPV is caused by neuronal migration, as in the ventromedial nucleus of the hypothalamus (27, 28). The results of previous studies, however, do not support steroid-dependent neural migration in the sexual differentiation of the AVPV or POA in general. Perinatal androgen decreases the nuclear volume of the AVPV of female rats through enhanced apoptosis (29). Apoptosis also is implicated in the establishment of the sexually dimorphic nucleus of the POA (30). Neuronal birth or death during development cannot be ruled out in the observed sex difference in the ERβ-positive cells.
The AVPV has been reported to contain sexually dimorphic populations of dopaminergic (15), peptidergic (31), or opiate (32) neurons, as well as those with different glutamate-receptor subunits (33). A greater density and number in the female rat AVPV have been demonstrated for dopaminergic (15) or opiate (32) neurons. In the present study, the distribution of ERβ-positive cells and dopaminergic neurons overlapped in the females but not in the males. This difference corroborates the interpretation that the observed sex difference in ERβ expression in this study was not merely a result of the sex difference in the total population of AVPV neurons. An enhanced dopaminergic activity in the POA preceding ovulation further implicates dopaminergic transmission in this process (34).
Lesions spreading over the AVPV induce persistent vaginal estrus, indicating that the AVPV is indispensable for regulation of the ovulatory surge of luteinizing hormone (16). The results of antisense oligonucleotide infusion, which prolonged vaginal estrus along with diminished ERβ expression in the AVPV, implicate ERβ-positive neurons in this structure in the induction of ovulatory surge of luteinizing hormone. ERs regulate gene transcription for luteinizing hormone secretion (35). In vitro studies have shown that ERα and ERβ exert alternate effects on gene transcription (36). ERβ may repress transcriptional activity of ERα (19, 37) presumably by forming a heterodimer (38, 39). In the present study, we found that a majority of ERβ neurons in the female rat AVPV coexpresses ERα, which enables possible interactions between ERα and ERβ (11). The presence of both ERα and ERβ in identical neurons may result in a unique regulation of transcription, which may be liable to disruption by antisense and culminates in the disruption of neural signals for ovulation.
In conclusion, this study shows that sexually dimorphic distribution of ERβ mRNA-positive neurons in the AVPV colocalized with ERα, suggesting that the ERβ-positive neurons in the AVPV may play an important role in controlling the secretion of luteinizing hormone and the ability of luteinizing hormone-releasing hormone neurons to respond to estrogen.
Acknowledgments
This work was supported by grants-in-aid for scientific research from the Japanese Ministry of Education, Science, Sports and Culture 13670071 (to C.O.) and 12878155 (to Y.S.).
Abbreviations
- ER
estrogen receptor
- POA
preoptic area
- AVPV
anteroventral periventricular nucleus
References
- 1.McEwen B S. Proc Natl Acad Sci USA. 1999;96:7128–7130. doi: 10.1073/pnas.96.13.7128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McEwen B S, Alves S E. Endocr Rev. 1999;20:279–307. doi: 10.1210/edrv.20.3.0365. [DOI] [PubMed] [Google Scholar]
- 3.Kuiper G G, Enmark E, Pelto-Huikko M, Nilsson S, Gustafsson J A. Proc Natl Acad Sci USA. 1996;93:5925–5930. doi: 10.1073/pnas.93.12.5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Laflamme N, Nappi R E, Drolet G, Labrie C, Rivest S. J Neurobiol. 1998;36:357–378. doi: 10.1002/(sici)1097-4695(19980905)36:3<357::aid-neu5>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 5.Li X, Schwartz P E, Rissman E F. Neuroendocrinology. 1997;66:63–67. doi: 10.1159/000127221. [DOI] [PubMed] [Google Scholar]
- 6.Shughrue P J, Scrimo P J, Merchenthaler I. Endocrinology. 1998;139:5267–5270. doi: 10.1210/endo.139.12.6525. [DOI] [PubMed] [Google Scholar]
- 7.Yokosuka M, Okamura H, Hayashi S. J Comp Neurol. 1997;389:81–93. doi: 10.1002/(sici)1096-9861(19971208)389:1<81::aid-cne6>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 8.Karolczak M, Beyer C. Neuroendocrinology. 1998;68:229–234. doi: 10.1159/000054370. [DOI] [PubMed] [Google Scholar]
- 9.Scott C J, Tilbrook A J, Simmons D M, Rawson J A, Chu S, Fuller P J, Ing N H, Clarke I J. Endocrinology. 2000;141:2951–2962. doi: 10.1210/endo.141.8.7622. [DOI] [PubMed] [Google Scholar]
- 10.Ogawa S, Inoue S, Orimo A, Hosoi T, Ouchi Y, Muramatsu M. FEBS Lett. 1998;423:129–132. doi: 10.1016/s0014-5793(98)00079-9. [DOI] [PubMed] [Google Scholar]
- 11.Hall J M, McDonnell D P. Endocrinology. 1999;140:5566–5578. doi: 10.1210/endo.140.12.7179. [DOI] [PubMed] [Google Scholar]
- 12.Lubahn D B, Moyer J S, Golding T S, Couse J F, Korach K S, Smithies O. Proc Natl Acad Sci USA. 1993;90:11162–11166. doi: 10.1073/pnas.90.23.11162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krege J H, Hodgin J B, Couse J F, Enmark E, Warner M, Mahler J F, Sar M, Korach K S, Gustafsson J A, Smithies O. Proc Natl Acad Sci USA. 1998;95:15677–15682. doi: 10.1073/pnas.95.26.15677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schomberg D W, Couse J F, Mukherjee A, Lubahn D B, Sar M, Mayo K E, Korach K S. Endocrinology. 1999;140:2733–2744. doi: 10.1210/endo.140.6.6823. [DOI] [PubMed] [Google Scholar]
- 15.Simerly R B. Mol Brain Res. 1989;6:297–310. doi: 10.1016/0169-328x(89)90075-2. [DOI] [PubMed] [Google Scholar]
- 16.Wiegand S J, Terasawa E, Bridson W E. Endocrinology. 1978;102:1645–1648. doi: 10.1210/endo-102-5-1645. [DOI] [PubMed] [Google Scholar]
- 17.Petersen S L, Barraclough C A. Brain Res. 1989;484:279–289. doi: 10.1016/0006-8993(89)90371-5. [DOI] [PubMed] [Google Scholar]
- 18.Orikasa C, McEwen B S, Hayashi H, Sakuma Y, Hayashi S. Dev Brain Res. 2000;120:245–254. doi: 10.1016/s0165-3806(00)00016-x. [DOI] [PubMed] [Google Scholar]
- 19.Hrabovszky E, Kallo I, Hajszan T, Shughrue P J, Merchenthaler I, Liposits Z. Endocrinology. 1998;139:2600–2604. doi: 10.1210/endo.139.5.6024. [DOI] [PubMed] [Google Scholar]
- 20.Shughrue P J, Komm B, Merchenthaler I. Steroids. 1996;61:678–681. doi: 10.1016/s0039-128x(96)00222-x. [DOI] [PubMed] [Google Scholar]
- 21.Gundlah C, Kohama S G, Mirkes S J, Garyfallou V T, Urbanski H F, Bethea C L. Mol Brain Res. 2000;76:191–204. doi: 10.1016/s0006-8993(99)02475-0. [DOI] [PubMed] [Google Scholar]
- 22.Shughrue P J, Lane M V, Merchenthaler I. Endocrinology. 1999;140:2613–2620. doi: 10.1210/endo.140.6.6876. [DOI] [PubMed] [Google Scholar]
- 23.MacLusky N F, Naftolin F. Science. 1981;211:1294–1302. doi: 10.1126/science.6163211. [DOI] [PubMed] [Google Scholar]
- 24.Ogawa S, Chan J, Chester A E, Gustafsson J A, Korach K S, Pfaff D W. Proc Natl Acad Sci USA. 1999;96:12887–12892. doi: 10.1073/pnas.96.22.12887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ogawa S, Lubahn D B, Korach K S, Pfaff D W. Proc Natl Acad Sci USA. 1997;94:1476–1481. doi: 10.1073/pnas.94.4.1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Simerly R B, Zee M C, Pendleton J W, Lubahn D B, Korach K S. Proc Natl Acad Sci USA. 1997;94:14077–14082. doi: 10.1073/pnas.94.25.14077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tobet S A, Henderson R G, Whiting P J, Sieghart W. J Comp Neurol. 1999;405:88–98. doi: 10.1002/(sici)1096-9861(19990301)405:1<88::aid-cne7>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 28.Dellovade T L, Young M, Ross E P, Henderson R, Caron K, Parker K, Tobet S A. J Comp Neurol. 2000;423:579–589. doi: 10.1002/1096-9861(20000807)423:4<579::aid-cne4>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 29.Arai Y, Murakami S, Nishizuka M. Horm Behav. 1994;28:313–319. doi: 10.1006/hbeh.1994.1027. [DOI] [PubMed] [Google Scholar]
- 30.Davis E C, Popper P, Gorski R A. Brain Res. 1996;734:10–18. [PubMed] [Google Scholar]
- 31.Gu G, Rojo A A, Zee M C, Yu J, Simerly R B. J Neurosci. 1996;16:3035–3044. doi: 10.1523/JNEUROSCI.16-09-03035.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Watson R E, Jr, Hoffmann G E, Wiegand S J. Brain Res. 1986;398:157–163. doi: 10.1016/0006-8993(86)91261-8. [DOI] [PubMed] [Google Scholar]
- 33.Gu G, Varoqueaux F, Simerly R B. J Neurosci. 1999;19:3213–3222. doi: 10.1523/JNEUROSCI.19-08-03213.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cruz M E, Villegas G, Dominguez-Gonzalez A, Chavira R, Dominguez R. Brain Res Bull. 2001;54:339–344. doi: 10.1016/s0361-9230(00)00378-6. [DOI] [PubMed] [Google Scholar]
- 35.Lauber A H, Romano G J, Pfaff D W. J Steroid Biochem Mol Biol. 1991;40:53–62. doi: 10.1016/0960-0760(91)90167-4. [DOI] [PubMed] [Google Scholar]
- 36.Paech K, Webb P, Kuiper G G, Nilsson S, Gustafsson J, Kushner P J, Scanlan T S. Science. 1997;277:1508–1510. doi: 10.1126/science.277.5331.1508. [DOI] [PubMed] [Google Scholar]
- 37.Weihua Z, Saji S, Makinen S, Cheng G, Jensen E V, Warner M, Gustafsson J A. Proc Natl Acad Sci USA. 2000;97:5936–5941. doi: 10.1073/pnas.97.11.5936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pettersson K, Grandien K, Kuiper G G, Gustafsson J A. Mol Endocrinol. 1997;11:1486–1496. doi: 10.1210/mend.11.10.9989. [DOI] [PubMed] [Google Scholar]
- 39.Cowley S M, Hoare S, Mosselman S, Parker M G. J Biol Chem. 1997;272:19858–19862. doi: 10.1074/jbc.272.32.19858. [DOI] [PubMed] [Google Scholar]