Abstract
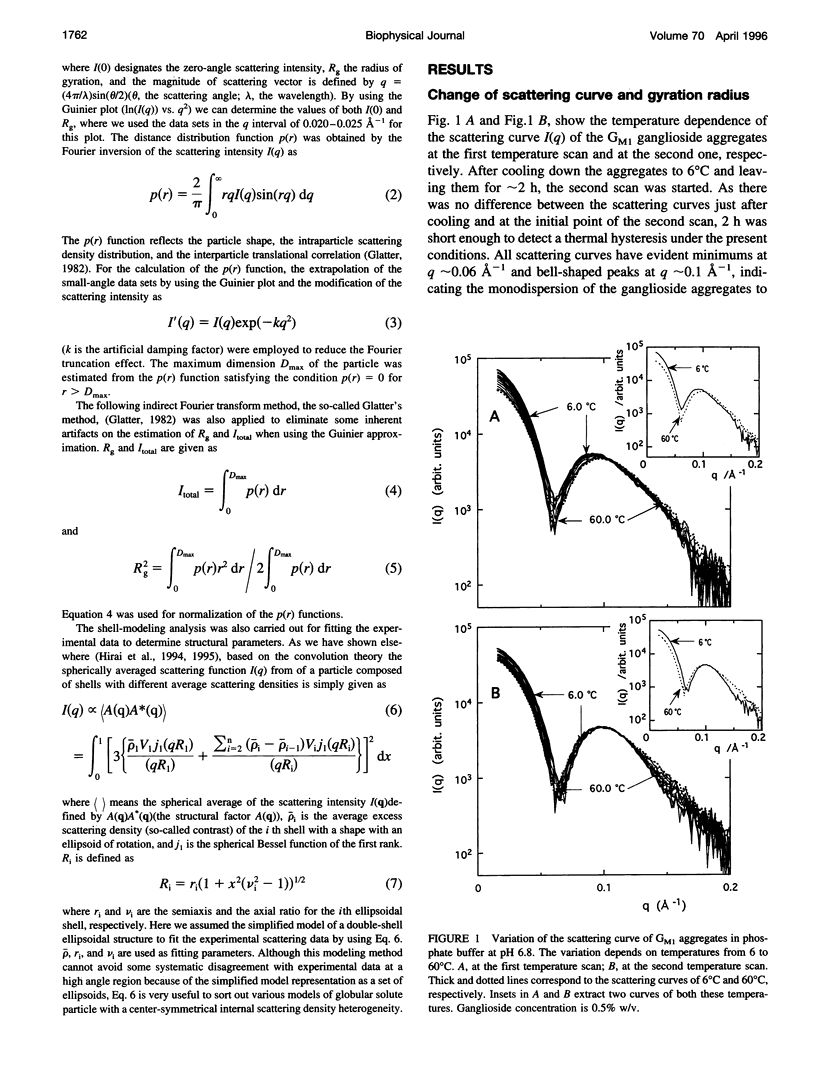
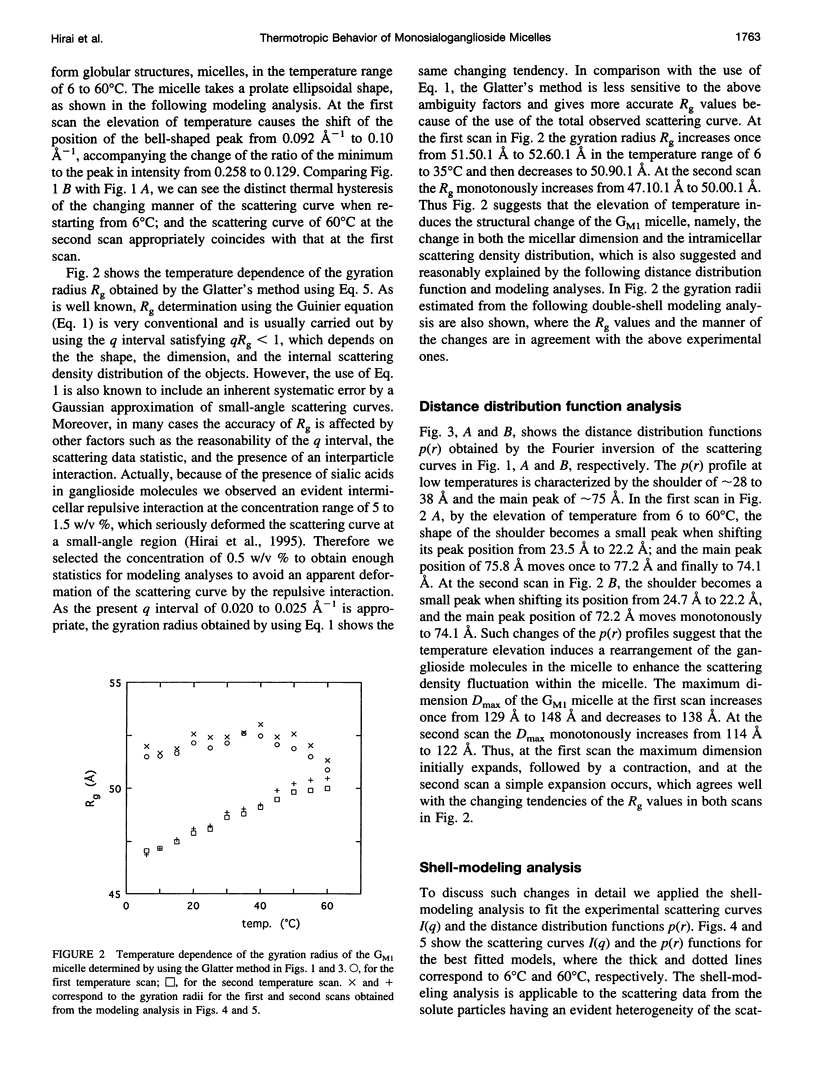
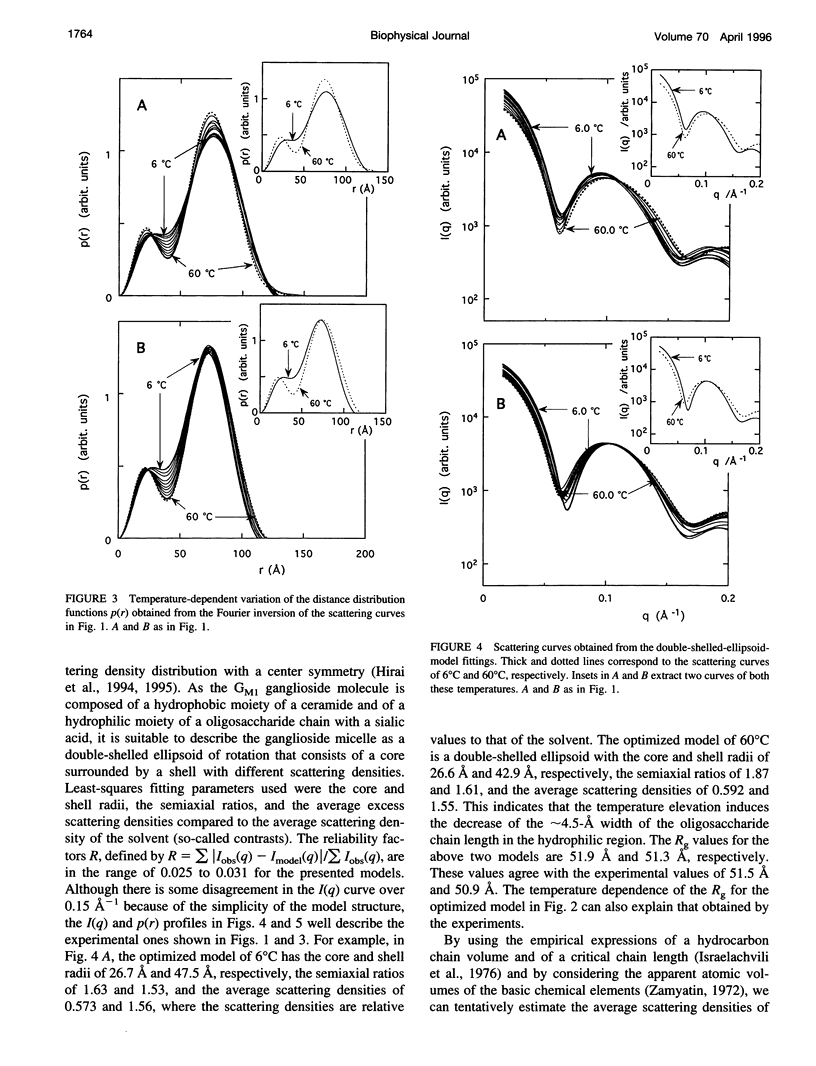
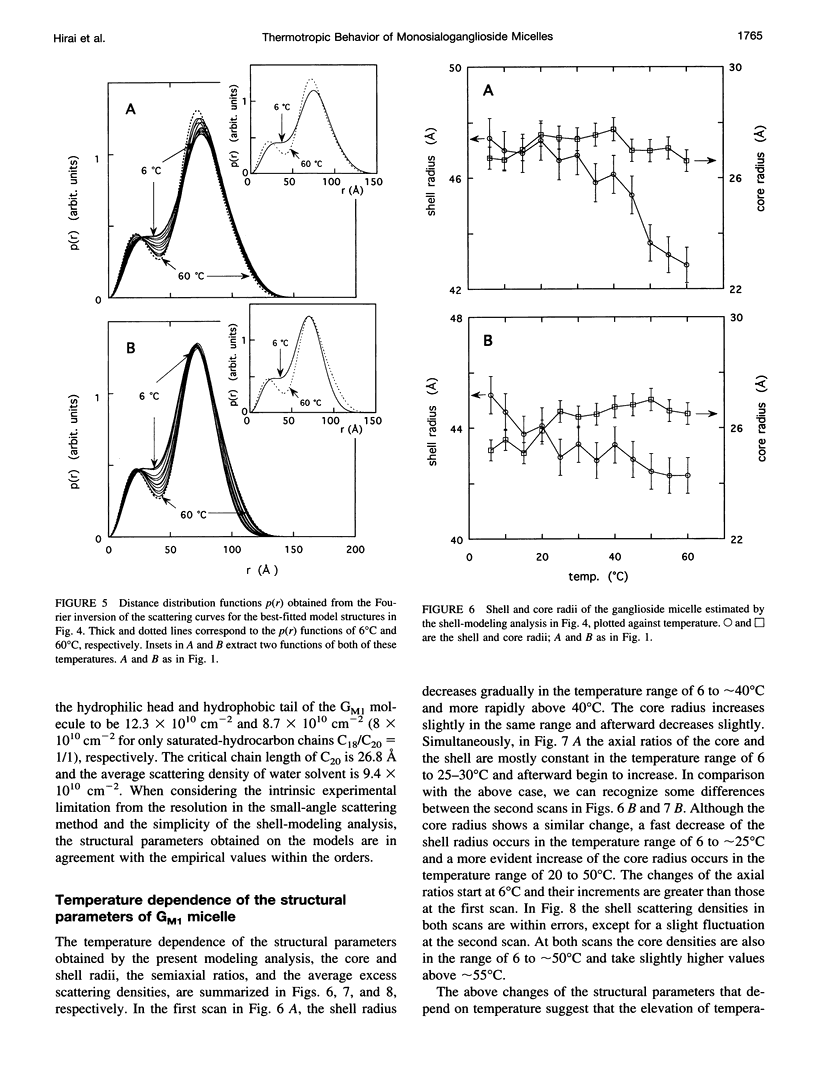
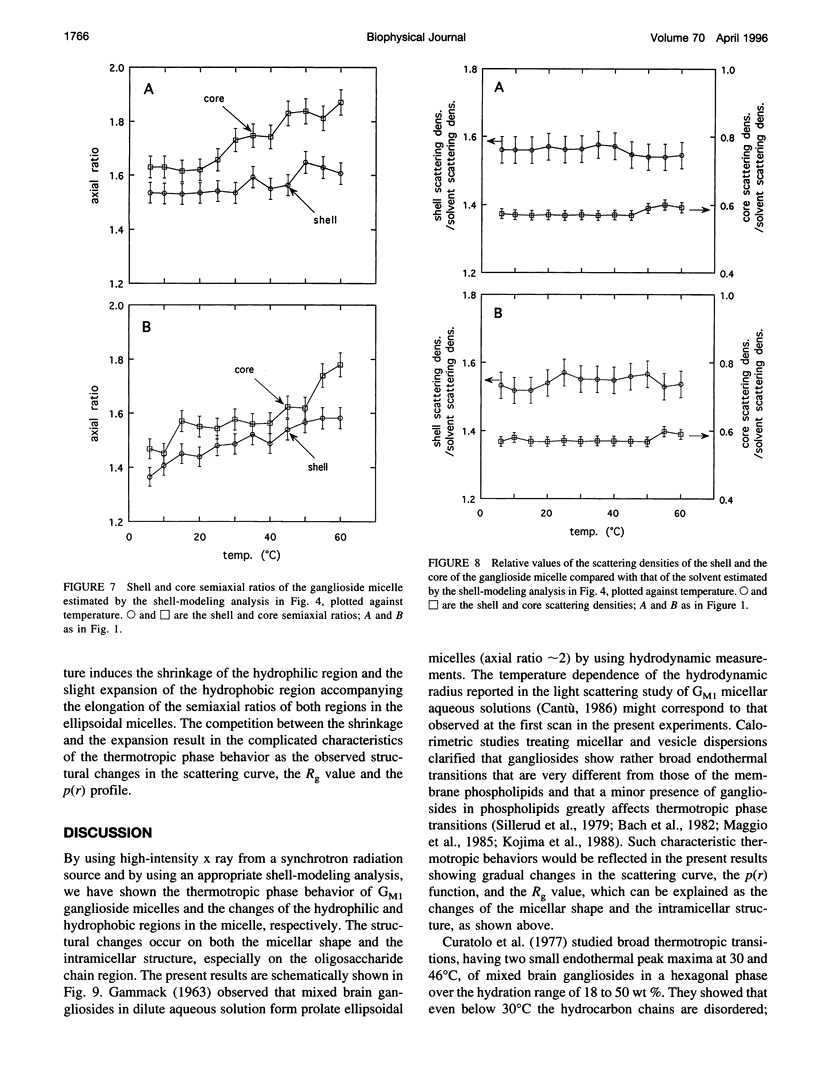
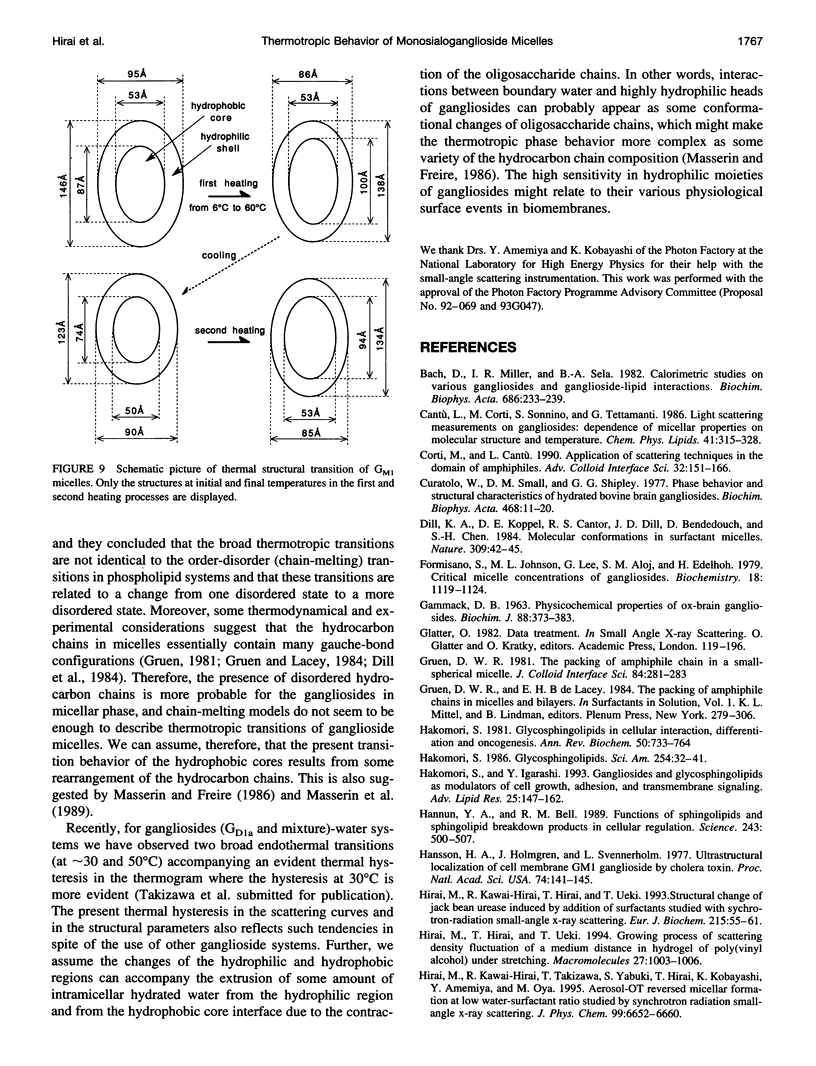
The thermotropic phase behavior of monosialoganglioside in a dilute aqueous dispersion at pH 6.8 was measured by using synchrotron radiation small-angle x-ray scattering and was analyzed by a shell-modeling method. Previous calorimetric studies on ganglioside systems have shown quite different thermotropic behaviors from other biological lipid systems, however, the details have still been ambiguous. Because of high statistical data and a shell-modeling analysis, we could elucidate the internal structural change of monosialoganglioside micelle induced by the elevation of temperature from 6 to 60 degrees C, that is, the shrinkage of the hydrophilic region and the slight expansion of the hydrophobic region occurring simultaneously, accompanying the elongation of the axial ratios of the ellipsoidal micelles. The model structures obtained explain the changes in the experimental scattering curves, the distance distribution functions, and the gyration radii. In addition we have also found an evident thermal hysteresis in the scattering curves and in the structural parameters. The present result suggests that the thickness of the hydrophilic region, namely, the conformation of oligosaccharide chains, is sensitive to a change of temperature.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bach D., Miller I. R., Sela B. A. Calorimetric studies on various gangliosides and ganglioside-lipid interactions. Biochim Biophys Acta. 1982 Apr 7;686(2):233–239. doi: 10.1016/0005-2736(82)90117-1. [DOI] [PubMed] [Google Scholar]
- Cantù L., Corti M., Sonnino S., Tettamanti G. Light scattering measurements on gangliosides: dependence of micellar properties on molecular structure and temperature. Chem Phys Lipids. 1986 Oct-Nov;41(3-4):315–328. doi: 10.1016/0009-3084(86)90029-0. [DOI] [PubMed] [Google Scholar]
- Corti M., Cantú L. Application of scattering techniques in the domain of amphiphiles. Adv Colloid Interface Sci. 1990 Aug;32(2-3):151–166. doi: 10.1016/0001-8686(90)80016-s. [DOI] [PubMed] [Google Scholar]
- Curatolo W., Small D. M., Shipley G. G. Phase behavior and structural characteristics of hydrated bovine brain gangliosides. Biochim Biophys Acta. 1977 Jul 4;468(1):11–20. doi: 10.1016/0005-2736(77)90147-x. [DOI] [PubMed] [Google Scholar]
- Formisano S., Johnson M. L., Lee G., Aloj S. M., Edelhoch H. Critical micelle concentrations of gangliosides. Biochemistry. 1979 Mar 20;18(6):1119–1124. doi: 10.1021/bi00573a028. [DOI] [PubMed] [Google Scholar]
- GAMMACK D. B. PHYSICOCHEMICAL PROPERTIES OF OX-BRAIN GANGLIOSIDES. Biochem J. 1963 Aug;88:373–383. doi: 10.1042/bj0880373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOWARD R. E., BURTON R. M. STUDIES ON THE GANGLIOSIDE MICELLE. Biochim Biophys Acta. 1964 Aug 5;84:435–440. doi: 10.1016/0926-6542(64)90007-1. [DOI] [PubMed] [Google Scholar]
- Hakomori S. Glycosphingolipids in cellular interaction, differentiation, and oncogenesis. Annu Rev Biochem. 1981;50:733–764. doi: 10.1146/annurev.bi.50.070181.003505. [DOI] [PubMed] [Google Scholar]
- Hakomori S., Igarashi Y. Gangliosides and glycosphingolipids as modulators of cell growth, adhesion, and transmembrane signaling. Adv Lipid Res. 1993;25:147–162. [PubMed] [Google Scholar]
- Hannun Y. A., Bell R. M. Functions of sphingolipids and sphingolipid breakdown products in cellular regulation. Science. 1989 Jan 27;243(4890):500–507. doi: 10.1126/science.2643164. [DOI] [PubMed] [Google Scholar]
- Hirai M., Kawai-Hirai R., Hirai T., Ueki T. Structural change of jack bean urease induced by addition of surfactants studied with synchrotron-radiation small-angle X-ray scattering. Eur J Biochem. 1993 Jul 1;215(1):55–61. doi: 10.1111/j.1432-1033.1993.tb18006.x. [DOI] [PubMed] [Google Scholar]
- Kojima H., Hanada-Yoshikawa K., Katagiri A., Tamai Y. Thermotropic behavior and electronmicroscopic structures of mixtures of gangliosides and dipalmitoylphosphatidylcholine. J Biochem. 1988 Jan;103(1):126–131. doi: 10.1093/oxfordjournals.jbchem.a122217. [DOI] [PubMed] [Google Scholar]
- Maggio B., Ariga T., Sturtevant J. M., Yu R. K. Thermotropic behavior of binary mixtures of dipalmitoylphosphatidylcholine and glycosphingolipids in aqueous dispersions. Biochim Biophys Acta. 1985 Aug 8;818(1):1–12. doi: 10.1016/0005-2736(85)90131-2. [DOI] [PubMed] [Google Scholar]
- Maggio B., Ariga T., Sturtevant J. M., Yu R. K. Thermotropic behavior of glycosphingolipids in aqueous dispersions. Biochemistry. 1985 Feb 26;24(5):1084–1092. doi: 10.1021/bi00326a003. [DOI] [PubMed] [Google Scholar]
- Marcus D. M. A review of the immunogenic and immuno-modulatory properties of glycosphingolipids. Mol Immunol. 1984 Nov;21(11):1083–1091. doi: 10.1016/0161-5890(84)90118-4. [DOI] [PubMed] [Google Scholar]
- Masserini M., Freire E. Thermotropic characterization of phosphatidylcholine vesicles containing ganglioside GM1 with homogeneous ceramide chain length. Biochemistry. 1986 Mar 11;25(5):1043–1049. doi: 10.1021/bi00353a014. [DOI] [PubMed] [Google Scholar]
- Masserini M., Palestini P., Freire E. Influence of glycolipid oligosaccharide and long-chain base composition on the thermotropic properties of dipalmitoylphosphatidylcholine large unilamellar vesicles containing gangliosides. Biochemistry. 1989 Jun 13;28(12):5029–5034. doi: 10.1021/bi00438a019. [DOI] [PubMed] [Google Scholar]
- Sillerud L. O., Schafer D. E., Yu R. K., Konigsberg W. H. Calorimetric properties of mixtures of ganglioside GM1 and dipalmitoylphosphatidylcholine. J Biol Chem. 1979 Nov 10;254(21):10876–10880. [PubMed] [Google Scholar]
- Spiegel S., Fishman P. H. Gangliosides as bimodal regulators of cell growth. Proc Natl Acad Sci U S A. 1987 Jan;84(1):141–145. doi: 10.1073/pnas.84.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueki T., Hiragi Y., Kataoka M., Inoko Y., Amemiya Y., Izumi Y., Tagawa H., Muroga Y. Aggregation of bovine serum albumin upon cleavage of its disulfide bonds, studied by the time-resolved small-angle X-ray scattering technique with synchrotron radiation. Biophys Chem. 1985 Nov;23(1-2):115–124. doi: 10.1016/0301-4622(85)80069-7. [DOI] [PubMed] [Google Scholar]
- Ulrich-Bott B., Wiegandt H. Micellar properties of glycosphingolipids in aqueous media. J Lipid Res. 1984 Nov;25(11):1233–1245. [PubMed] [Google Scholar]
- Zamyatnin A. A. Protein volume in solution. Prog Biophys Mol Biol. 1972;24:107–123. doi: 10.1016/0079-6107(72)90005-3. [DOI] [PubMed] [Google Scholar]


