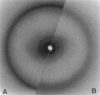
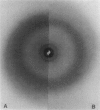
Abstract
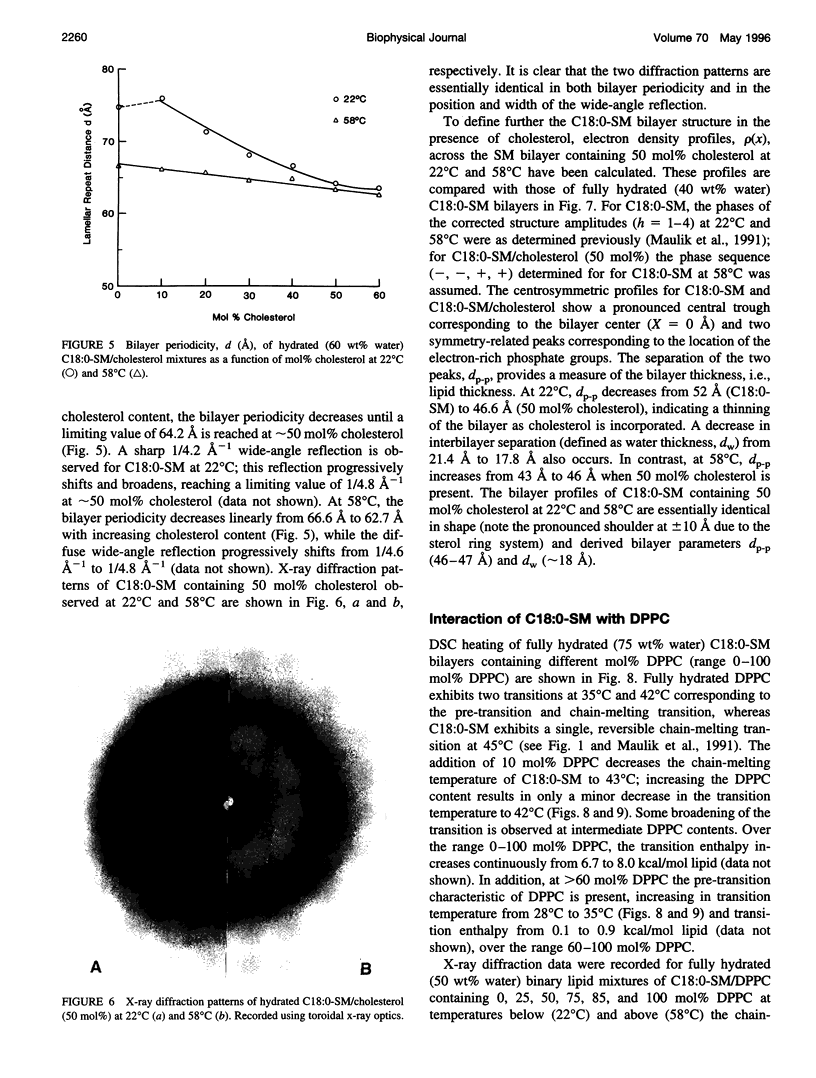
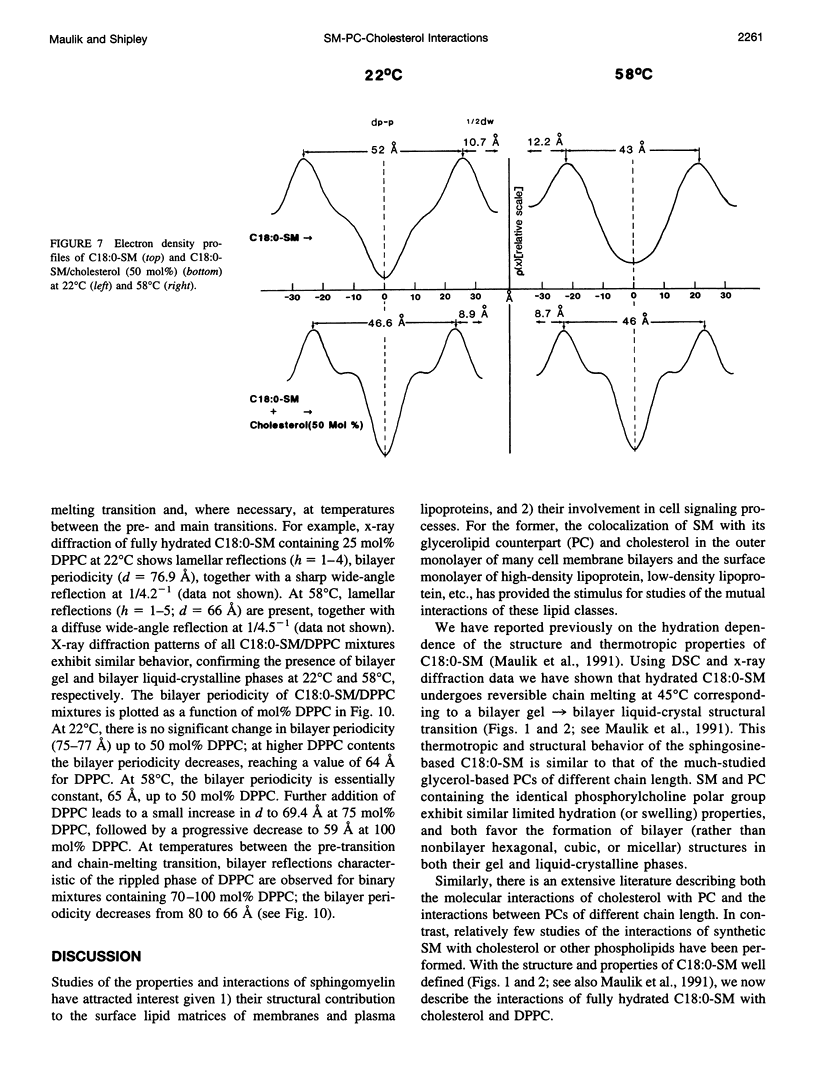
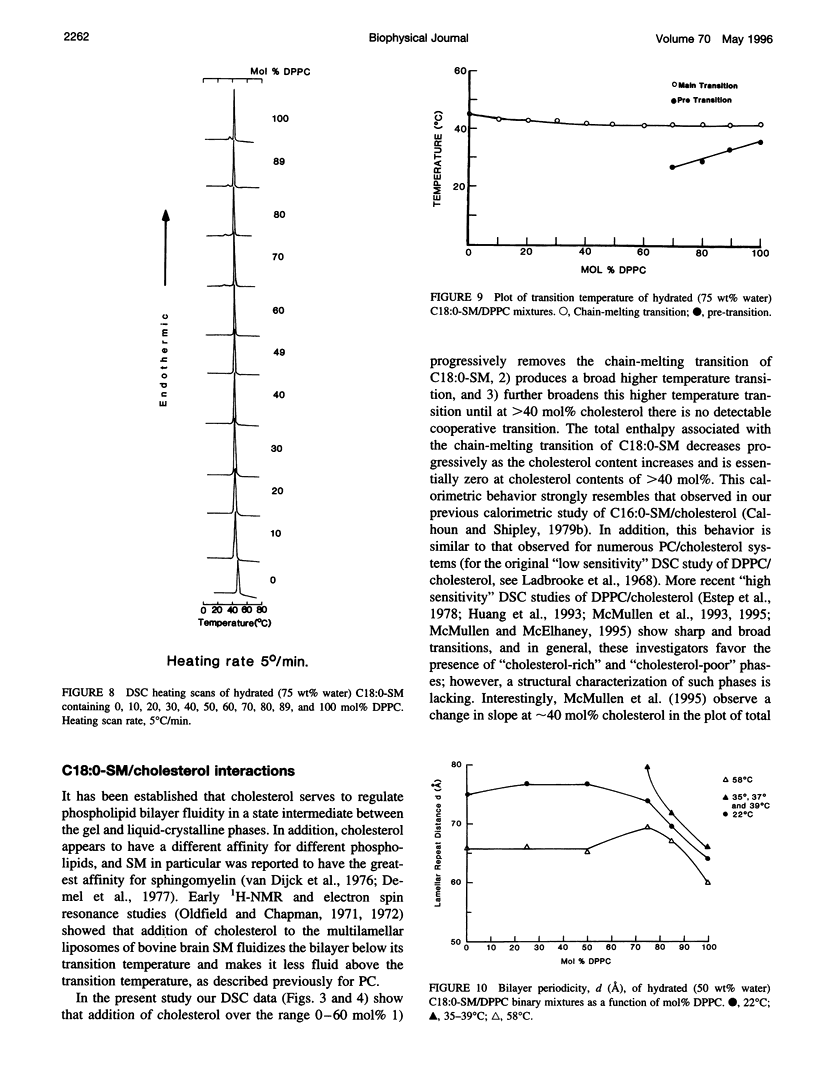
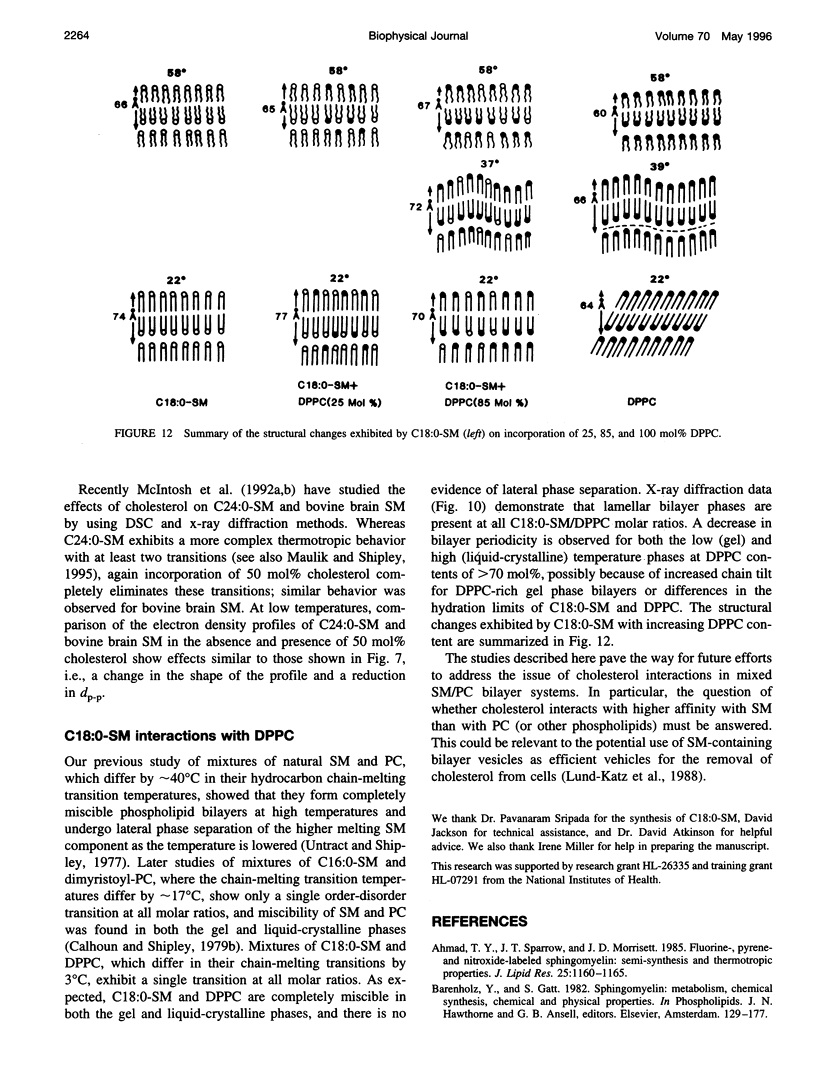
Differential scanning calorimetry and x-ray diffraction have been utilized to investigate the interaction of N-stearoylsphingomyelin (C18:0-SM) with cholesterol and dipalmitoylphosphatidylcholine (DPPC). Fully hydrated C18:0-SM forms bilayers that undergo a chain-melting (gel -->liquid-crystalline) transition at 45 degrees C, delta H = 6.7 kcal/mol. Addition of cholesterol results in a progressive decrease in the enthalpy of the transition at 45 degrees C and the appearance of a broad transition centered at 46.3 degrees C; this latter transition progressively broadens and is not detectable at cholesterol contents of >40 mol%. X-ray diffraction and electron density profiles indicate that bilayers of C18:0-SM/cholesterol (50 mol%) are essentially identical at 22 degrees C and 58 degrees C in terms of bilayer periodicity (d = 63-64 A), bilayer thickness (d rho-p = 46-47 A), and lateral molecular packing (wide-angle reflection, 1/4.8 A-(1)). These data show that cholesterol inserts into C18:0-SM bilayers, progressively removing the chain-melting transition and altering the bilayer structural characteristics. In contrast, DPPC has relatively minor effects on the structure and thermotropic properties of C18:0-SM. DPPC and C18:0-SM exhibit complete miscibility in both the gel and liquid-crystalline bilayer phases, but the pre-transition exhibited by DPPC is eliminated at >30 mol% C18:0-SM. The bilayer periodicity in both the gel and liquid-crystalline phases decreases significantly at high DPPC contents, probably reflecting differences in hydration and/or chain tilt (gel phase) of C18:0-SM and DPPC.
Full text
PDF

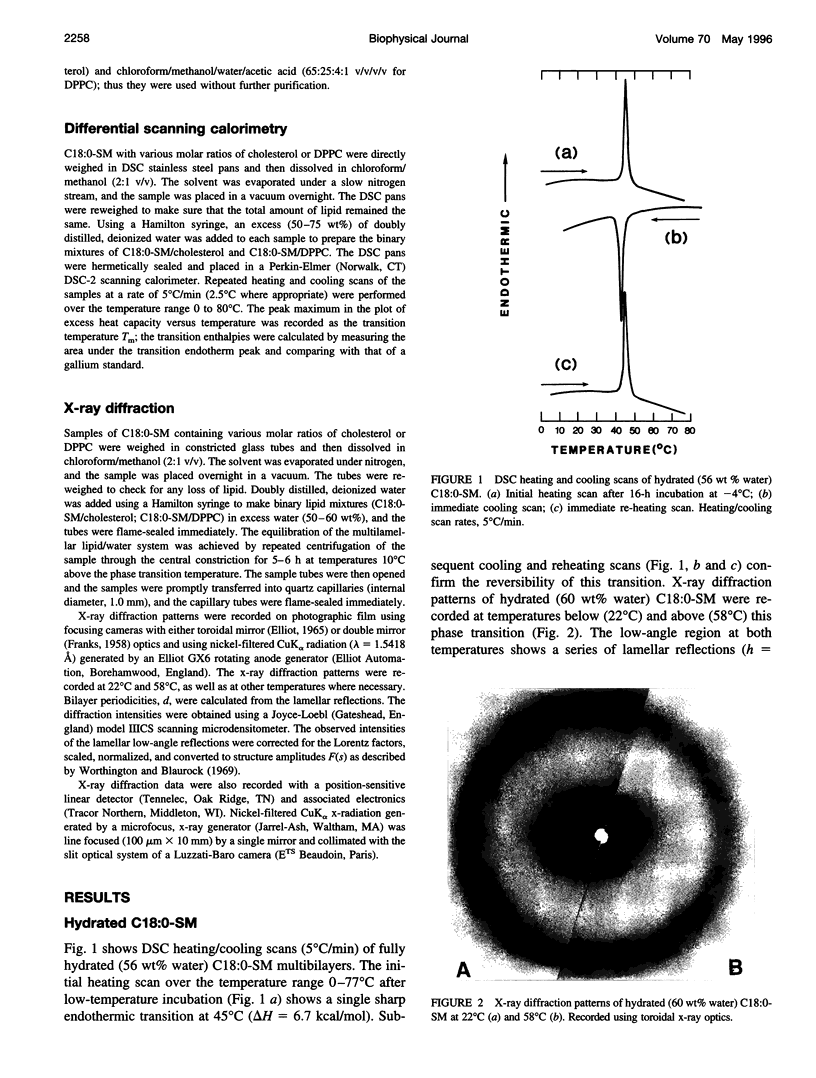
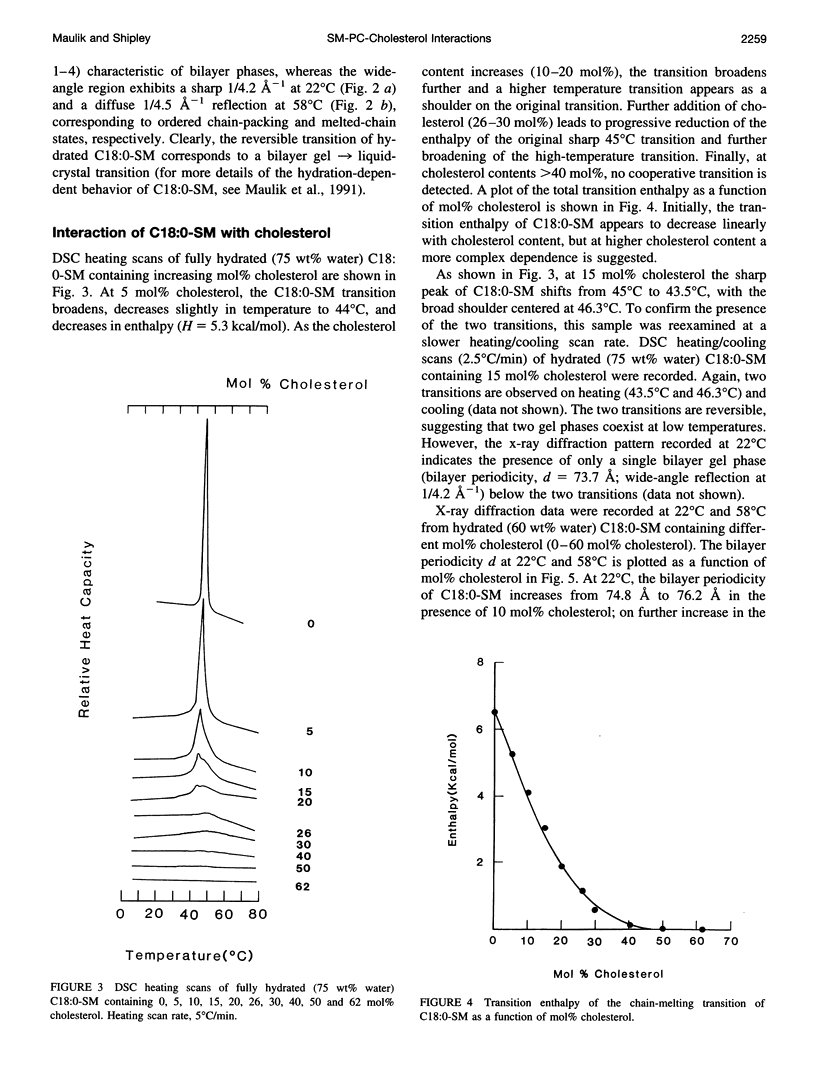


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmad T. Y., Sparrow J. T., Morrisett J. D. Fluorine-, pyrene-, and nitroxide-labeled sphingomyelin: semi-synthesis and thermotropic properties. J Lipid Res. 1985 Sep;26(9):1160–1165. [PubMed] [Google Scholar]
- Barenholz Y., Suurkuusk J., Mountcastle D., Thompson T. E., Biltonen R. L. A calorimetric study of the thermotropic behavior of aqueous dispersions of natural and synthetic sphingomyelins. Biochemistry. 1976 Jun 1;15(11):2441–2447. doi: 10.1021/bi00656a030. [DOI] [PubMed] [Google Scholar]
- Barenholz Y., Thompson T. E. Sphingomyelins in bilayers and biological membranes. Biochim Biophys Acta. 1980 Sep 30;604(2):129–158. doi: 10.1016/0005-2736(80)90572-6. [DOI] [PubMed] [Google Scholar]
- Bruzik K. S. Conformation of the polar headgroup of sphingomyelin and its analogues. Biochim Biophys Acta. 1988 Apr 7;939(2):315–326. doi: 10.1016/0005-2736(88)90076-4. [DOI] [PubMed] [Google Scholar]
- Bruzik K. S., Sobon B., Salamonczyk G. M. Nuclear magnetic resonance study of sphingomyelin bilayers. Biochemistry. 1990 Apr 24;29(16):4017–4021. doi: 10.1021/bi00468a032. [DOI] [PubMed] [Google Scholar]
- Bruzik K. S., Tsai M. D. A calorimetric study of the thermotropic behavior of pure sphingomyelin diastereomers. Biochemistry. 1987 Aug 25;26(17):5364–5368. doi: 10.1021/bi00391a022. [DOI] [PubMed] [Google Scholar]
- Calhoun W. I., Shipley G. G. Fatty acid composition and thermal behavior of natural sphingomyelins. Biochim Biophys Acta. 1979 Aug 23;555(3):436–441. doi: 10.1016/0005-2736(79)90397-3. [DOI] [PubMed] [Google Scholar]
- Calhoun W. I., Shipley G. G. Sphingomyelin--lecithin bilayers and their interaction with cholesterol. Biochemistry. 1979 May 1;18(9):1717–1722. doi: 10.1021/bi00576a013. [DOI] [PubMed] [Google Scholar]
- Cohen R., Barenholz Y., Gatt S., Dagan A. Preparation and characterization of well defined D-erythro sphingomyelins. Chem Phys Lipids. 1984 Oct;35(4):371–384. doi: 10.1016/0009-3084(84)90079-3. [DOI] [PubMed] [Google Scholar]
- Demel R. A., Jansen J. W., van Dijck P. W., van Deenen L. L. The preferential interaction of cholesterol with different classes of phospholipids. Biochim Biophys Acta. 1977 Feb 14;465(1):1–10. doi: 10.1016/0005-2736(77)90350-9. [DOI] [PubMed] [Google Scholar]
- Dong Z., Butcher J. A., Jr An efficient route to N-palmitoyl-D-erythro-sphingomyelin and its 13C-labeled derivatives. Chem Phys Lipids. 1993 Nov;66(1-2):41–46. doi: 10.1016/0009-3084(93)90029-3. [DOI] [PubMed] [Google Scholar]
- Estep T. N., Calhoun W. I., Barenholz Y., Biltonen R. L., Shipley G. G., Thompson T. E. Evidence for metastability in stearoylsphingomyelin bilayers. Biochemistry. 1980 Jan 8;19(1):20–24. doi: 10.1021/bi00542a004. [DOI] [PubMed] [Google Scholar]
- Estep T. N., Freire E., Anthony F., Barenholz Y., Biltonen R. L., Thompson T. E. Thermal behavior of stearoylsphingomyelin-cholesterol dispersions. Biochemistry. 1981 Dec 8;20(25):7115–7118. doi: 10.1021/bi00528a010. [DOI] [PubMed] [Google Scholar]
- Estep T. N., Mountcastle D. B., Barenholz Y., Biltonen R. L., Thompson T. E. Thermal behavior of synthetic sphingomyelin-cholesterol dispersions. Biochemistry. 1979 May 15;18(10):2112–2117. doi: 10.1021/bi00577a042. [DOI] [PubMed] [Google Scholar]
- Estep T. N., Mountcastle D. B., Biltonen R. L., Thompson T. E. Studies on the anomalous thermotropic behavior of aqueous dispersions of dipalmitoylphosphatidylcholine-cholesterol mixtures. Biochemistry. 1978 May 16;17(10):1984–1989. doi: 10.1021/bi00603a029. [DOI] [PubMed] [Google Scholar]
- Hannun Y. A., Obeid L. M. Ceramide: an intracellular signal for apoptosis. Trends Biochem Sci. 1995 Feb;20(2):73–77. doi: 10.1016/s0968-0004(00)88961-6. [DOI] [PubMed] [Google Scholar]
- Hannun Y. A. The sphingomyelin cycle and the second messenger function of ceramide. J Biol Chem. 1994 Feb 4;269(5):3125–3128. [PubMed] [Google Scholar]
- Huang T. H., Lee C. W., Das Gupta S. K., Blume A., Griffin R. G. A 13C and 2H nuclear magnetic resonance study of phosphatidylcholine/cholesterol interactions: characterization of liquid-gel phases. Biochemistry. 1993 Dec 7;32(48):13277–13287. doi: 10.1021/bi00211a041. [DOI] [PubMed] [Google Scholar]
- Ladbrooke B. D., Williams R. M., Chapman D. Studies on lecithin-cholesterol-water interactions by differential scanning calorimetry and X-ray diffraction. Biochim Biophys Acta. 1968 Apr 29;150(3):333–340. doi: 10.1016/0005-2736(68)90132-6. [DOI] [PubMed] [Google Scholar]
- Long R. A., Hruska F. E., Gesser H. D. Phase transitions in sphingomyelin thin filsm. A spin label study. Biochem Biophys Res Commun. 1971 Oct 1;45(1):167–173. doi: 10.1016/0006-291x(71)90065-9. [DOI] [PubMed] [Google Scholar]
- Lund-Katz S., Laboda H. M., McLean L. R., Phillips M. C. Influence of molecular packing and phospholipid type on rates of cholesterol exchange. Biochemistry. 1988 May 3;27(9):3416–3423. doi: 10.1021/bi00409a044. [DOI] [PubMed] [Google Scholar]
- Maulik P. R., Atkinson D., Shipley G. G. X-ray scattering of vesicles of N-acyl sphingomyelins. Determination of bilayer thickness. Biophys J. 1986 Dec;50(6):1071–1077. doi: 10.1016/S0006-3495(86)83551-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maulik P. R., Shipley G. G. X-ray diffraction and calorimetric study of N-lignoceryl sphingomyelin membranes. Biophys J. 1995 Nov;69(5):1909–1916. doi: 10.1016/S0006-3495(95)80061-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maulik P. R., Sripada P. K., Shipley G. G. Structure and thermotropic properties of hydrated N-stearoyl sphingomyelin bilayer membranes. Biochim Biophys Acta. 1991 Feb 25;1062(2):211–219. doi: 10.1016/0005-2736(91)90395-o. [DOI] [PubMed] [Google Scholar]
- McIntosh T. J., Simon S. A., Needham D., Huang C. H. Interbilayer interactions between sphingomyelin and sphingomyelin/cholesterol bilayers. Biochemistry. 1992 Feb 25;31(7):2020–2024. doi: 10.1021/bi00122a018. [DOI] [PubMed] [Google Scholar]
- McIntosh T. J., Simon S. A., Needham D., Huang C. H. Structure and cohesive properties of sphingomyelin/cholesterol bilayers. Biochemistry. 1992 Feb 25;31(7):2012–2020. doi: 10.1021/bi00122a017. [DOI] [PubMed] [Google Scholar]
- McIntosh T. J. The effect of cholesterol on the structure of phosphatidylcholine bilayers. Biochim Biophys Acta. 1978 Oct 19;513(1):43–58. doi: 10.1016/0005-2736(78)90110-4. [DOI] [PubMed] [Google Scholar]
- McMullen T. P., Lewis R. N., McElhaney R. N. Differential scanning calorimetric study of the effect of cholesterol on the thermotropic phase behavior of a homologous series of linear saturated phosphatidylcholines. Biochemistry. 1993 Jan 19;32(2):516–522. doi: 10.1021/bi00053a016. [DOI] [PubMed] [Google Scholar]
- McMullen T. P., McElhaney R. N. New aspects of the interaction of cholesterol with dipalmitoylphosphatidylcholine bilayers as revealed by high-sensitivity differential scanning calorimetry. Biochim Biophys Acta. 1995 Mar 8;1234(1):90–98. doi: 10.1016/0005-2736(94)00266-r. [DOI] [PubMed] [Google Scholar]
- McMullen T. P., Vilchèze C., McElhaney R. N., Bittman R. Differential scanning calorimetric study of the effect of sterol side chain length and structure on dipalmitoylphosphatidylcholine thermotropic phase behavior. Biophys J. 1995 Jul;69(1):169–176. doi: 10.1016/S0006-3495(95)79887-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieva J. L., Bron R., Corver J., Wilschut J. Membrane fusion of Semliki Forest virus requires sphingolipids in the target membrane. EMBO J. 1994 Jun 15;13(12):2797–2804. doi: 10.1002/j.1460-2075.1994.tb06573.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield E., Chapman D. Effects of cholesterol and cholesterol derivatives on hydrocarbon chain mobility in lipids. Biochem Biophys Res Commun. 1971 May 7;43(3):610–616. doi: 10.1016/0006-291x(71)90658-9. [DOI] [PubMed] [Google Scholar]
- Oldfield E., Chapman D. Molecular dynamics of cerebroside-cholesterol and sphingomyelin-cholesterol interactions: Implications for myelin membrane structure. FEBS Lett. 1972 Apr 1;21(3):303–306. doi: 10.1016/0014-5793(72)80189-3. [DOI] [PubMed] [Google Scholar]
- Shipley G. G., Avecilla L. S., Small D. M. Phase behavior and structure of aqueous dispersions of sphingomyelin. J Lipid Res. 1974 Mar;15(2):124–131. [PubMed] [Google Scholar]
- Sripada P. K., Maulik P. R., Hamilton J. A., Shipley G. G. Partial synthesis and properties of a series of N-acyl sphingomyelins. J Lipid Res. 1987 Jun;28(6):710–718. [PubMed] [Google Scholar]
- Untrach S. H., Shipley G. G. Molecular interactions between lecithin and sphingomyelin. Temperature- and composition-dependent phase separation. J Biol Chem. 1977 Jul 10;252(13):4449–4457. [PubMed] [Google Scholar]
- Van Dijck P. W., De Kruijff B., Van Deenen L. L., De Gier J., Demel R. A. The preference of cholesterol for phosphatidylcholine in mixed phosphatidylcholine-phosphatidylethanolamine bilayers. Biochim Biophys Acta. 1976 Dec 2;455(2):576–587. doi: 10.1016/0005-2736(76)90326-6. [DOI] [PubMed] [Google Scholar]
- Worthington C. R., Blaurock A. E. A structural analysis of nerve myelin. Biophys J. 1969 Jul;9(7):970–990. doi: 10.1016/S0006-3495(69)86431-3. [DOI] [PMC free article] [PubMed] [Google Scholar]