Abstract
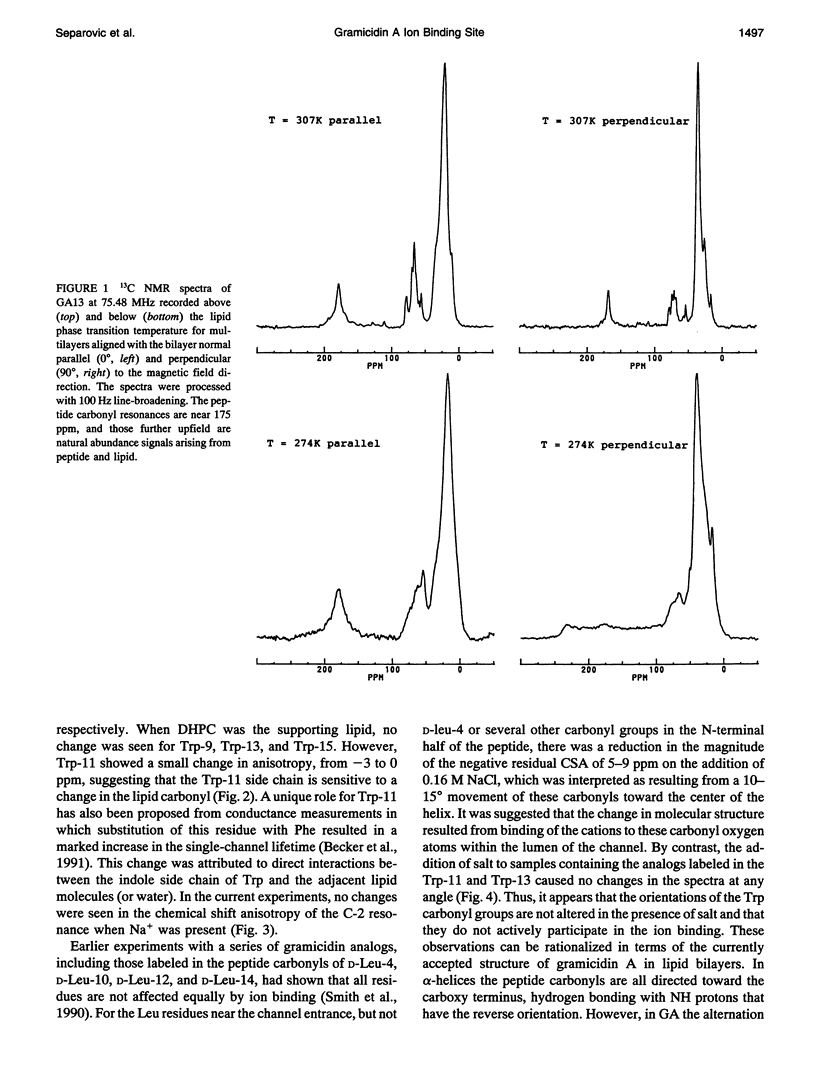
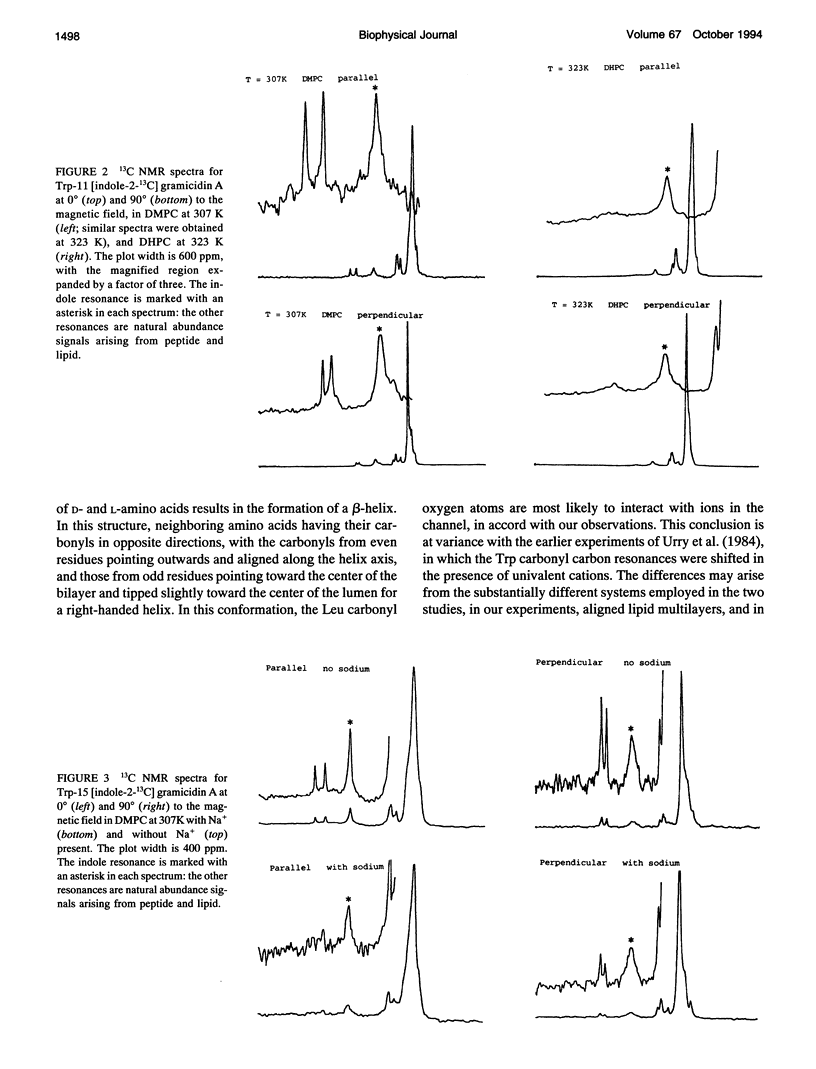
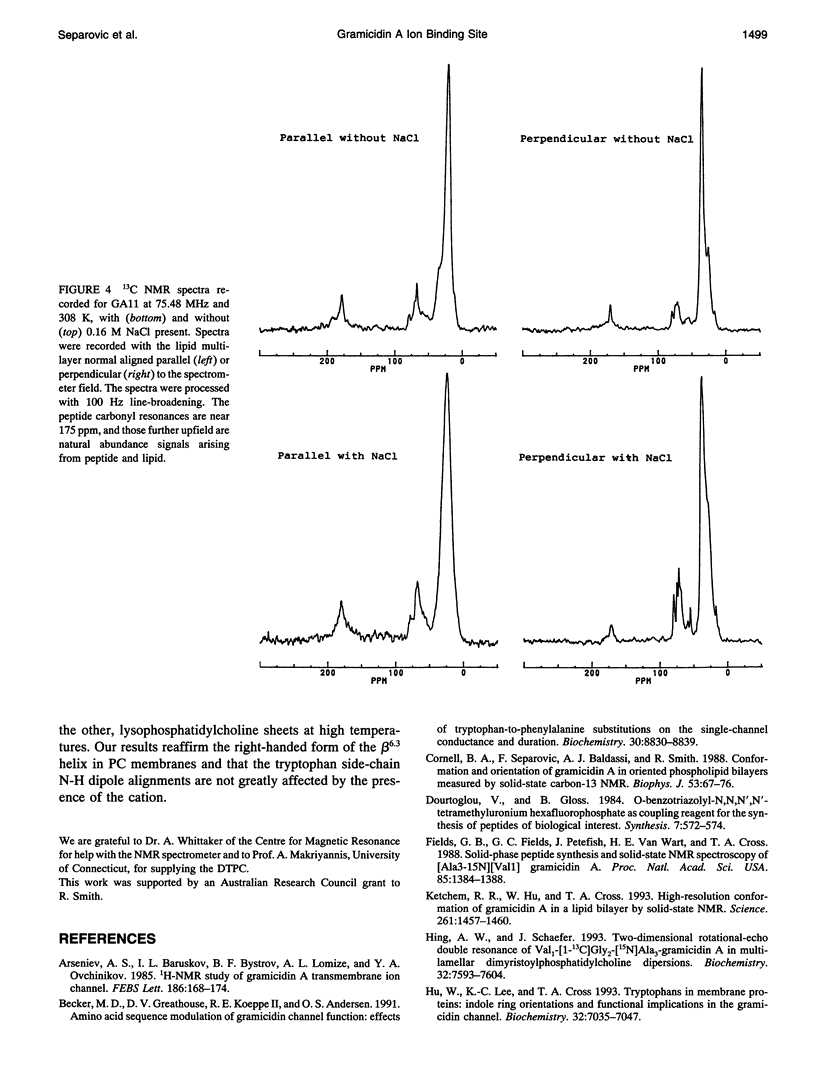
Gramicidin A analogs, labeled with 13C in the backbone carbonyl groups and the C-2 indole carbons of the tryptophan-11 and tryptophan-13 residues, were synthesized using t-Boc-protected amino acids. The purified analogs were incorporated into phosphatidylcholine bilayers at a 1:15 molar ratio and macroscopically aligned between glass coverslips. The orientations of the labeled groups within the channel were investigated using solid-state NMR and the effect of a monovalent ion (Na+) on the orientation of these groups determined. The presence of sodium ions did not perturb the 13C spectra of the tryptophan carbonyl groups. These results contrast with earlier results in which the Leu-10, Leu-12, and Leu-14 carbonyl groups were found to be significantly affected by the presence of sodium ions and imply that the tryptophan carbonyl groups are not directly involved in ion binding. The channel form of gramicidin A has been demonstrated to be the right-handed form of the beta 6.3 helix: consequently, the tryptophan carbonyls would be directed away from the entrance to the channel and take part in internal hydrogen bonding, so that the presence of cations in the channel would have less effect than on the outer leucine residues. Sodium ions also had no effect on the C-2 indole resonance of the tryptophan side chains. However, a small change was observed in Trp-11 when the ether lipid, ditetradecylphosphatidylcholine, was substituted for the ester lipid, dimyristoylphosphatidylcholine, indicating some sensitivity of the gramicidin side chains to the surrounding lipid.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arseniev A. S., Barsukov I. L., Bystrov V. F., Lomize A. L., Ovchinnikov YuA 1H-NMR study of gramicidin A transmembrane ion channel. Head-to-head right-handed, single-stranded helices. FEBS Lett. 1985 Jul 8;186(2):168–174. doi: 10.1016/0014-5793(85)80702-x. [DOI] [PubMed] [Google Scholar]
- Becker M. D., Greathouse D. V., Koeppe R. E., 2nd, Andersen O. S. Amino acid sequence modulation of gramicidin channel function: effects of tryptophan-to-phenylalanine substitutions on the single-channel conductance and duration. Biochemistry. 1991 Sep 10;30(36):8830–8839. doi: 10.1021/bi00100a015. [DOI] [PubMed] [Google Scholar]
- Cornell B. A., Separovic F., Baldassi A. J., Smith R. Conformation and orientation of gramicidin a in oriented phospholipid bilayers measured by solid state carbon-13 NMR. Biophys J. 1988 Jan;53(1):67–76. doi: 10.1016/S0006-3495(88)83066-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields G. B., Fields C. G., Petefish J., Van Wart H. E., Cross T. A. Solid-phase peptide synthesis and solid-state NMR spectroscopy of [Ala3-15N][Val1]gramicidin A. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1384–1388. doi: 10.1073/pnas.85.5.1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hing A. W., Schaefer J. Two-dimensional rotational-echo double resonance of Val1-[1-13C]Gly2-[15N]Ala3-gramicidin A in multilamellar dimyristoylphosphatidylcholine dispersions. Biochemistry. 1993 Jul 27;32(29):7593–7604. doi: 10.1021/bi00080a035. [DOI] [PubMed] [Google Scholar]
- Hu W., Lee K. C., Cross T. A. Tryptophans in membrane proteins: indole ring orientations and functional implications in the gramicidin channel. Biochemistry. 1993 Jul 13;32(27):7035–7047. doi: 10.1021/bi00078a032. [DOI] [PubMed] [Google Scholar]
- Ketchem R. R., Hu W., Cross T. A. High-resolution conformation of gramicidin A in a lipid bilayer by solid-state NMR. Science. 1993 Sep 10;261(5127):1457–1460. doi: 10.1126/science.7690158. [DOI] [PubMed] [Google Scholar]
- Mai W., Hu W., Wang C., Cross T. A. Orientational constraints as three-dimensional structural constraints from chemical shift anisotropy: the polypeptide backbone of gramicidin A in a lipid bilayer. Protein Sci. 1993 Apr;2(4):532–542. doi: 10.1002/pro.5560020405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazet J. L., Andersen O. S., Koeppe R. E., 2nd Single-channel studies on linear gramicidins with altered amino acid sequences. A comparison of phenylalanine, tryptophane, and tyrosine substitutions at positions 1 and 11. Biophys J. 1984 Jan;45(1):263–276. doi: 10.1016/S0006-3495(84)84153-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson L. K., Moll F., Mixon T. E., LoGrasso P. V., Lay J. C., Cross T. A. Solid-state 15N NMR of oriented lipid bilayer bound gramicidin A'. Biochemistry. 1987 Oct 20;26(21):6621–6626. doi: 10.1021/bi00395a009. [DOI] [PubMed] [Google Scholar]
- O'Connell A. M., Koeppe R. E., 2nd, Andersen O. S. Kinetics of gramicidin channel formation in lipid bilayers: transmembrane monomer association. Science. 1990 Nov 30;250(4985):1256–1259. doi: 10.1126/science.1700867. [DOI] [PubMed] [Google Scholar]
- Olah G. A., Huang H. W., Liu W. H., Wu Y. L. Location of ion-binding sites in the gramicidin channel by X-ray diffraction. J Mol Biol. 1991 Apr 20;218(4):847–858. doi: 10.1016/0022-2836(91)90272-8. [DOI] [PubMed] [Google Scholar]
- Prosser R. S., Davis J. H., Dahlquist F. W., Lindorfer M. A. 2H nuclear magnetic resonance of the gramicidin A backbone in a phospholipid bilayer. Biochemistry. 1991 May 14;30(19):4687–4696. doi: 10.1021/bi00233a008. [DOI] [PubMed] [Google Scholar]
- Ruocco M. J., Makriyannis A., Siminovitch D. J., Griffin R. G. Deuterium NMR investigation of ether- and ester-linked phosphatidylcholine bilayers. Biochemistry. 1985 Aug 27;24(18):4844–4851. doi: 10.1021/bi00339a018. [DOI] [PubMed] [Google Scholar]
- Sarin V. K., Kent S. B., Tam J. P., Merrifield R. B. Quantitative monitoring of solid-phase peptide synthesis by the ninhydrin reaction. Anal Biochem. 1981 Oct;117(1):147–157. doi: 10.1016/0003-2697(81)90704-1. [DOI] [PubMed] [Google Scholar]
- Sawyer D. B., Williams L. P., Whaley W. L., Koeppe R. E., 2nd, Andersen O. S. Gramicidins A, B, and C form structurally equivalent ion channels. Biophys J. 1990 Nov;58(5):1207–1212. doi: 10.1016/S0006-3495(90)82461-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R., Thomas D. E., Atkins A. R., Separovic F., Cornell B. A. Solid-state 13C-NMR studies of the effects of sodium ions on the gramicidin A ion channel. Biochim Biophys Acta. 1990 Jul 24;1026(2):161–166. doi: 10.1016/0005-2736(90)90059-w. [DOI] [PubMed] [Google Scholar]
- Smith R., Thomas D. E., Separovic F., Atkins A. R., Cornell B. A. Determination of the structure of a membrane-incorporated ion channel. Solid-state nuclear magnetic resonance studies of gramicidin A. Biophys J. 1989 Aug;56(2):307–314. doi: 10.1016/S0006-3495(89)82677-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urry D. W., Alonso-Romanowski S., Venkatachalam C. M., Bradley R. J., Harris R. D. Temperature dependence of single channel currents and the peptide libration mechanism for ion transport through the gramicidin A transmembrane channel. J Membr Biol. 1984;81(3):205–217. doi: 10.1007/BF01868714. [DOI] [PubMed] [Google Scholar]
- Urry D. W., Alonso-Romanowski S., Venkatachalam C. M., Trapane T. L., Harris R. D., Prasad K. U. Shortened analog of the gramicidin A channel argues for the doubly occupied channel as the dominant conducting state. Biochim Biophys Acta. 1984 Aug 8;775(1):115–119. doi: 10.1016/0005-2736(84)90242-6. [DOI] [PubMed] [Google Scholar]
- Urry D. W., Prasad K. U., Trapane T. L. Location of monovalent cation binding sites in the gramicidin channel. Proc Natl Acad Sci U S A. 1982 Jan;79(2):390–394. doi: 10.1073/pnas.79.2.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urry D. W., Trapane T. L., Prasad K. U. Is the gramicidin a transmembrane channel single-stranded or double-stranded helix? A simple unequivocal determination. Science. 1983 Sep 9;221(4615):1064–1067. doi: 10.1126/science.221.4615.1064. [DOI] [PubMed] [Google Scholar]
- Urry D. W., Trapane T. L., Romanowski S., Bradley R. J., Prasad K. U. Use of synthetic gramicidins in the determination of channel structure and mechanism. Int J Pept Protein Res. 1983 Jan;21(1):16–23. doi: 10.1111/j.1399-3011.1983.tb03073.x. [DOI] [PubMed] [Google Scholar]
- Urry D. W., Trapane T. L., Walker J. T., Prasad K. U. On the relative lipid membrane permeability of Na+ and Ca2+. A physical basis for the messenger role of Ca2+. J Biol Chem. 1982 Jun 25;257(12):6659–6661. [PubMed] [Google Scholar]
- Urry D. W., Venkatachalam C. M., Spisni A., Bradley R. J., Trapane T. L., Prasad K. U. The malonyl gramicidin channel: NMR-derived rate constants and comparison of calculated and experimental single-channel currents. J Membr Biol. 1980 Jun 30;55(1):29–51. doi: 10.1007/BF01926368. [DOI] [PubMed] [Google Scholar]
- Urry D. W., Walker J. T., Trapane T. L. Ion interactions in (1-13C)D-Val8 and D-Leu14 analogs of gramicidin A, the helix sense of the channel and location of ion binding sites. J Membr Biol. 1982;69(3):225–231. doi: 10.1007/BF01870401. [DOI] [PubMed] [Google Scholar]


