Abstract
In order to clarify by what mechanism the lipid bilayer membrane changes its potential under the stimulation of bitter substances, a microscopic model for the effects of the substances on the membrane is presented and studied theoretically. It is assumed that the substances are adsorbed on the membrane and change the partition coefficients of ions between the membrane and the stimulation solution, the dipole orientation in the polar head, and the diffusion constants of ions in the membrane. It is shown, based on the comparison of the calculated results with the experimental ones, that the response arises mainly from a change in the partition coefficients. Protons play an essential role in the membrane potential variation due to the change in their partition coefficients. The present model reproduces the following observed unique properties in the response of lipid bilayers to bitter substances, which cannot be accounted for by the usual channel model for the membrane potential: 1) the response of the membrane potential appears even under the condition that there is no ion gradient across the membrane, 2) the response remains even when the salt in the stimulating solution is replaced with the salt made of an impermeable cation, and 3) the direction of the polarization of the potential is not reversed, even when the ion gradient across the bilayer is reversed.
Full text
PDF
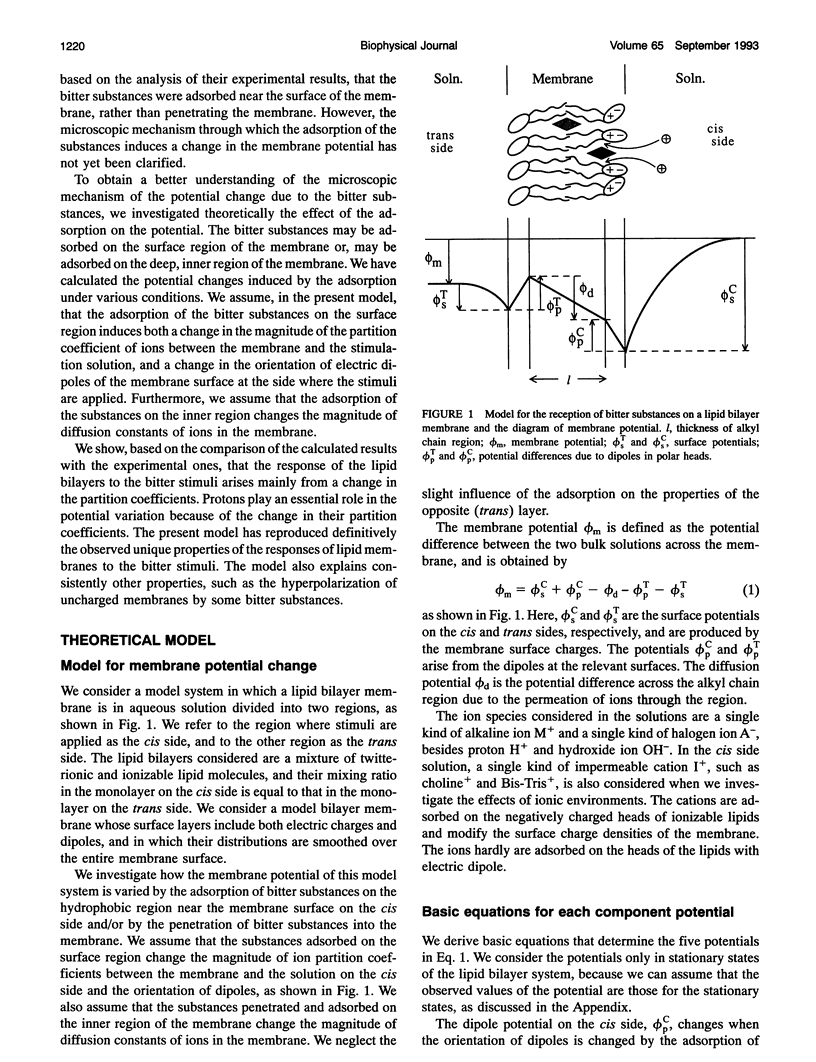



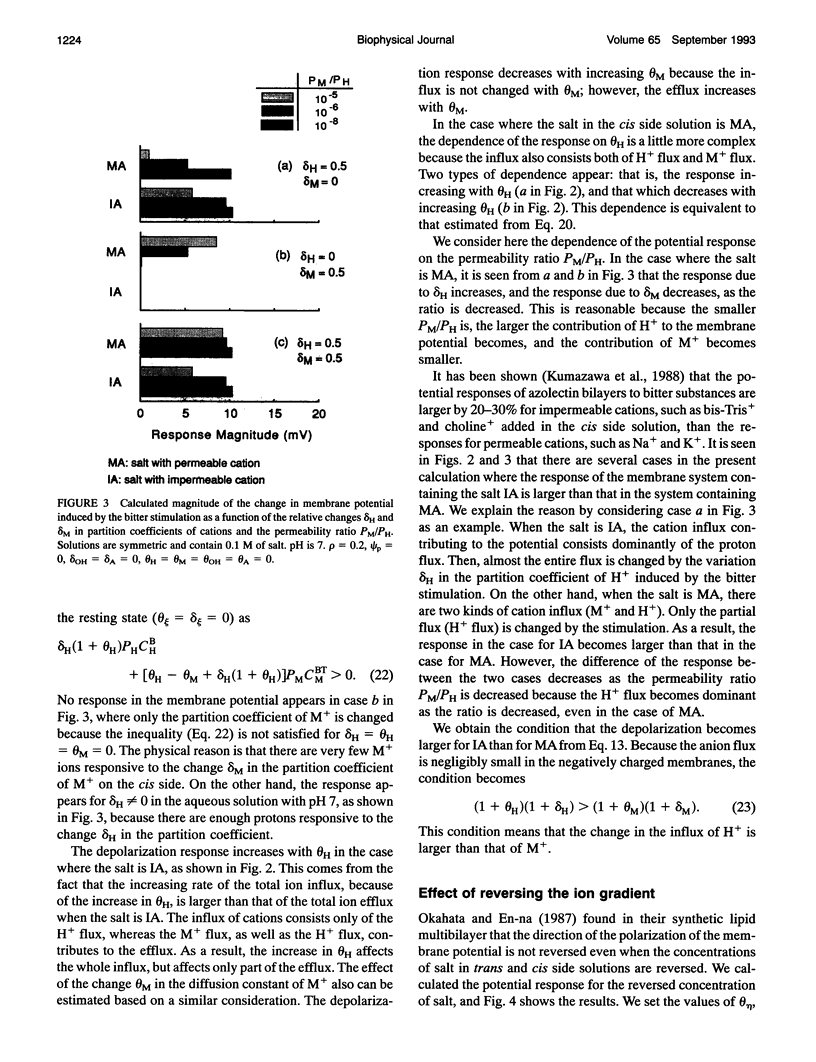



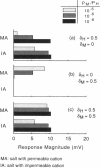
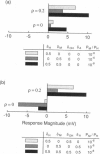
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akabas M. H., Dodd J., Al-Awqati Q. A bitter substance induces a rise in intracellular calcium in a subpopulation of rat taste cells. Science. 1988 Nov 18;242(4881):1047–1050. doi: 10.1126/science.3194756. [DOI] [PubMed] [Google Scholar]
- Akaike N., Noma A., Sato M. Electrical responses to frog taste cells to chemical stimuli. J Physiol. 1976 Jan;254(1):87–107. doi: 10.1113/jphysiol.1976.sp011223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avenet P., Lindemann B. Patch-clamp study of isolated taste receptor cells of the frog. J Membr Biol. 1987;97(3):223–240. doi: 10.1007/BF01869225. [DOI] [PubMed] [Google Scholar]
- Cafiso D. S., Hubbell W. L. Electrogenic H+/OH- movement across phospholipid vesicles measured by spin-labeled hydrophobic ions. Biophys J. 1983 Oct;44(1):49–57. doi: 10.1016/S0006-3495(83)84276-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deamer D. W., Nichols J. W. Proton-hydroxide permeability of liposomes. Proc Natl Acad Sci U S A. 1983 Jan;80(1):165–168. doi: 10.1073/pnas.80.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman D. E. POTENTIAL, IMPEDANCE, AND RECTIFICATION IN MEMBRANES. J Gen Physiol. 1943 Sep 20;27(1):37–60. doi: 10.1085/jgp.27.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grzesiek S., Dencher N. A. Dependency of delta pH-relaxation across vesicular membranes on the buffering power of bulk solutions and lipids. Biophys J. 1986 Aug;50(2):265–276. doi: 10.1016/S0006-3495(86)83460-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., KATZ B. The effect of sodium ions on the electrical activity of giant axon of the squid. J Physiol. 1949 Mar 1;108(1):37–77. doi: 10.1113/jphysiol.1949.sp004310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser H., Oldani D., Phillips M. C. Mechanism of ion escape from phosphatidylcholine and phosphatidylserine single bilayer vesicles. Biochemistry. 1973 Oct 23;12(22):4507–4517. doi: 10.1021/bi00746a032. [DOI] [PubMed] [Google Scholar]
- Johnson S. M., Bangham A. D. Potassium permeability of single compartment liposomes with and without valinomycin. Biochim Biophys Acta. 1969 Oct 14;193(1):82–91. doi: 10.1016/0005-2736(69)90061-3. [DOI] [PubMed] [Google Scholar]
- Kell D. B., Morris J. G. Formulation and some biological uses of a buffer mixture whose buffering capacity is relatively independent of pH in the range pH 4-9. J Biochem Biophys Methods. 1980 Sep;3(3):143–150. doi: 10.1016/0165-022x(80)90013-5. [DOI] [PubMed] [Google Scholar]
- Kinnamon S. C., Roper S. D. Membrane properties of isolated mudpuppy taste cells. J Gen Physiol. 1988 Mar;91(3):351–371. doi: 10.1085/jgp.91.3.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnamoorthy G., Hinkle P. C. Non-ohmic proton conductance of mitochondria and liposomes. Biochemistry. 1984 Apr 10;23(8):1640–1645. doi: 10.1021/bi00303a009. [DOI] [PubMed] [Google Scholar]
- Kumazawa T., Kashiwayanagi M., Kurihara K. Contribution of electrostatic and hydrophobic interactions of bitter substances with taste receptor membranes to generation of receptor potentials. Biochim Biophys Acta. 1986 Aug 29;888(1):62–69. doi: 10.1016/0167-4889(86)90071-6. [DOI] [PubMed] [Google Scholar]
- Kumazawa T., Kashiwayanagi M., Kurihara K. Neuroblastoma cell as a model for a taste cell: mechanism of depolarization in response to various bitter substances. Brain Res. 1985 Apr 29;333(1):27–33. doi: 10.1016/0006-8993(85)90120-9. [DOI] [PubMed] [Google Scholar]
- Kumazawa T., Nomura T., Kurihara K. Liposomes as model for taste cells: receptor sites for bitter substances including N-C=S substances and mechanism of membrane potential changes. Biochemistry. 1988 Feb 23;27(4):1239–1244. doi: 10.1021/bi00404a025. [DOI] [PubMed] [Google Scholar]
- Kurihara K., Yoshii K., Kashiwayanagi M. Transduction mechanisms in chemoreception. Comp Biochem Physiol A Comp Physiol. 1986;85(1):1–22. doi: 10.1016/0300-9629(86)90455-x. [DOI] [PubMed] [Google Scholar]
- Kurihara Y. Effect of taste stimuli on the extraction of lipids from bovine taste papillae. Biochim Biophys Acta. 1973 Jun 21;306(3):478–482. doi: 10.1016/0005-2760(73)90186-0. [DOI] [PubMed] [Google Scholar]
- MacDonald R. C., Simon S. A., Baer E. Ionic influences on the phase transition of dipalmitoylphosphatidylserine. Biochemistry. 1976 Feb 24;15(4):885–891. doi: 10.1021/bi00649a025. [DOI] [PubMed] [Google Scholar]
- Naito M., Fuchikami N., Sasaki N., Kambara T. Model for the dynamic responses of taste receptor cells to salty stimuli. I. Function of lipid bilayer membranes. Biophys J. 1991 Jun;59(6):1218–1234. doi: 10.1016/S0006-3495(91)82337-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols J. W., Deamer D. W. Net proton-hydroxyl permeability of large unilamellar liposomes measured by an acid-base titration technique. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2038–2042. doi: 10.1073/pnas.77.4.2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols J. W., Hill M. W., Bangham A. D., Deamer D. W. Measurement of net proton-hydroxyl permeability of large unilamellar liposomes with the fluorescent pH probe, 9-aminoacridine. Biochim Biophys Acta. 1980 Mar 13;596(3):393–403. doi: 10.1016/0005-2736(80)90126-1. [DOI] [PubMed] [Google Scholar]
- Nomura T., Kurihara K. Liposomes as a model for olfactory cells: changes in membrane potential in response to various odorants. Biochemistry. 1987 Sep 22;26(19):6135–6140. doi: 10.1021/bi00393a028. [DOI] [PubMed] [Google Scholar]
- Nozaki Y., Tanford C. Proton and hydroxide ion permeability of phospholipid vesicles. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4324–4328. doi: 10.1073/pnas.78.7.4324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohki S., Kurland R. Surface potential of phosphatidylserine monolayers. II. Divalent and monovalent ion binding. Biochim Biophys Acta. 1981 Jul 20;645(2):170–176. doi: 10.1016/0005-2736(81)90187-5. [DOI] [PubMed] [Google Scholar]
- Okada Y., Miyamoto T., Sato T. Ionic mechanism of generation of receptor potential in response to quinine in frog taste cell. Brain Res. 1988 May 31;450(1-2):295–302. doi: 10.1016/0006-8993(88)91568-5. [DOI] [PubMed] [Google Scholar]
- Ozeki M. Conductance change associated with receptor potentials of gustatory cells in rat. J Gen Physiol. 1971 Dec;58(6):688–699. doi: 10.1085/jgp.58.6.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papahadjopoulos D., Nir S., Oki S. Permeability properties of phospholipid membranes: effect of cholesterol and temperature. Biochim Biophys Acta. 1972 Jun 20;266(3):561–583. doi: 10.1016/0006-3002(72)90001-7. [DOI] [PubMed] [Google Scholar]
- Pike M. M., Simon S. R., Balschi J. A., Springer C. S., Jr High-resolution NMR studies of transmembrane cation transport: use of an aqueous shift reagent for 23Na. Proc Natl Acad Sci U S A. 1982 Feb;79(3):810–814. doi: 10.1073/pnas.79.3.810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Träuble H., Eibl H. Electrostatic effects on lipid phase transitions: membrane structure and ionic environment. Proc Natl Acad Sci U S A. 1974 Jan;71(1):214–219. doi: 10.1073/pnas.71.1.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- el-Mashak E. M., Tsong T. Y. Ion selectivity of temperature-induced and electric field induced pores in dipalmitoylphosphatidylcholine vesicles. Biochemistry. 1985 Jun 4;24(12):2884–2888. doi: 10.1021/bi00333a010. [DOI] [PubMed] [Google Scholar]