Abstract
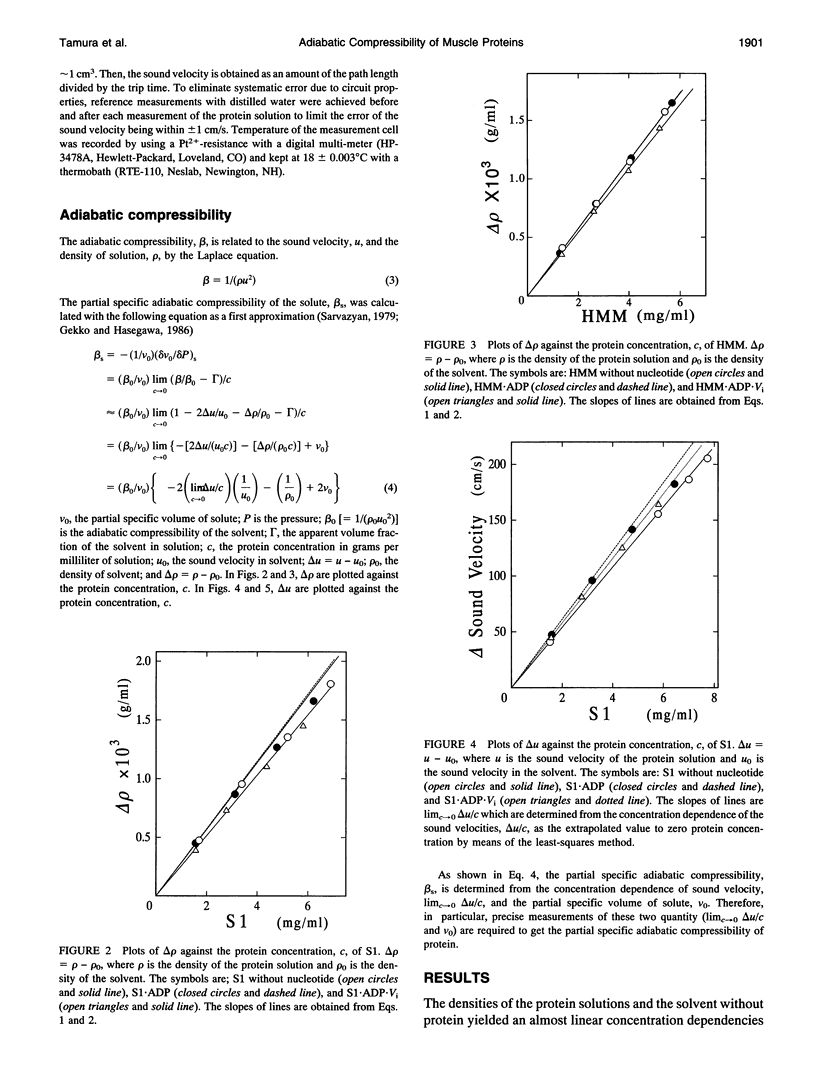
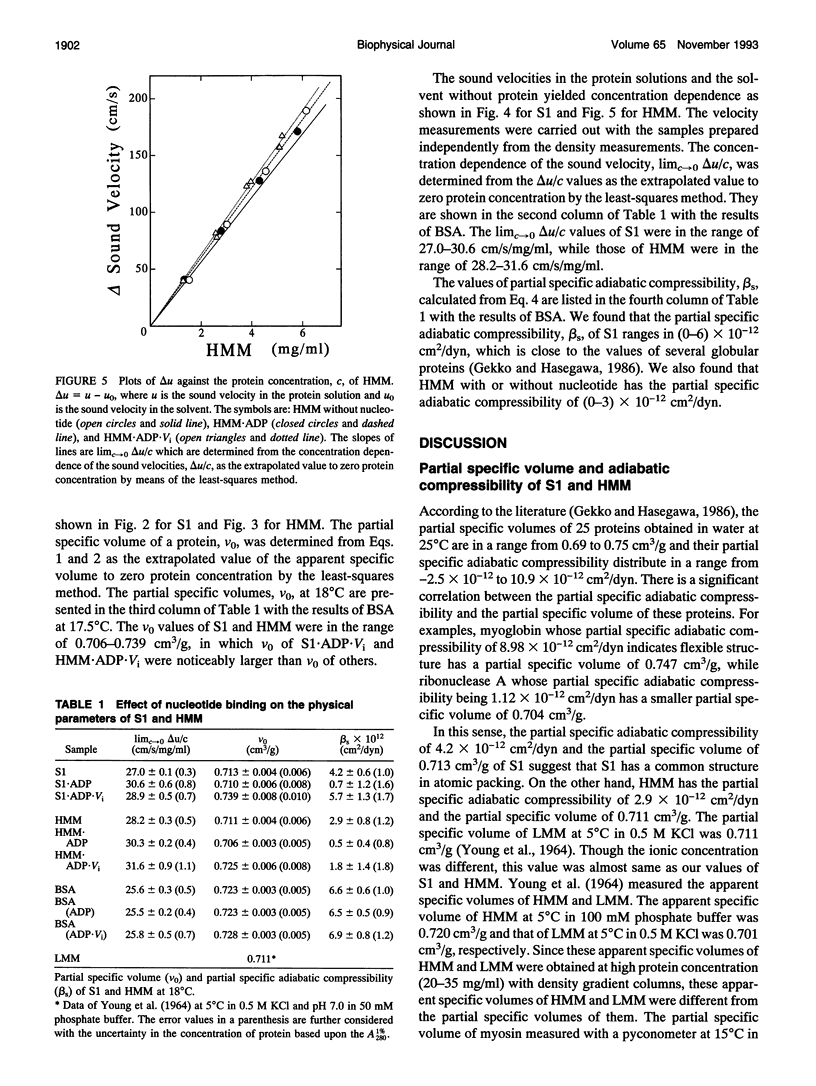
The partial specific adiabatic compressibilities of myosin subfragment-1 (S1) and heavy meromyosin (HMM) of skeletal muscle in solution were determined by measuring the density and the sound velocity of the solution. The partial specific volumes of S1 and HMM were 0.713 and 0.711 cm3/g, respectively. The partial specific adiabatic compressibilities of S1 and HMM were 4.2 x 10(-12) and 2.9 x 10(-12) cm2/dyn, respectively. These values are in the same range as the most of globular proteins so far studied. The result indicates that the flexibility of S1 region almost equals to that of HMM. After binding to ADP.orthovanadate, S1 and HMM became softer than their complexes with ADP. The bulk moduli of S1 and HMM were of the order of (4-6) x 10(10) dyn/cm2, which are very comparable with the bulk modulus of muscle fiber.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aguirre R., Lin S. H., Gonsoulin F., Wang C. K., Cheung H. C. Characterization of the ethenoadenosine diphosphate binding site of myosin subfragment 1. Energetics of the equilibrium between two states of nucleotide.S1 and vanadate-induced global conformation changes detected by energy transfer. Biochemistry. 1989 Jan 24;28(2):799–807. doi: 10.1021/bi00428a058. [DOI] [PubMed] [Google Scholar]
- Applegate D., Flicker P. New states of actomyosin. J Biol Chem. 1987 May 15;262(14):6856–6863. [PubMed] [Google Scholar]
- Brenner B. Mechanical and structural approaches to correlation of cross-bridge action in muscle with actomyosin ATPase in solution. Annu Rev Physiol. 1987;49:655–672. doi: 10.1146/annurev.ph.49.030187.003255. [DOI] [PubMed] [Google Scholar]
- Cooke R. Structure of the myosin head. Cell Motil Cytoskeleton. 1989;14(2):183–186. doi: 10.1002/cm.970140204. [DOI] [PubMed] [Google Scholar]
- Craig R., Greene L. E., Eisenberg E. Structure of the actin-myosin complex in the presence of ATP. Proc Natl Acad Sci U S A. 1985 May;82(10):3247–3251. doi: 10.1073/pnas.82.10.3247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eden D., Matthew J. B., Rosa J. J., Richards F. M. Increase in apparent compressibility of cytochrome c upon oxidation. Proc Natl Acad Sci U S A. 1982 Feb;79(3):815–819. doi: 10.1073/pnas.79.3.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg E., Greene L. E. The relation of muscle biochemistry to muscle physiology. Annu Rev Physiol. 1980;42:293–309. doi: 10.1146/annurev.ph.42.030180.001453. [DOI] [PubMed] [Google Scholar]
- Eisenberg E., Hill T. L. Muscle contraction and free energy transduction in biological systems. Science. 1985 Mar 1;227(4690):999–1006. doi: 10.1126/science.3156404. [DOI] [PubMed] [Google Scholar]
- Ford L. E., Huxley A. F., Simmons R. M. Tension responses to sudden length change in stimulated frog muscle fibres near slack length. J Physiol. 1977 Jul;269(2):441–515. doi: 10.1113/jphysiol.1977.sp011911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gekko K., Hasegawa Y. Compressibility-structure relationship of globular proteins. Biochemistry. 1986 Oct 21;25(21):6563–6571. doi: 10.1021/bi00369a034. [DOI] [PubMed] [Google Scholar]
- Goodno C. C. Myosin active-site trapping with vanadate ion. Methods Enzymol. 1982;85(Pt B):116–123. doi: 10.1016/0076-6879(82)85014-3. [DOI] [PubMed] [Google Scholar]
- Goodno C. C., Taylor E. W. Inhibition of actomyosin ATPase by vanadate. Proc Natl Acad Sci U S A. 1982 Jan;79(1):21–25. doi: 10.1073/pnas.79.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasan H., Mason P. Pulse propagation in muscle. Phys Med Biol. 1978 Sep;23(5):917–927. doi: 10.1088/0031-9155/23/5/008. [DOI] [PubMed] [Google Scholar]
- Hatta I., Sugi H., Tamura Y. Stiffness changes in frog skeletal muscle during contraction recorded using ultrasonic waves. J Physiol. 1988 Sep;403:193–209. doi: 10.1113/jphysiol.1988.sp017245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Highsmith S., Eden D. Ligand-induced myosin subfragment 1 global conformational change. Biochemistry. 1990 May 1;29(17):4087–4093. doi: 10.1021/bi00469a010. [DOI] [PubMed] [Google Scholar]
- Huston E. E., Grammer J. C., Yount R. G. Flexibility of the myosin heavy chain: direct evidence that the region containing SH1 and SH2 can move 10 A under the influence of nucleotide binding. Biochemistry. 1988 Dec 13;27(25):8945–8952. doi: 10.1021/bi00425a011. [DOI] [PubMed] [Google Scholar]
- Huxley A. F. Muscular contraction. J Physiol. 1974 Nov;243(1):1–43. [PMC free article] [PubMed] [Google Scholar]
- Huxley H. E. The mechanism of muscular contraction. Science. 1969 Jun 20;164(3886):1356–1365. doi: 10.1126/science.164.3886.1356. [DOI] [PubMed] [Google Scholar]
- Jung D. W., Blangé T., de Graaf H., Treijtel B. W. Elastic properties of relaxed, activated, and rigor muscle fibers measured with microsecond resolution. Biophys J. 1988 Nov;54(5):897–908. doi: 10.1016/S0006-3495(88)83026-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAY C. M. The partial specific volume of muscle proteins. Biochim Biophys Acta. 1960 Mar 11;38:420–427. doi: 10.1016/0006-3002(60)91277-4. [DOI] [PubMed] [Google Scholar]
- Katayama E. The effects of various nucleotides on the structure of actin-attached myosin subfragment-1 studied by quick-freeze deep-etch electron microscopy. J Biochem. 1989 Nov;106(5):751–770. doi: 10.1093/oxfordjournals.jbchem.a122928. [DOI] [PubMed] [Google Scholar]
- Kuntz I. D., Jr, Kauzmann W. Hydration of proteins and polypeptides. Adv Protein Chem. 1974;28:239–345. doi: 10.1016/s0065-3233(08)60232-6. [DOI] [PubMed] [Google Scholar]
- Leung W. P., Cho K. C., Lo Y. M., Choy C. L. Adiabatic compressibility of myoglobin. Effect of axial ligand and denaturation. Biochim Biophys Acta. 1986 Mar 7;870(1):148–153. doi: 10.1016/0167-4838(86)90018-x. [DOI] [PubMed] [Google Scholar]
- Maita T., Yajima E., Nagata S., Miyanishi T., Nakayama S., Matsuda G. The primary structure of skeletal muscle myosin heavy chain: IV. Sequence of the rod, and the complete 1,938-residue sequence of the heavy chain. J Biochem. 1991 Jul;110(1):75–87. doi: 10.1093/oxfordjournals.jbchem.a123546. [DOI] [PubMed] [Google Scholar]
- Margossian S. S., Lowey S. Interaction of myosin subfragments with F-actin. Biochemistry. 1978 Dec 12;17(25):5431–5439. doi: 10.1021/bi00618a017. [DOI] [PubMed] [Google Scholar]
- Margossian S. S., Lowey S. Preparation of myosin and its subfragments from rabbit skeletal muscle. Methods Enzymol. 1982;85(Pt B):55–71. doi: 10.1016/0076-6879(82)85009-x. [DOI] [PubMed] [Google Scholar]
- Mason P. Dynamic stiffness and crossbridge action in muscle. Biophys Struct Mech. 1977 Dec 27;4(1):15–25. doi: 10.1007/BF00538837. [DOI] [PubMed] [Google Scholar]
- Morita F. Temperature induced analog reaction of adenylyl imidodiphosphate to an intermediate step of heavy meromyosin adenosine triphosphatase. J Biochem. 1977 Feb;81(2):313–320. doi: 10.1093/oxfordjournals.jbchem.a131460. [DOI] [PubMed] [Google Scholar]
- PARRISH R. G., MOMMAERTS W. F. Studies on myosin. II. Some molecular-kinetic data. J Biol Chem. 1954 Aug;209(2):901–913. [PubMed] [Google Scholar]
- Schoenberg M., Wells J. B., Podolsky R. J. Muscle compliance and the longitudinal transmission of mechanical impulses. J Gen Physiol. 1974 Dec;64(6):623–642. doi: 10.1085/jgp.64.6.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura Y., Hatta I., Matsuda T., Sugi H., Tsuchiya T. Changes in muscle stiffness during contraction recorded using ultrasonic waves. Nature. 1982 Oct 14;299(5884):631–633. doi: 10.1038/299631a0. [DOI] [PubMed] [Google Scholar]
- Teller D. C. Accessible area, packing volumes and interaction surfaces of globular proteins. Nature. 1976 Apr 22;260(5553):729–731. doi: 10.1038/260729a0. [DOI] [PubMed] [Google Scholar]
- Trentham D. R., Eccleston J. F., Bagshaw C. R. Kinetic analysis of ATPase mechanisms. Q Rev Biophys. 1976 May;9(2):217–281. doi: 10.1017/s0033583500002419. [DOI] [PubMed] [Google Scholar]
- Truong X. T. Viscoelastic wave propagation and rheologic properties of skeletal muscle. Am J Physiol. 1974 Feb;226(2):256–264. doi: 10.1152/ajplegacy.1974.226.2.256. [DOI] [PubMed] [Google Scholar]
- Weeds A. G., Taylor R. S. Separation of subfragment-1 isoenzymes from rabbit skeletal muscle myosin. Nature. 1975 Sep 4;257(5521):54–56. doi: 10.1038/257054a0. [DOI] [PubMed] [Google Scholar]
- YOUNG D. M., HIMMELFARB S., HARRINGTON W. F. THE RELATIONSHIP OF THE MEROMYOSINS TO THE MOLECULAR STRUCTURE OF MYOSIN. J Biol Chem. 1964 Sep;239:2822–2829. [PubMed] [Google Scholar]
- Zamyatnin A. A. Protein volume in solution. Prog Biophys Mol Biol. 1972;24:107–123. doi: 10.1016/0079-6107(72)90005-3. [DOI] [PubMed] [Google Scholar]


