Abstract
Because of its importance in the chemiosmotic theory, mitochondrial membrane potential has been the object of many investigations. Significantly, however, quantitative data on how energy transduction might be regulated or perturbed by the physiological state of the cell has only been gathered via indirect studies on isolated mitochondrial suspensions; quantitative studies on individual mitochondria in situ have not been possible because of their small size, their intrinsic motility, and the absence of appropriate analytical reagents. In this article, we combine techniques for rapid, high resolution, quantitative three-dimensional imaging microscopy and mathematical modeling to determine accurate distributions of a potentiometric fluorescent probe between the cytosol and individual mitochondria inside a living cell. Analysis of this distribution via the Nernst equation permits assignment of potentials to each of the imaged mitochondrial membranes. The mitochondrial membrane potentials are distributed over a narrow range centered at -150 mV within the neurites of differentiated neuroblastoma cells. We find that the membrane potential of a single mitochondrion is generally remarkably stable over times of 40-80 s, but significant fluctuations can occasionally be seen. The motility of individual mitochondria is not directly correlated to membrane potential, but mitochondria do become immobile after prolonged treatment with respiratory inhibitors or uncouplers. Thus, three spatial dimensions, a key physiological parameter, and their changes over time are all quantitated for objects at the resolution limit of light microscopy. The methods described may be readily extended to permit investigations of how mitochondrial function is integrated with other processes in the intact cell.
Full text
PDF

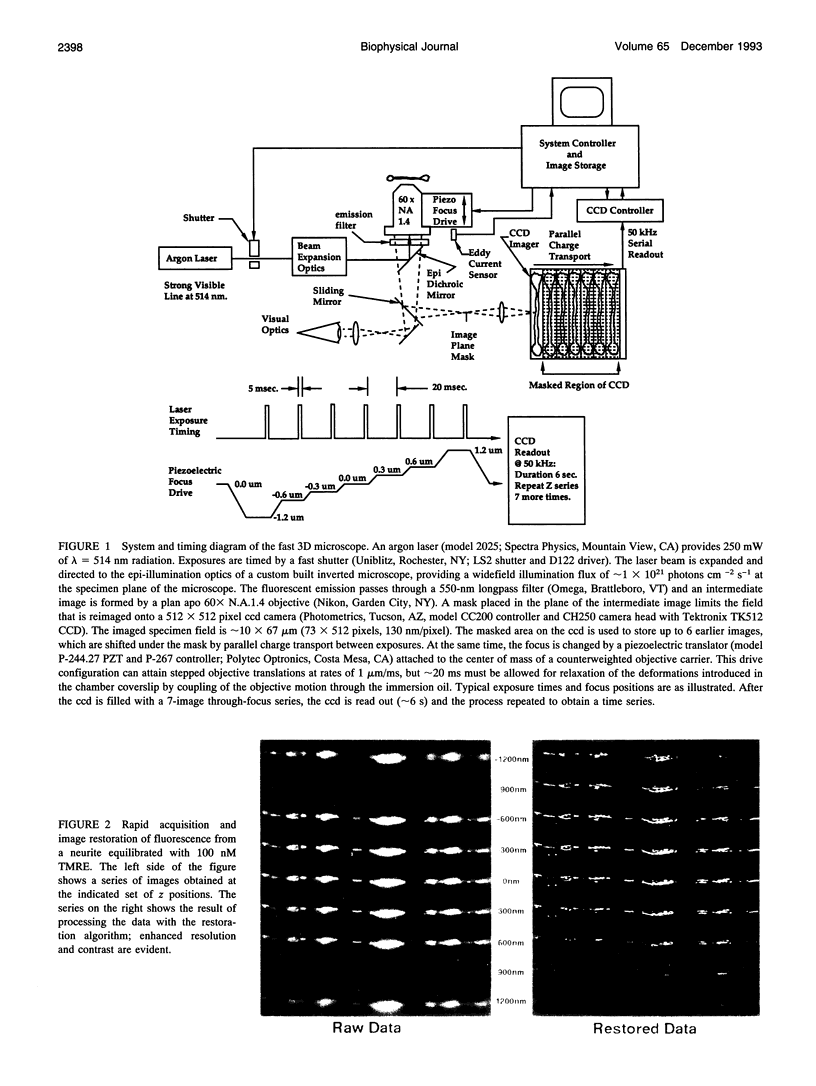





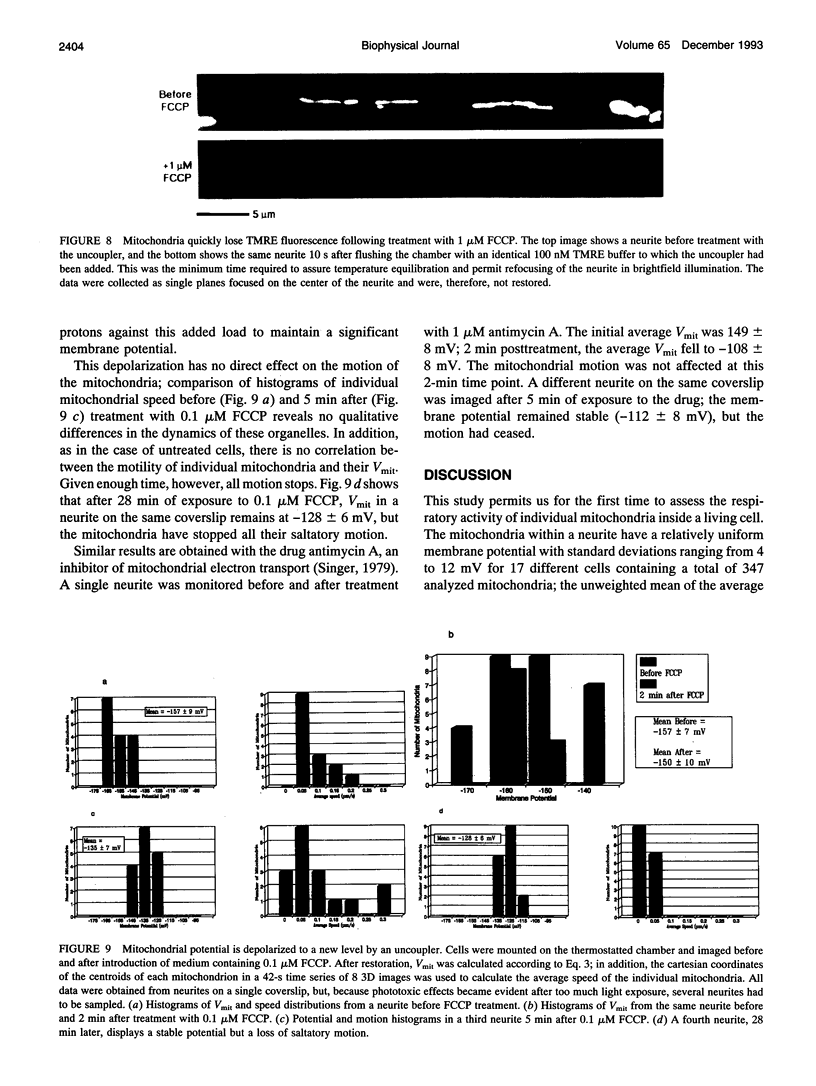



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agard D. A., Hiraoka Y., Shaw P., Sedat J. W. Fluorescence microscopy in three dimensions. Methods Cell Biol. 1989;30:353–377. doi: 10.1016/s0091-679x(08)60986-3. [DOI] [PubMed] [Google Scholar]
- Agard D. A. Optical sectioning microscopy: cellular architecture in three dimensions. Annu Rev Biophys Bioeng. 1984;13:191–219. doi: 10.1146/annurev.bb.13.060184.001203. [DOI] [PubMed] [Google Scholar]
- Beyer W., Imlay J., Fridovich I. Superoxide dismutases. Prog Nucleic Acid Res Mol Biol. 1991;40:221–253. doi: 10.1016/s0079-6603(08)60843-0. [DOI] [PubMed] [Google Scholar]
- Boveris A., Chance B. The mitochondrial generation of hydrogen peroxide. General properties and effect of hyperbaric oxygen. Biochem J. 1973 Jul;134(3):707–716. doi: 10.1042/bj1340707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boveris A. Mitochondrial production of superoxide radical and hydrogen peroxide. Adv Exp Med Biol. 1977;78:67–82. doi: 10.1007/978-1-4615-9035-4_5. [DOI] [PubMed] [Google Scholar]
- Brand M. D., Murphy M. P. Control of electron flux through the respiratory chain in mitochondria and cells. Biol Rev Camb Philos Soc. 1987 May;62(2):141–193. doi: 10.1111/j.1469-185x.1987.tb01265.x. [DOI] [PubMed] [Google Scholar]
- Chen L. B. Mitochondrial membrane potential in living cells. Annu Rev Cell Biol. 1988;4:155–181. doi: 10.1146/annurev.cb.04.110188.001103. [DOI] [PubMed] [Google Scholar]
- Ehrenberg B., Montana V., Wei M. D., Wuskell J. P., Loew L. M. Membrane potential can be determined in individual cells from the nernstian distribution of cationic dyes. Biophys J. 1988 May;53(5):785–794. doi: 10.1016/S0006-3495(88)83158-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farkas D. L., Wei M. D., Febbroriello P., Carson J. H., Loew L. M. Simultaneous imaging of cell and mitochondrial membrane potentials. Biophys J. 1989 Dec;56(6):1053–1069. doi: 10.1016/S0006-3495(89)82754-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fay F. S., Carrington W., Fogarty K. E. Three-dimensional molecular distribution in single cells analysed using the digital imaging microscope. J Microsc. 1989 Feb;153(Pt 2):133–149. [PubMed] [Google Scholar]
- Forman D. S., Lynch K. J., Smith R. S. Organelle dynamics in lobster axons: anterograde, retrograde and stationary mitochondria. Brain Res. 1987 May 26;412(1):96–106. doi: 10.1016/0006-8993(87)91443-0. [DOI] [PubMed] [Google Scholar]
- Gooch V. D., Packer L. Oscillatory systems in mitochondria. Biochim Biophys Acta. 1974 Dec 30;346(3-4):245–260. doi: 10.1016/0304-4173(74)90002-0. [DOI] [PubMed] [Google Scholar]
- Johnson L. V., Walsh M. L., Bockus B. J., Chen L. B. Monitoring of relative mitochondrial membrane potential in living cells by fluorescence microscopy. J Cell Biol. 1981 Mar;88(3):526–535. doi: 10.1083/jcb.88.3.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimhi Y., Palfrey C., Spector I., Barak Y., Littauer U. Z. Maturation of neuroblastoma cells in the presence of dimethylsulfoxide. Proc Natl Acad Sci U S A. 1976 Feb;73(2):462–466. doi: 10.1073/pnas.73.2.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loew L. M. Confocal microscopy of potentiometric fluorescent dyes. Methods Cell Biol. 1993;38:195–209. doi: 10.1016/s0091-679x(08)61003-1. [DOI] [PubMed] [Google Scholar]
- Martz D., Lasek R. J., Brady S. T., Allen R. D. Mitochondrial motility in axons: membranous organelles may interact with the force generating system through multiple surface binding sites. Cell Motil. 1984;4(2):89–101. doi: 10.1002/cm.970040203. [DOI] [PubMed] [Google Scholar]
- Morris R. L., Hollenbeck P. J. The regulation of bidirectional mitochondrial transport is coordinated with axonal outgrowth. J Cell Sci. 1993 Mar;104(Pt 3):917–927. doi: 10.1242/jcs.104.3.917. [DOI] [PubMed] [Google Scholar]
- Reers M., Smith T. W., Chen L. B. J-aggregate formation of a carbocyanine as a quantitative fluorescent indicator of membrane potential. Biochemistry. 1991 May 7;30(18):4480–4486. doi: 10.1021/bi00232a015. [DOI] [PubMed] [Google Scholar]
- Scalettar B. A., Abney J. R., Hackenbrock C. R. Dynamics, structure, and function are coupled in the mitochondrial matrix. Proc Natl Acad Sci U S A. 1991 Sep 15;88(18):8057–8061. doi: 10.1073/pnas.88.18.8057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer T. P. Mitochondrial electron-transport inhibitors. Methods Enzymol. 1979;55:454–462. doi: 10.1016/0076-6879(79)55059-9. [DOI] [PubMed] [Google Scholar]
- Slater E. C. Mechanism of oxidative phosphorylation. Annu Rev Biochem. 1977;46:1015–1026. doi: 10.1146/annurev.bi.46.070177.005055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smiley S. T., Reers M., Mottola-Hartshorn C., Lin M., Chen A., Smith T. W., Steele G. D., Jr, Chen L. B. Intracellular heterogeneity in mitochondrial membrane potentials revealed by a J-aggregate-forming lipophilic cation JC-1. Proc Natl Acad Sci U S A. 1991 May 1;88(9):3671–3675. doi: 10.1073/pnas.88.9.3671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tedeschi H. The mitochondrial membrane potential. Biol Rev Camb Philos Soc. 1980 May;55(2):171–206. doi: 10.1111/j.1469-185x.1980.tb00692.x. [DOI] [PubMed] [Google Scholar]