Abstract
In the adult brain, neural stem cells (NSC) must migrate to express their neuroplastic potential. The addition of recombinant reelin to human NSC (HNSC) cultures facilitates neuronal retraction in the neurospheroid. Because we detected reelin, α3-integrin receptor subunits, and disabled-1 immunoreactivity in HNSC cultures, it is possible that integrin-mediated reelin signal transduction is operative in these cultures. To investigate whether reelin is important in the regulation of NSC migration, we injected HNSCs into the lateral ventricle of null reeler and wild-type mice. Four weeks after transplantation, we detected symmetrical migration and extensive neuronal and glial differentiation of transplanted HNSCs in wild-type, but not in reeler mice. In reeler mice, most of the injected HNSCs failed to migrate or to display the typical differentiation pattern. However, a subpopulation of transplanted HNSCs expressing reelin did show a pattern of chain migration in the reeler mouse cortex. We also analyzed the endogenous NSC population in the reeler mouse using bromodeoxyuridine injections. In reeler mice, the endogenous NSC population in the hippocampus and olfactory bulb was significantly reduced compared with wild-type mice; in contrast, endogenous NSCs expressed in the subventricular zonewere preserved. Hence, it seems likely that the lack of endogenous reelin may have disrupted the migration of the NSCs that had proliferated in the SVZ. We suggest that a possible inhibition of NSC migration in psychiatric patients with a reelin deficit may be a potential problem in successful NSC transplantation in these patients.
Neurogenesis depends on a specific population of cells, termed “neural stem cells” (NSCs). Islands of NSCs have been detected in embryonic and adult mammalian brains (1, 2). These cells possess pluripotent differentiation potential; in fact, they can become astrocytes, neurons, or oligodendrocytes. Recent studies have observed NSCs in the anterior subventricular zone (SVZ) and dentate gyrus of the adult brain (3–5), indicating that neurogenesis may occur throughout life. Although pluripotency of adult NSCs is regionally and temporally restricted, these cells retain their ability to migrate and differentiate in response to environmental cues. In a previous report, human NSCs (HNSCs) injected into the lateral ventricle of 24-month-old rats showed a symmetrical migration in the host brain, followed by differentiation into neurons and glial cells (6). This result indicates that the aged brain maintains regulatory mechanisms to guide the migration of NSCs, which may be indispensable for proper adult brain neuroplasticity.
Recent studies (7, 8) have revealed two distinct neuronal migration patterns, radial and tangential. Each pattern consists of two different neuronal populations that participate in corticogenesis. One neuronal population proliferates from the embryonic SVZ and migrates along the radial glia to reach the subcortical plate, detaches from radial glia scaffolding, and then penetrates the subcortical plate guided by reelin gradient secreted by γ amino butyric acid (GABA)-ergic Cajal-Retzius cells (9). A second neuronal population consists of tangentially migrating neuroblasts that proliferate in the SVZ in the mantle of ganglionic telencephalic eminences and give rise to GABA-producing interneurons (10). In the adult brain, host glial cells respond to the transplantation of NSCs (11) and lesions (12) by becoming transient radial glia-type cells, which may serve to guide migration in adult neurogenesis. In contrast, subsets of cells expressed in the ventral piriform cortex and olfactory bulb migrate long distances without a radial glia connection (13), suggesting that specific regulatory mechanisms guide NSC migration in the adult brain, and that some of these mechanisms are very likely analogous to those operating during development.
Reelin is a large extracellular matrix (ECM) protein of approximately 400 kDa (9) which binds to the α3 subunit of integrin receptors that are expressed on neuronal cell surfaces (14, 15), very low density lipoprotein receptor (VLDLR), and Apolipoprotein E receptor 2 (ApoER2; refs. 16–18), triggering the adaptor function of the disabled-1 (Dab-1) cytosolic protein (14, 16). The clustering of integrin receptor subunits after reelin binding activates a tyrosine kinase (focal adhesion kinase) to phosphorylate Dab-1. This phosphorylated Dab-1 binds and transports soluble tyrosine kinases and transcription factors to functional cellular compartments (19). In the null reeler mouse, migrating neurons fail to penetrate the subcortical plate, perhaps because of a deficiency of serine protease activity associated with reelin (20). Although much attention has been focused on the role of reelin in neuronal migration during corticogenesis, we now know that reelin is expressed in several neuronal populations in the adult brain (21, 22). This protein may be operative in other important functions still under examination; e.g., (i) the reelin haploinsufficient heterozygous reeler mouse exhibits decreased dendritic spine expression density in pyramidal neurons of the cortex and hippocampus (23); and (ii) reelin and α3-integrin receptor subunit immunoreactivities colocalize to dendritic spine postsynaptic densities (24), supporting a hypothetical role for reelin in adult brain neuroplasticity (spine formation and synaptogenesis) that requires protein synthesis.
A serum-free defined HNSC culture system was developed (25) to expand and differentiate HNSCs in vitro. This technique allows examination of the above-mentioned complex molecular interactions during differentiation in the absence of many identified and unidentified molecules expressed in the serum. In this culture, glia-differentiating HNSCs migrate out from the neurosphere, which is a cluster of undifferentiated HNSCs. These cells and their processes provide a scaffold for neurally differentiating HNSCs, which migrate out and back into the neurosphere. Thus, we can speculate that reelin-mediated migration within the neurosphere may be associated with HNSC differentiation. After injection of HNSCs into the brain lateral ventricles, these cells penetrate the existing brain structures and migrate to their destinations (6).
By using these HNSC culture systems and transplantation models in reeler mice, we have investigated whether reelin has a role in HNSC biology. Here, we present evidence that HNSCs express reelin, α3-integrin receptor subunits, and Dab-1, and that the reelin transduction signaling pathway should be regarded as an indispensable factor in NSC migration.
Materials and Methods
This research was conducted under Institutional Review Board protocol no. 2001–0316 for human subjects and protocol no. 01–197 for animal use approved by the Office for the Protection of Research Subjects at University of Illinois at Chicago.
Cell Cultures.
A method for the long-term growth of human neural precursor cells used in this study was published by Svendsen et al. (26). Briefly, HNSCs (25) proliferated in a defined media containing epidermal growth factor (20 ng/ml, R & D Systems), fibroblast growth factor (FGF, 20 ng/ml, R & D Systems), B27 (1:50, GIBCO), heparin (5 μg/ml, Sigma), antibiotic-antimycotic mixture (1:100, GIBCO), DMEM and Ham's F-12 (both from GIBCO). Thereafter, HNSCs were differentiated for 5–7 days in a humidified incubator with 10% (vol/vol) FBS-supplemented Eagle's medium (GIBCO) or serum-free (basal) media (GIBCO).
Immunocytochemistry.
For immunocytochemistry, HNSCs were fixed in 100% methanol at −20°C for 20 min. After washing, cells were blocked for 1 h at 4°C with PBS containing 0.25% Triton X-100 and 2.0% normal donkey serum (PBSTS, Jackson ImmunoResearch). Next, cells were incubated overnight at 4°C with the primary antibody solution diluted in PBSTS. Then, the cells were incubated with secondary antibody diluted in PBSTS for 1 h at 4°C in the dark. After washing with PBS, the cells were coverslipped with Vectashield 4′,6-diamidino-2-phenylindole (DAPI) Mounting Media (Vector Laboratories). Microscopic images were taken by an Axiocam digital camera mounted on an Axioscop 2 with AXIOVISION software (Zeiss).
Immunocytochemistry for Electron Microscopy.
The differentiated NSCs were fixed with 4% (wt/vol) paraformaldehyde fixative (pH 7.4 with 0.1 mM phosphate buffer) for 30 min at room temperature (RT). After washing, the cells were blocked for 1 h in PBS with 1.5% goat serum. The cells then were transferred to the primary antibody solution diluted in PBS-serum solution overnight at 4°C. The following day, the cells were sequentially incubated with a biotinylated secondary antibody and avidin-biotin complex solution (Vector Laboratories) for 1 h each at RT. The cells then were incubated with a diaminobenzidine solution (Sigma).
Immunoprecipitation.
For immunoprecipitation, both cell lysates and cell media were sampled under native conditions after a 7-day differentiation period. Cells were lysed for 30 min with constant agitation in ice-cold immunoprecipitation buffer containing 10 mM Tris at pH 7.5, 150 mM NaCl, 2 mM EDTA, and 1% Triton X-100. Insoluble material was removed from the cell lysates by centrifugation for 30 sec at 14,000 × g. The cell lysates were incubated with monoclonal anti-reelin 142 antibody overnight at 4°C on a rotator. The antigen–antibody complex then was incubated with 50 μl of IgG Sepharose bead suspension (Amersham Pharmacia) overnight at 4°C on a rotator. The third day included washing the cells with immunoprecipitation buffer three times, with 10 mM Tris at pH 7.5, 500 mM NaCl, 2 mM EDTA, and 1% Triton X-100 twice, and with 10 mM Tris at pH 7.5 three times. After each wash, the cell-attached beads were centrifuged for 30 s at 14,000 × g. After the last wash, the supernatant was carefully removed, and the beads were heated for 3–5 min at 95°C with 100 μl of SDS sample buffer (Invitrogen) for elution of the protein sample.
Western Blot.
HNSCs differentiated under both serum and nonserum (basal) conditions for 7 days. Protein samples were prepared with Tri reagent according to the manufacturer's protocol (Molecular Research Center, Cincinnati). Protein sample concentrations were measured using the Bio-Rad protein analysis kit according to the manufacturer's protocol. Equal amounts of protein samples were loaded onto four 12% bis/Tris gels (Invitrogen). Proteins were transferred onto poly(vinylidene difluoride) membranes (Invitrogen) overnight at 4°C at a constant 15 mV. The membranes were blocked with 5% milk in PBS, .05% Tween 20 (Sigma) for 8 h. Thereafter, membranes were incubated with primary antibody solution diluted in 5% milk/PBS-Tween 20 mixture overnight at 4°C. Membranes were washed three times with 5% milk/PBS-Tween 20 for 5 min each. Next, the membranes incubated in the secondary antibody solution also were diluted in 5% milk/PBS-Tween 20 mixture for 1 h at 4°C. The proteins of interest were detected by enhanced chemiluminescence with the SuperSignal West Pico Chemiluminescent Substrate (Pierce).
Antibodies.
Primary antibodies were used in the following dilutions: mouse anti-reelin 142 monoclonal antibody (1:100, a gift of A. M. Goffinet, Univ. of Namur, Brussels, Belgium); mouse anti-α3-integrin receptor subunit monoclonal antibody (1:500, Chemicon); rabbit anti-Dab-1 polyclonal antibody (1:500, from Pascale Lacor at Univ. of Illinois at Chicago); mouse anti-βIII tubulin antibody (1:1,000, Sigma); and goat anti-glial filament protein antibody (GFAP, 1:500, Research Diagnostics, Flanders, NJ)
Secondary antibodies purchased from Jackson ImmunoResearch were used with the following dilutions: fluorescein (FITC)-conjugated donkey anti-mouse IgG (1:200); rhodamine (TRITC)-conjugated donkey anti-goat IgG (1:200); and peroxidase-conjugated donkey anti-mouse IgG (1:5,000).
Transplantation of HNSCs into Reeler and Wild-Type Mice.
Three-month-old male mice were anesthetized with 50 mg/kg pentobarbital (i.p.) and mounted in a stereotaxic apparatus (David Kopf Instruments, Tujunga, CA). After the hair removal from the surgical site by an electric razor, a 0.5-cm surgical incision was made caudal to rostral in the skin at the surface of the cranium. The ventricles were stereotaxically localized by using the following coordinates: AP = −0.1 mm from bregma, ML = ±1 mm, and 1.4 mm below dura. A 0.4-mm hole was made on the cranium using a precision drill bit. NSCs (5 × 104) in 5 μl of PBS were injected into the ventricle by using a microsyringe attached to the stereotaxic apparatus. The injection was delivered over a period of 10 min, and the needle was left in place for an additional 5 min after the injection. After the injection, the surgically incised skin was closed by 1 Michel suture clips (2.5 × 1.75 mm). Four weeks after the surgery, the animals were deeply anesthetized by 70 mg/kg pentobarbital (i.p.) and perfused with physiological saline and 4% paraformaldehyde fixative. The brains were removed and further processed for immunohistochemical analysis.
In Vivo Cell Proliferation Analysis.
Adult (4-month-old) mice were injected i.p. with bromodeoxyuridine (BrdUrd, 100 mg/kg/day; Sigma) for 3 days. Twenty-four hours after the last injection, the animals were deeply anesthetized by 70 mg/kg pentobarbital (i.p.) and perfused with physiological saline and 4% paraformaldehyde fixative. The brains were removed and further processed for immunohistochemical analysis.
Immunohistochemistry of the Mouse Brain.
The removed brains were placed into 4% (wt/vol) paraformaldehyde fixative containing 20% (wt/vol) sucrose overnight. The brains were sliced into 20-μm coronal sections using a cryostat-microtome. The sections were washed briefly in PBS and treated with 1M HCl for 30 min at RT to increase the accessibility of the anti-BrdUrd antibody to the cell nuclei. After rinsing with PBS, sections were transferred to a PBS containing 0.25% Triton X-100 (PBST) for 30 min. Then, the sections were blocked in PBST containing 3% donkey normal serum for 1 h and incubated with sheep anti-BrdUrd overnight at 4°C. After rinsing in PBS, donkey anti-sheep conjugated to rhodamine IgG was added at a 1:200 dilution in PBST for 2 h incubation at RT in the dark. Then, the sections were washed with PBS and incubated with mouse IgG2b monoclonal anti-human βIII-tubulin, clone SDL3D10 (1:500, Sigma), and goat anti human-GFAP, N-terminal human affinity purified (1:200, Research Diagnostics), respectively, overnight at 4°C in the dark. The corresponding secondary antibodies for these sections were donkey anti-mouse or donkey anti-goat IgG conjugated with FITC or rhodamine. After a brief PBS washing, the secondary antibodies were added into sections for a 2-h incubation at RT in the dark. Sections then were washed with PBS thoroughly before mounting on glass slides and covered with Vectashield with DAPI (Vector Laboratories) for fluorescent microscopic observation.
Production and Purification of Recombinant Reelin.
Full-length mouse reelin cDNA constructs, with or without an internal Myc epitope insert, were a gift of G. D'Arcangelo (Baylor College of Medicine, Houston, TX; ref. 27). Reelin cDNA was transfected into 293T cells at ≈90% confluence using Lipofectamine 2000 (Life Technologies, Rockville, MD) for 6 h in protein-free DMEM/F12 medium, then replaced with serum-free neurobasal/B-27 medium and refed every 2 days. Conditioned medium was spun at low speed to remove cellular debris, then aliquoted and stored at −80°C. As a negative control, conditioned medium was taken from nontransfected cells or from cells exposed to lipofectamine only (no plasmid).
Results
Effect of Recombinant Reelin on HNSC Migration Under Serum-Free Conditions.
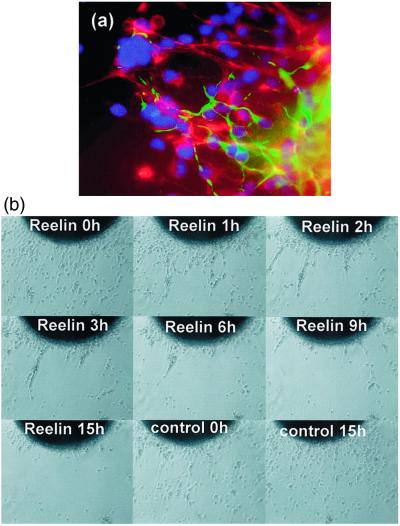
To investigate the role of reelin in HNSC migration, we applied recombinant reelin (200–400 pM) to HNSCs differentiated under serum-free defined conditions in vitro. We previously have reported that HNSCs not only survive at least 3 weeks under these conditions, but they also fully differentiate into βIII-tubulin, GFAP, or O4-immunopositive cells (25). Fig. 1a shows the typical differentiation pattern of HNSCs at 5 days under serum-free conditions with immunocytochemistry with anti-βIII-tubulin and anti-GFAP antibodies. Normal migration consists of glial cells differentiating out in a “spoke-wheel” pattern. These cells and their processes provide a scaffold for later differentiating neurons. Neurons moving back and forth from the neurospheroid migrate in association with the radial processes and cell bodies of glial cells. Fig. 1b shows time-lapse photo microscopy of the effect of recombinant reelin on HNSCs differentiated under serum-free conditions. Addition of recombinant reelin induced retraction of differentiating neurons, glial cells, and their projections back to the spheroid in about 3 h, whereas addition of conditioned media from 293T cells, which were not transfected with plasmid, failed to elicit a significant change in the movement of HNSCs (Fig. 1b). We speculate that reelin may be operative in regulating cell movement in our cultures and very likely are necessary for differentiation. The effect of reelin on the differentiation of HNSCs requires further investigation. This result indicates that reelin may regulate HNSC biology through a responsive system requiring reelin expression in these cells.
Figure 1.
Effect of recombinant reelin on HNSCs differentiated without serum. (a) βIII-tubulin (green) and GFAP (red) at time 0 (×400). (b) Typical time-lapse image of HNSC movements in the presence and absence of recombinant reelin (×50). quicktime movie files of these time-lapse images are published as Movies 1 and 2 as supporting information on the PNAS web site, www.pnas.org.
Expression of Reelin, α3-Integrin Receptor Subunits, and Dab-1 in HNSCs.
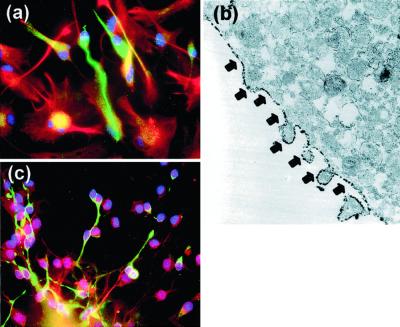
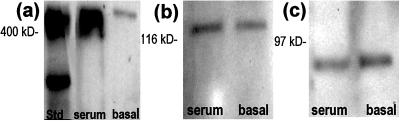
To study the role that reelin and its signaling pathway may have in the differentiation and migration of HNSCs, we performed immunocytochemical and Western blot analysis using antibodies recognizing reelin, α3-integrin receptor subunits, and Dab-1. Fig. 2 shows that HNSCs differentiated in vitro for 5 days express reelin immunoreactivity in small bipolar cells, which exhibited a morphological phenotype suggestive of neuronal differentiation. However, reelin immunoreactivity was not detectable in GFAP-positive cells (Fig. 2a). Immunoreactivity to α3-integrin receptor subunits also was expressed by those small bipolar cells but not by GFAP-positive cells, indicating expression of α3-integrin receptor subunits that was higher in neuronal phenotypes than in glial phenotypes (Fig. 2b). By using electron microscopy, we detected that α3-integrin receptor subunit immunoreactivity was localized on the surface of the plasma membrane of differentiating neurons (Fig. 2c). Western blot analysis of protein extracts prepared by immunoprecipitation with the monoclonal antibody reelin 142 from HNSCs after 7 days of differentiation allowed the identification of reelin, the α3-integrin receptor subunit, and the Dab-1 immunopositive bands (Fig. 3).
Figure 2.
Expression of reelin and α3-integrin receptor subunits in cultures of HNSCs. (a) Reelin immunoreactivity (green) expressed by neuronal-like phenotypes and not by GFAP-positive cells (red) (×400). (b) Electron-microscopic image (×17,500) of α3−integrin receptor subunit immunoreactivity located on HNSC plasma membrane (arrows). (c) α3-Integrin receptor subunit immunoreactivity (green) expressed specifically by neuron-like phenotypes (×400). Nuclei were counterstained with DAPI (blue).
Figure 3.
Western blot analysis of reelin (a), α3-integrin (b), and Dab-1 protein (c) extracted from HNSCs by immunoprecipitation with anti-reelin 142 antibody after nonserum (basal) or serum differentiation.
Transplantation of Stem Cells to Reeler Mice.
A previous study with rodents using the HNSC intraventricular injection model (6) not only demonstrated that the brain of senile rats provides the environment necessary for successful HNSC migration and differentiation, but also that HNSC transplantation improved the cognitive function of the aged hosts.
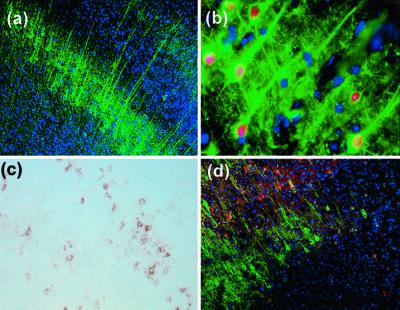
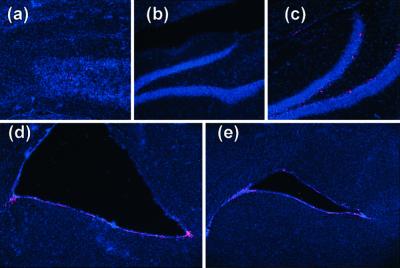
To investigate the effect of reelin on the migration and differentiation patterns of HNSCs in vivo, HNSCs were expanded without differentiation in vitro as described (25) and labeled via a BrdUrd incorporation into nuclear DNA. The BrdUrd-tagged HNSCs were subsequently injected into the lateral ventricles (6) of 3-month-old wild-type (B6C3F) and null reeler mice (Edimburg mutation; ref. 23). We analyzed the brain sections with immunohistochemistry for βIII-tubulin, GFAP, and BrdUrd 4 weeks after the injection. Wild-type mice showed a differentiation and distribution pattern of transplanted HNSCs similar to that previously detected in rat transplantation experiments (Fig. 4). In contrast, HNSCs transplanted into reeler mice failed to migrate in the host brain. Most BrdUrd-positive cells remained at the site of injection in the ventricle area. In the cortex, a few GFAP-positive cells were detected (Fig. 5d), but βIII-tubulin-positive HNSCs were absent (Fig. 5a). Additionally, βIII-tubulin-positive HNSCs located near the needle track displayed a particular morphology characterized by a severe deficit of neuronal process expression (Fig. 5 b and e). However, a population of reelin-positive HNSCs located near the SVZ showed a chain-migration pattern of neuron-like phenotypes along a reelin trail (Fig. 5c). These results indicate that ECM reelin is necessary to promote migration of stem cells, and also that cells expressing reelin can migrate in the reeler mouse.
Figure 4.
Immunohistochemistry of cerebral cortex in a wild-type mouse 4 weeks after HNSC transplantation into the brain. (a) HNSCs migrated into the cortex and differentiated into neuron-like phenotypes expressing βIII-tubulin immunoreactivity (green) (×100). (b) Pyramidal neuron-like phenotypes, with the presence of apical dendrites (green) expressing BrdUrd-positive nuclei (red) (×400). (c) Note a layer of GFAP-positive HNSCs (brown) (×100). (d) Double GFAP and βIII-tubulin immunostaining revealing a layer of GFAP-positive (red) and a layer of βIII-tubulin-positive (green) cells (×100). Nuclei were counterstained with DAPI (blue).
Figure 5.
Immunohistochemistry of a reeler mouse brain 4 weeks after HNSC transplantation. (a) Low magnification (×100) of the cortex stained with anti-βIII-tubulin antibody and counterstained with DAPI to reveal nuclei. Note that βIII-tubulin-positive (green) cells are virtually undetectable. (b) Groups of BrdUrd-positive cells near the injection site (×400). Note that the morphology of these cells differs from that of cells expressed in the cortex of wild-type mice (see Fig. 4). (c) Chain migration of transplanted cells expressing reelin (green) in the cortex (×400). (d) Few GFAP-positive HNSCs (brown) detected in the cortex (×100). (e) Differentiation of HNSCs into βIII-tubulin-positive cells (green) near the injection site (×400). Nuclei were counterstained with DAPI (blue).
Analysis of Stem Cell Population in Reeler Mice.
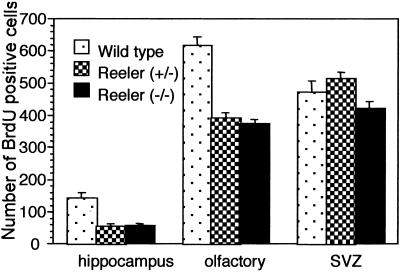
Because our study indicated the ineffective migratory ability of stem cells transplanted in reeler mice, we hypothesized that the endogenous stem cells of reeler mice might have migratory deficiencies. To compare migration of stem cells expressed in reeler mice with that of wild-type mice, we injected BrdUrd into homozygous reeler, heterozygous reeler (haploinsufficient for reelin; ref. 23), and wild-type mice for 4 days. Subsequently, an immunofluorescent BrdUrd staining of brain sections including hippocampal formation, olfactory bulb, and SVZ was performed. The mouse NSCs that proliferated during and after the BrdUrd injection can be identified by nuclei BrdUrd-fluorescence staining. We found that the number of BrdUrd-positive cells in the hippocampus and olfactory bulb of both homozygous and heterozygous reeler mice was smaller than in wild-type mice (Fig. 6 and Fig. 7). In contrast, stem cell population density in the SVZ of homozygous and heterozygous reeler mice was similar to that of wild-type mice (Figs. 6 and 7). These results indicate that the proliferation of stem cells was not affected in reeler mice; however, migration of these cells from the SVZ to the olfactory bulb and hippocampus was dramatically decreased, presumably because of the lack of reelin.
Figure 6.
Comparison of endogenous NSC populations in reeler and wild-type mice (×100). BrdUrd incorporated into the nuclei of proliferating cells was detected by fluorescent immunohistochemistry (red), and all of the nuclei were counterstained with DAPI (blue). (Upper) Dramatically decreased population of stem cells in the hippocampus of homozygous (a) and heterozygous (b) reeler mice compared with wild-type mice (c). (Lower) Similar population of stem cells in the SVZ of homozygous reeler (d) and wild-type mice (e). Note the unorganized structure of the dentate gyrus in homozygous reeler mice (a). Nuclei were counterstained with DAPI (blue).
Figure 7.
Number of BrdUrd-positive cells in heterozygous reeler (+/−), homozygous reeler (−/−), and wild-type mice in the hippocampus, olfactory bulb, and SVZ. Significant reduction of the stem cell population was observed in the hippocampus (P < 0.001) and olfactory bulb (P < 0.01) but not in the SVZ of reeler (+/−) and reeler (−/−) mice compared with wild-type mice by Fisher's Protected LSD post hoc analysis after ANOVA.
Discussion
We have provided evidence that reelin, Dab-1, and α3-integrin receptor subunits are expressed in HNSCs, indicating that HNSCs can use reelin signaling to self-regulate migration during differentiation. Addition of recombinant reelin to differentiating HNSC cultures with defined medium caused an immediate retraction of their processes and also caused the cells to migrate back to the neurospheroid. These findings not only indicate that reelin, α3-integrin receptor subunits, and Dab-1 are present in HNSCs, but also demonstrate the need for future studies to elucidate the functional role of reelin signaling cascades, including VLDLR and ApoER2 in HNSC biology. These results agree with a recent report that reelin expression in HEK 293T cells impairs their ability to adhere to a fibronectin-coated dish surface (20). The authors suggest that the serine protease activity of reelin may be an important factor to produce this antagonism. The HNSC differentiated in a defined medium consist of a spheroid, which is a cluster of undifferentiated cells in the center, and of migrating and differentiated cells in the periphery. Neurally differentiated HNSCs migrate back and forth on a glial network laid on the outside of the spheroid in a manner that is quite similar to the movement of the cells in the gastrula. During gastrulation, progenitor cells generate an “organizer,” which induces a second neural axis in the host embryo (28). In Fig. 2a, reelin seems to be released from reelin-positive cells and reaches the neighboring cells, suggesting that reelin released from a subpopulation of these stem cells may coordinate their movement as a regulator of their biology. Although we do not have direct evidence for the presence of an organizer in HNSC cultures, it would be interesting to examine the expression of reelin in the embryo organizer.
Studies have pointed to at least two reelin functions. Morphological analysis of brain sections derived from the reeler mouse cortex reveals the inability of migrating neurons to penetrate the subplate. Consequently, these neurons push the subplate toward the marginal zone forming a “superplate.” Thus, one function of reelin would be to allow migrating neurons to pass between subplate neurons. In this study, HNSCs transplanted into homozygous reeler mouse brain did not migrate, whereas HNSCs transplanted into wild-type mouse brain showed symmetrical migration and incorporation into the host brain. The only cells that migrated in homozygous reeler mice were reelin-positive HNSCs. It is probable that the serine protease activity of reelin is important in regulating the duration of the interactions between reelin and its receptors (e.g., integrin, VLDLR, or ApoER2; ref. 20). One may speculate that to penetrate into the previously laid down cells of the subplate during embryonic corticogenesis, migrating neurons or NSCs may have to digest ECM to make space for cell movement. However, one cannot dismiss the importance of reelin receptors in the coordination of reelin-mediated cell migration.
In fact, although reelin is expressed in α3-integrin receptor subunit-deficient mice (14), in VLDLR and ApoER2 double-knockout mice (16–18), and in “scrambler” and “yotari” mice (29), which fail to express functional Dab-1, all these mice display a phenotype similar to the reeler mouse, indicating involvement of the reelin signaling cascade in neuronal movement. Reelin not only induces tyrosine phosphorylation of Dab-1 but also modulates tau phosphorylation (17). Because the cooperative functions of tau and neuronal microtubule-associated proteins are reported in axonal elongation and neuronal migration (30), reelin may regulate neuronal movement by changing microtubule organization.
Analysis of cell populations incorporating BrdUrd into nuclei, which has been used as a marker of stem cells in the brain (31, 32), revealed significantly fewer BrdUrd-labeled cells in the olfactory bulb and hippocampus of the reeler mouse than wild-type mice. These results indicate that either the reeler mouse has a deficit in migration or that there is less proliferation of NSCs because of the lack of reelin. As we did not find a significant difference in the number of proliferating cells in the SVZ between reeler and wild-type mice, the rate of NSC proliferation seems to be similar in these genetic models. High levels of reelin are expressed in the adult olfactory bulb (33, 34), which includes physiologically active cell migration from the SVZ during their life span. Thus, the significant reduction of BrdUrd-positive cells in the olfactory bulb and hippocampus of the reeler mouse (Figs. 6 and 7) may be caused by a reelin-related deficiency of SVZ cell migration.
The deficit in NSC migration also may take place in the schizophrenia brain, which has significantly reduced reelin expression (35). Because reelin is preferentially expressed in GABAergic neurons in the adult cortex (21), some of the loss of GABAergic interneurons in the neocortex of schizophrenia (35, 36) may be explained by this mechanism. In fact, heterozygous reeler mice that only have a haploinsufficiency of reelin (similar to schizophrenia brain) also have a deficiency of cell migration similar to the reeler mouse (Figs. 6 and 7). This paper posits a number of interesting questions that require further investigation. One of these questions concerns the putative role of reelin in the function of the “organizer” in inducing formation of mesenchymal components.
Supplementary Material
Acknowledgments
We thank Dr. Jeffrey D. Rothstein, Johns Hopkins University, and Dr. Gabriella D'Arcangelo, Baylor College of Medicine, for constructive criticisms and suggestions in the preparation of the manuscript. We also thank Ms. Roberta Baruch for copy editing and Mr. Andrew K. Sugaya for technical support. The work was supported in part by National Institute of Mental Health Grants MH062188 (to A.G.) and MH062090 (to E.C.).
Abbreviations
- NSC
neural stem cell
- SVZ
subventricular zone
- HNSC
human NSC
- DAPI
4′,6-diamidino-2-phenylindole
- RT
room temperature
- BrdUrd
bromodeoxyuridine
References
- 1.Doetsch F, Caille I, Lim D A, Garcia-Verdugo J M, Alvarez-Buylla A. Cell. 1999;97:703–716. doi: 10.1016/s0092-8674(00)80783-7. [DOI] [PubMed] [Google Scholar]
- 2.Johansson C B, Momma S, Clarke D L, Risling M, Lendahl U, Frisen J. Cell. 1999;96:25–34. doi: 10.1016/s0092-8674(00)80956-3. [DOI] [PubMed] [Google Scholar]
- 3.Gould E, Reeves A J, Fallah M, Tanapat P, Gross C G, Fuchs E. Proc Natl Acad Sci USA. 1999;96:5263–5267. doi: 10.1073/pnas.96.9.5263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kornack D R, Rakic P. Proc Natl Acad Sci USA. 1999;96:5768–5773. doi: 10.1073/pnas.96.10.5768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gage F H. Science. 2000;287:1433–1438. doi: 10.1126/science.287.5457.1433. [DOI] [PubMed] [Google Scholar]
- 6.Qu T, Brannen C L, Kim H M, Sugaya K. NeuroReport. 2001;12:1127–1132. doi: 10.1097/00001756-200105080-00016. [DOI] [PubMed] [Google Scholar]
- 7.Corbin J G, Nery S, Fishell G. Nat Neurosci. 2001;4, Suppl. 1:1177–1182. doi: 10.1038/nn749. [DOI] [PubMed] [Google Scholar]
- 8.Marin O, Rubenstein J L. Nat Rev Neurosci. 2001;2:780–790. doi: 10.1038/35097509. [DOI] [PubMed] [Google Scholar]
- 9.D'Arcangelo G, Miao G G, Chen S C, Soares H D, Morgan J I, Curran T. Nature (London) 1995;374:719–723. doi: 10.1038/374719a0. [DOI] [PubMed] [Google Scholar]
- 10.Anderson S A, Marin O, Horn C, Jennings K, Rubenstein J L. Development (Cambridge, UK) 2001;128:353–363. doi: 10.1242/dev.128.3.353. [DOI] [PubMed] [Google Scholar]
- 11.Leavitt B R, Hernit-Grant C S, Macklis J D. Exp Neurol. 1999;157:43–57. doi: 10.1006/exnr.1999.6982. [DOI] [PubMed] [Google Scholar]
- 12.Yang H Y, Lieska N, Kriho V, Wu C M, Pappas G D. Exp Neurol. 1997;146:199–205. doi: 10.1006/exnr.1997.6518. [DOI] [PubMed] [Google Scholar]
- 13.Durbec P, Rougon G. Mol Cell Neurosci. 2001;17:561–576. doi: 10.1006/mcne.2000.0951. [DOI] [PubMed] [Google Scholar]
- 14.Dulabon L, Olson E C, Taglienti M G, Eisenhuth S, McGrath B, Walsh C A, Kreidberg J A, Anton E S. Neuron. 2000;27:33–44. doi: 10.1016/s0896-6273(00)00007-6. [DOI] [PubMed] [Google Scholar]
- 15.Costa E, Davis J, Grayson D R, Guidotti A, Pappas G D, Pesold C. Neurobiol Dis. 2001;8:723–742. doi: 10.1006/nbdi.2001.0436. [DOI] [PubMed] [Google Scholar]
- 16.D'Arcangelo G, Homayouni R, Keshvara L, Rice D S, Sheldon M, Curran T. Neuron. 1999;24:471–479. doi: 10.1016/s0896-6273(00)80860-0. [DOI] [PubMed] [Google Scholar]
- 17.Hiesberger T, Trommsdorff M, Howell B W, Goffinet A, Mumby M C, Cooper J A, Herz J. Neuron. 1999;24:481–489. doi: 10.1016/s0896-6273(00)80861-2. [DOI] [PubMed] [Google Scholar]
- 18.Trommsdorff M, Gotthardt M, Hiesberger T, Shelton J, Stockinger W, Nimpf J, Hammer R E, Richardson J A, Herz J. Cell. 1999;97:689–701. doi: 10.1016/s0092-8674(00)80782-5. [DOI] [PubMed] [Google Scholar]
- 19.Howell B W, Hawkes R, Soriano P, Cooper J A. Nature (London) 1997;389:733–737. doi: 10.1038/39607. [DOI] [PubMed] [Google Scholar]
- 20.Quattrocchi C C, Wannenes F, Persico A M, Ciafre S A, D'Arcangelo G, Farace M G, Keller F. J Biol Chem. 2001;31:31. doi: 10.1074/jbc.M106996200. [DOI] [PubMed] [Google Scholar]
- 21.Pesold C, Impagnatiello F, Pisu M G, Uzunov D P, Costa E, Guidotti A, Caruncho H J. Proc Natl Acad Sci USA. 1998;95:3221–3226. doi: 10.1073/pnas.95.6.3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pesold C, Liu W S, Guidotti A, Costa E, Caruncho H J. Proc Natl Acad Sci USA. 1999;96:3217–3222. doi: 10.1073/pnas.96.6.3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu W S, Pesold C, Rodriguez M A, Carboni G, Auta J, Lacor P, Larson J, Condie B G, Guidotti A, Costa E. Proc Natl Acad Sci USA. 2001;98:3477–3482. doi: 10.1073/pnas.051614698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rodriguez M A, Pesold C, Liu W S, Kriho V, Guidotti A, Pappas G D, Costa E. Proc Natl Acad Sci USA. 2000;97:3550–3555. doi: 10.1073/pnas.050589797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brannen C L, Sugaya K. NeuroReport. 2000;11:1123–1128. doi: 10.1097/00001756-200004070-00042. [DOI] [PubMed] [Google Scholar]
- 26.Svendsen C N, ter Borg M G, Armstrong R J, Rosser A E, Chandran S, Ostenfeld T, Caldwell M A. J Neurosci Methods. 1998;85:141–152. doi: 10.1016/s0165-0270(98)00126-5. [DOI] [PubMed] [Google Scholar]
- 27.D'Arcangelo G, Nakajima K, Miyata T, Ogawa M, Mikoshiba K, Curran T. J Neurosci. 1997;17:23–31. doi: 10.1523/JNEUROSCI.17-01-00023.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kinder S J, Tsang T E, Wakamiya M, Sasaki H, Behringer R R, Nagy A, Tam P P. Development (Cambridge, UK) 2001;128:3623–3634. doi: 10.1242/dev.128.18.3623. [DOI] [PubMed] [Google Scholar]
- 29.Sheldon M, Rice D S, D'Arcangelo G, Yoneshima H, Nakajima K, Mikoshiba K, Howell B W, Cooper J A, Goldowitz D, Curran T. Nature (London) 1997;389:730–733. doi: 10.1038/39601. [DOI] [PubMed] [Google Scholar]
- 30.Takei Y, Teng J, Harada A, Hirokawa N. J Cell Biol. 2000;150:989–1000. doi: 10.1083/jcb.150.5.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yagita Y, Kitagawa K, Ohtsuki T, Takasawa K, Miyata T, Okano H, Hori M, Matsumoto M. Stroke. 2001;32:1890–1896. doi: 10.1161/01.str.32.8.1890. [DOI] [PubMed] [Google Scholar]
- 32.Zhang R L, Zhang Z G, Zhang L, Chopp M. Neuroscience. 2001;105:33–41. doi: 10.1016/s0306-4522(01)00117-8. [DOI] [PubMed] [Google Scholar]
- 33.Alcantara S, Ruiz M, D'Arcangelo G, Ezan F, de Lecea L, Curran T, Sotelo C, Soriano E. J Neurosci. 1998;18:7779–7799. doi: 10.1523/JNEUROSCI.18-19-07779.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ikeda Y, Terashima T. Dev Dyn. 1997;210:157–172. doi: 10.1002/(SICI)1097-0177(199710)210:2<157::AID-AJA8>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 35.Guidotti A, Auta J, Davis J M, DiGiorgi Gerevini V, Dwivedi Y, Grayson D R, Impagnatiello F, Pandey G, Pesold C, Sharma R, et al. Arch Gen Psychiatry. 2000;57:1061–1069. doi: 10.1001/archpsyc.57.11.1061. [DOI] [PubMed] [Google Scholar]
- 36.Reynolds G P, Zhang Z J, Beasley C L. Brain Res Bull. 2001;55:579–584. doi: 10.1016/s0361-9230(01)00526-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.