Abstract
Ferritin concentrates iron as a hydrous ferric oxide in a protein cavity (8 nm in diameter) by using eight pores along the threefold symmetry axes of the octahedral supramolecular structure. The role of ligand exchange in the entry of Fe(II) hexahydrate into ferritin protein has been studied with [Cr(TREN)(H2O)(OH)]2+ [TREN = N(CH2CH2NH2)3], a model for Fe(H2O)62+ with only two exchangeable ligands. The results show that five different ferritin proteins, varying in pore structure, oxidation sites, and nucleation sites, bind Cr(TREN) at functional protein sites, based on inhibition of iron mineralization and oxidation. Properties of Cr(TREN)–ferritin adducts include an increased isoelectric point, a shift in the Cr(TREN) UV/vis spectrum consistent with exchange of water for protein carboxylate or thiolate ligands, binding affinities of 50–250 μM, and a slow rate of dissociation (k = 4 × 10−6 sec−1). The relationship of Cr(TREN) inhibition of iron oxidation and mineralization by Cr(TREN) to the known structures of the various ferritins tested suggests that Cr(TREN) plugs the ferritin pores, obstructing Fe(II) entry in folded and unfolded pores. Because only two exchangeable waters are sufficient for pore binding of Cr(TREN), the physiological Fe(II) donor must bind to the pore with few exchangeable ligands. These results show the advantage of using stable model complexes to explore properties of transient Fe–protein complexes during Fe mineralization in ferritin.
Ferritin, the intracellular protein that concentrates up to 4,500 iron atoms as Fe2O3·H2O, self-assembles from 24 subunits into a hollow protein (usually 12 nm in diameter with an 8-nm-diameter cavity) with octahedral symmetry (rotational point group symmetry O, Fig. 1). Ferritin concentrates iron 100 billion times above the solubility of ferric ion in a nontoxic, accessible form (1, 2). The subunits, four α-helix bundles, contain a catalytic center that converts two Fe(II) atoms to an Fe(III)-oxo bridged dimer intermediate in mineralization (Fig. 1). In vertebrates, a subunit (L) with an inactive catalytic center coassembles with the catalytically active subunits (H) with tissue-specific H/L ratios (3, 4) to modulate rapid H subunit iron uptake; this has the cost of peroxide production (2, 5–7). In addition to concentrating iron, ferritin plays an important role in scavenging intracellular iron to modulate the cellular labile iron pool (8). The primary route of iron transit into and out of the protein is through eight hydrophilic pores in the protein shell, which lead to the ferroxidase site (1.0 to 1.5 nm away) and from the ferric oxide nanoparticle (2 nm away). Clusters of glutamate residues located at the subunit dimer interfaces on the ferritin cavity nucleate the mineral (Fig. 1). When catalytic sites are few (low H/L ratio) some iron oxidation also occurs at the surface of the growing iron mineral.
Figure 1.
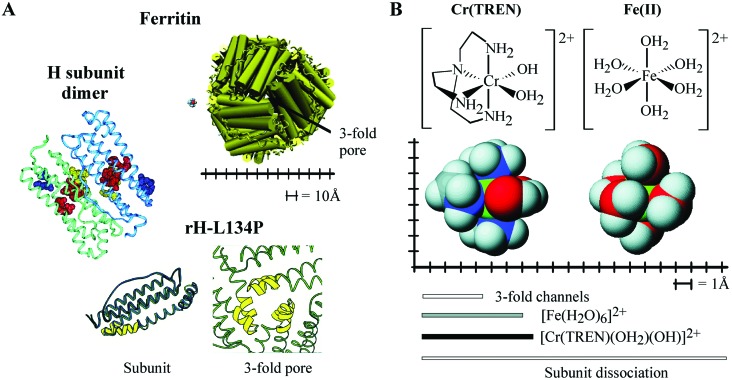
Ferritin structure, functional sites, and relative sizes of Cr(TREN) and Fe(II) to ferritin pore. (A Upper) A cartoon of ferritin viewed down the pore along the threefold axis where subunit triples meet, with the Cr(TREN) complex shown to scale. (Left) Ribbon diagram of an H-subunit dimer, with functional residues highlighted as space-filling models [in purple, threefold channels (Asp-127, Glu-130); in red, ferroxidase site (Glu-23, Glu-58, His-61, and Glu-103), and in yellow, nucleation site at the dimer interface (Glu-57, Glu-60, and Glu-63)]. (Lower) The subunit monomer and pore structure in rH-L134P: the rH-L134P subunit (green) is structurally identical to the rL subunit (dark blue), with the exception of a disordered region at the end of two helices (yellow); the disordered region is limited to the pore. The figure of the dimer was generated using the application software insight ii and the coordinates for human H ferritin. The figure of rH-L134P subunit monomer and pore are reproduced from ref. 3. Accession numbers for the human H ferritin and frog rH-L134P coordinates in the Brookhaven Protein Database are 2FHA and 1BG7 (17, 20). (B) Cr(TREN) structure compared with hydrated Fe(II) and size of the ferritin pore. The Fe(II) analogue Cr(TREN) has approximately the same size, shape, and charge as [Fe(H2O)6]2+. The figure is calibrated in Angstroms for size comparisons, and the bars show the sizes of the resting diameter of the pores, and of the Fe(II) and Cr(TREN) complexes. Dissociation of a ferritin subunit is the only way to make a large aperture (20 Å). Space-filling models were prepared using CAChe; metal ligand bond distances used during minimization are from crystal structures for Fe(II) (49, 50) and similar Cr(III)TREN compounds (51).
Several iron-binding sites have been identified by a combination of protein crystallography, ion competition, and mutagenesis. Three categories of iron sites can be defined according to function (1): (i) pore residues§ (Asp-127, Glu-130, Cys-126,¶ His-114, Leu-110 and -134, and Arg-72/Asp-122) (9–18); (ii) protein-catalyzed iron oxidation (Glu-23, Glu-57, Glu-58, His-61, Glu-103, Gln-137, and Asp-140) (4, 19–23); and (iii) Fe(III) nucleation/chelation in the interior surface of the protein cavity (Glu-56, Glu-57, Glu-60, and Glu-63 in H subunits and Glu-53, Glu-56, Glu-57, Glu-60, and Glu-63 in L subunits) (24, 25).
The extent of ligand exchange that occurs during iron entry into the protein is unknown. However, it is clear from the crystal structure of ferritin that the threefold pores are too narrow (3–4) Å to accommodate [Fe(H2O)6]2+ without ligand exchange or a conformational change of the protein to widen the pores (26). Evidence of at least momentary larger pore size is shown by the diffusion of large molecules in and out of the ferritin cavity, exemplified by sugars as large as 13 Å (27), iron chelators (28, 29), spin probes (28), and large iron complexes (up to 13 Å) (28, 29). Evidence for protein flexibility is the observation of alternate side chain conformations during ferritin crystallization (3) and localized pore unfolding with preservation of global folding and supramolecular assembly (17, 18). Microscopy studies indicate that short lived holes in ferritin of 2.0–3.0 nm might occur by dynamic dissociation/association of subunits (ref. 30; Fig. 1).
To probe the requirements for ligand exchange of ferrous ions entering ferritin, a kinetically inert analogue of the labile ferrous hexaaquo ion, [Cr(TREN)(H2O)(OH)]2+ (TREN = N(CH2CH2NH2)3), abbreviated here as Cr(TREN), was chosen. The half-life of water exchange for the exchange inert aqua chromium (III) ion (nearly 2 weeks) remains 7–9 orders of magnitude slower than corresponding complexes of high-spin Fe(III) at a variety of values of pH and much slower still than for complexes of high-spin Fe(II). Thus the expected rate of ligand exchange for similar Cr(III) and Fe(II) complexes are expected to differ by at least 10 orders of magnitude. Although Cr(TREN) has a size, shape, and charge similar to [Fe(H2O)6]2+ at pH 7 the TREN ligand cannot be exchanged, which leaves only two labile water/hydroxyl for substitution with protein residues. The results of these studies indicate that Cr(TREN) binds to ferritin and inhibits both ferrous oxidation and mineralization, indicating that two exchangeable ligands are sufficient for metal ion binding to ferritin. Cr(TREN) binding affinities and stoichiometries are in the range 50–250 μM, whether active ferroxidase or iron nucleation/chelation sites are present, but ferritins without nucleation sites are much more sensitive to Cr(TREN) inhibition, suggesting that the active Fe site protects against Cr(TREN) inhibition. Ferritins with unfolded entry pores bind Cr(TREN) with significantly higher affinity and stoichiometry, indicating that the main effect of Cr(TREN) is to decrease (in the more rigid ferritins) or completely block (in the more flexible proteins) iron entry through the eight pores along the ferritin cluster threefold symmetry axes.
Experimental Methods
General.
All starting materials were purchased from Sigma, Aldrich, Bio-Rad, or Strem Chemicals and were used without further purification, unless otherwise specified. The water was purified to 18 MΩ (reverse osmosis-charcoal demineralization-Millipore). Apoferritin concentrations, determined by the Bradford method [BSA standard (31)] were confirmed spectrophotometrically (molar absorptivity of ɛ280nm = 4.8 × 105 M−1⋅cm−1 per ferritin). The 51CrCl3, obtained from New England Nuclear and incorporated into Cr(TREN), was analyzed by scintillation spectrometry [TRI-CARB 2100-TR (Packard)]. Elemental analyses, performed at the Microanalysis Facility (University of California, Berkeley), used samples digested in concentrated nitric acid (1:1) and boiled for 1 h. Electronic absorption spectra were recorded on a Hewlett–Packard HP8452A spectrophotometer (1-cm path length) and stopped-flow UV-vis data were collected on an Applied Photophysics instrument (Surrey, U.K.). See Supporting Text, which is published as supporting information on the PNAS web site, www.pnas.org, for other details.
Results
Ferritin Binding of Cr(TREN) Alters the UV-vis Absorbance Spectrum.
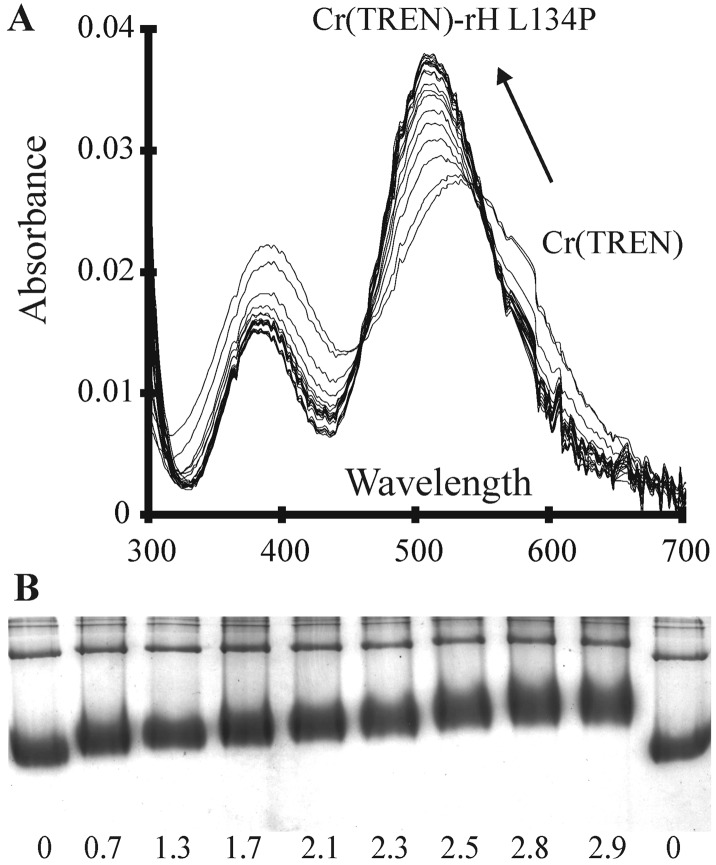
Binding of Cr(TREN) to apoferritins was monitored through the change in the UV/Vis spectrum of the Cr(TREN) with increasing Cr(TREN):protein ratio (Fig. 2). A shift in the main Cr(TREN) absorbance peak from 534 nm to 510 nm and extinction coefficient increase from 69 to 95 was observed on protein binding, along with a shift in a smaller absorbance peak from 394 nm to 380 nm and an extinction coefficient decrease from 58 to 39. Two isosbestic points, indicating the conversion of free Cr(TREN) to ferritin-bound Cr(TREN), were observed in the range 440–460 and 540–550 nm. The energy of the d-d transition in the Cr(TREN)–ferritin complex is consistent with retention of the TREN backbone and substitution of coordinated water by protein side chains such as carboxylate or sulfur, but not histidine (32–35).
Figure 2.
Cr(TREN)–ferritin Interactions. (A) The effect of ferritin on the Cr(TREN) spectrum. UV-vis spectra showing the shift in the Cr(TREN) d-d bands on complexation to rH-L134P, with two apparent isosbestic points at 544 and 460 nm. Cr(TREN) (0.4 mM, 2 equivalents per subunit) was incubated with rH-L134P (8 μM) at 25°C. Spectra were taken at 20-min intervals. The absorption spectrum of the protein in buffer, before Cr(TREN) addition, was used as a reference. (B) The effect of Cr(TREN) on protein surface charge (electrophoretic mobility). Migration of rH-L134P, with varying amounts of bound Cr(TREN) during electrophoresis in native polyacrylamide gels (6%). Values shown are Cr(TREN) per subunit after removing unbound Cr(TREN) by dialysis.
Cr(TREN) Binding to Ferritin Decreases the Protein Electrophoretic Mobility.
Formation of a Cr(TREN)-apoferritin complex changed the protein mobility in nondenaturing gels (6% polyacrylamide). (The types of protein studied are described in Table 1, which is published as supporting information on the PNAS web site.) When Cr(TREN):protein increased, the mobility decreased proportionately, up to three Cr(TREN) per subunit [72 Cr(TREN)] bound per protein.
Increased mass and size would only account for minor changes in electrophoretic mobility. The negative charge of the protein is diminished when Cr(TREN) binds, because Cr(TREN) is a dication,‖ and can mask resident charges in the protein. The pI values of the ferritin–Cr(TREN) complexes, analyzed by isoelectric focusing, increased linearly with the equivalents of Cr(TREN) binding (correlation coefficient for rH-L134P mobility shifts, for example, was 0.98), showing that masking of the negative charge accounts for most of the change in mobility of ferritin in the Cr(TREN) complex.
Stoichiometry and Stability of the Cr(TREN)–Ferritin Complex.
To determine the effect of Cr(TREN) binding in ferritin function, four recombinant ferritins (rH, rH-L134P, rL, and rL-E2) varying in pore structure, oxidation sites, and nucleation sites were used in these studies. (The properties of these different ferritins are listed in Table 1.) In addition, the effect of mixed subunits in chromium binding was studied in native horse spleen ferritin, which has an approximate H4L20 subunit ratio. The threefold pores are disorganized in rH-L134P (Fig. 1; ref. 17) but are well ordered in the parent protein (rH) as well as in the other ferritins tested (HoSF, rL, and rL-E2). The nucleation/chelation sites on the inner protein surface are altered in the mutant ferritin rL-E2 (four conserved glutamates have been replaced with alanine residues (3), but are intact in the parent protein (rL) as well as in the remaining three ferritins tested (rH, rH-L134P, and HoSF). Finally, the ferroxidase sites are present in three of the ferritins examined (rH, rH-L134P, and 16% of the HoSF subunits), but are absent in the L-type proteins (rL and rL-E2).
The measured rate of dissociation of bound Cr(TREN) from Cr(TREN)-HoSF is 4.1 × 10−6 s−1 and is expected to be within the same order of magnitude for the other proteins. This slow dissociation permitted the separation of unbound Cr(TREN) from the ferritin–Cr(TREN) mixture by dialysis and then measurement of the amount of Cr(TREN) bound to each ferritin. Binding constants were determined after dialysis for the five Cr(TREN)–ferritin complexes, using 2.1 μM solutions of ferritin and Cr(TREN) concentrations ranging from 0 to 1.5 mM (720 per molecule).
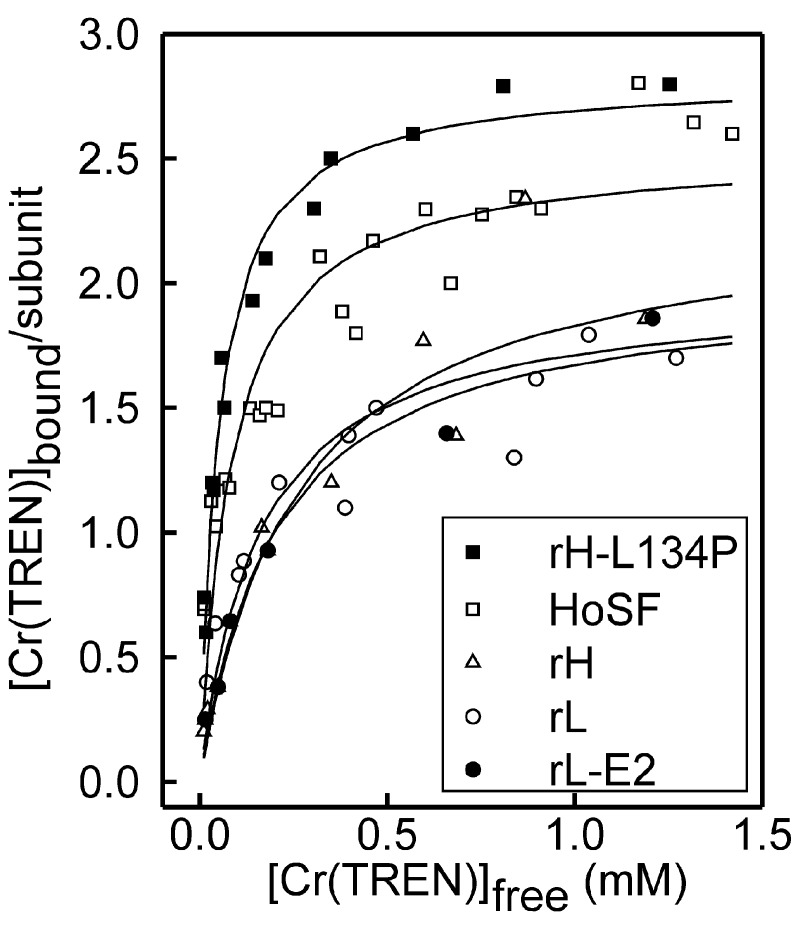
The number of Cr(TREN)-binding sites on ferritin ranged from ≈2.0 to 2.8 among the various ferritins (Fig. 3). No significant difference in binding was observed between recombinant frog ferritins with active ferroxidase and nucleation sites (rH), ferritins lacking ferroxidase sites (rL), or ferritins lacking both ferroxidase and nucleation sites (rL-E2) (Table 1). Importantly, ferritins with locally disordered helices around the threefold axis pores (rH-L134P) bound significantly more Cr(TREN) and with a much greater affinity (5-fold) than the parent protein, rH. The native ferritin from horse spleen (HoSF) bound almost as much Cr(TREN) as the protein with flexible entry pores, rH-L134P.
Figure 3.
Stoichiometry of Cr(TREN)-binding to ferritin. Saturation curves are shown for Cr(TREN) binding to apoferritin after dialysis as a function of free Cr(TREN) added (mM). No significant effect in Cr(TREN) binding was observed when rH-L134P and HoSF were partially filled with 1,000 and 2,000 Fe atoms per protein, respectively (data not shown). Solid lines correspond to nonlinear regression of the data to a one-site model (according to Eq. 1 of Supporting Text) The number of data sets averaged (and of different protein preparations used): rH-L134P = 4 (2); rH = 2 (1); HoSF = 4 (2); rL = 2 (2), E2 = 2 (1); rH-L134P+Fe = 1; HoSF+Fe = 2 (1) (see Table 1).
Stability constants for Cr(TREN) binding to the proteins varied from 50 to 250 μM among the five proteins, with the most stable Cr(TREN)–ferritin complexes formed in rH-L134P and HoSF. In all cases, the data were fitted to a single type of binding site (Fig. 3); however, in the case of HoSF, an improved fit to the data was obtained when using a two-site model [with an overall stoichiometry of 72 Cr(TREN) per subunit]. Whereas good fits to the data could be obtained for HoSF and rH-L134P, much poorer fits were obtained for the rH, rL, and rL-E2 proteins, because these data were noisier and there were not enough data points in the near saturation part of the curve as a result of the weaker affinity for Cr(TREN).
Cr(TREN) Binding Inhibits both Iron Oxidation and Iron Mineralization.
To determine whether Cr(TREN) bound to a site(s) required for ferritin function, the effect of Cr(TREN) binding on oxidation and mineralization was examined in Cr(TREN)–ferritins. Iron oxidation was analyzed on Cr(TREN)–ferritins immediately following the addition of ferrous ion to the protein, and was only examined in ferritins containing H subunits (rH, rH-L134P, and HoSF); the L-type ferritins rL and rL-E2 were not assayed because their rates of oxidation are too slow to distinguish from iron autooxidation outside the protein shell. The effect of Cr(TREN) on iron mineralization was analyzed in all Cr(TREN)–ferritin complexes 2 h after Fe(II) addition. The percentage of Cr(TREN) lost during the oxidation and mineralization experiments was negligible (0.05% and 3%, respectively).
Oxidation of iron was inhibited by Cr(TREN) binding in the three ferritin proteins tested (Fig. 4). Rates of oxidation were compared using measurements of the absorbance at 350 nm of ferric–oxo complexes and mineral. This assay could be used whether or not the H-subunit-specific, blue diferric peroxo complex formed. In horse spleen ferritin, where the contribution of H-subunits (H/L ≈ 1:5) is small, oxidation cannot be monitored by changes in the absorbance at 650 nm.
Figure 4.
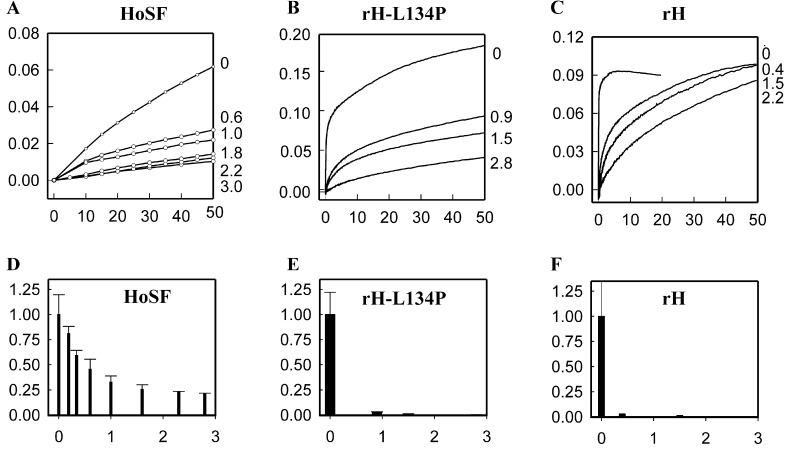
Inhibition of Fe(II) oxidation in Cr(TREN)–ferritin complexes. (Upper) The rates of Fe oxidation as the diferric oxo and ferric oxo mineral (A350nm) for HoSF (H/L ≈ 0.2), and for rH, rH-L134P, and plotted vs. Cr(TREN) bound per subunit (shown at right). In HoSF iron oxidation was monitored 10 s after addition of Fe2+; final concentrations: [Fe2+] 0.25 mM, protein 2 μM, [NaCl] 0.2 M, pH 7.0 [0.1 M 4-morpholinepropanesulfonic acid (Mops)]. In rH-L134P and rH iron oxidation was monitored within 10 ms after addition of Fe2+ by using stopped-flow spectrophotometry; final concentrations: [Fe2+] 80 μM, protein 1.6 μM, [NaCl] 0.2 M, pH 7.0 (0.1 M Mops). Unbound Cr(TREN) was removed by dialysis. (Lower) The initial rate (vi = ΔA350nm/min) in the presence of varying amounts of Cr(TREN) per subunit. This is normalized to the free protein (vo) and shown as a function of Cr(TREN) bound per subunit.
In contrast to the ease of monitoring effects of Cr(TREN) on function of rH ferritins at low Fe/protein ratios (2 per subunit or 48 per molecule), higher Fe/protein ratios had to be used for the L-rich HoSF (5.2 Fe per subunit or 125 per protein) to achieve rates readily analyzed, but the stoichiometries of Cr(TREN) inhibition were comparable.** [The inhibition of iron oxidation by Cr(TREN) for different types of ferritin is shown in Table 1. When the Fe per subunit increases above ≈0.5 oxidation in HoSF, in contrast to H-ferritins, oxidation shifts from sites on the protein to sites on the mineral itself (36–38).]
The formation of the colored Fe(III) dimer intermediate in rH and rH-L134P was also inhibited because of Cr(TREN) binding, even at stoichiometries of <1 Cr(TREN) bound per subunit. Whereas the Fe(III) intermediate in rH is transient, forming and decaying within milliseconds (with a maximum absorbance at 650 nm), in rH-L134P the Fe(III) intermediate is long-lived (maximum absorbance at 550 nm) and decays slowly over several hours (37, 39, 40). In Cr(TREN)-rH, no Fe(III) transient intermediate was detected at any Cr(TREN)/protein ratio (data not shown).
Because the Fe(III) intermediate in rH-L134P is long-lived, it was possible to monitor the number of active ferroxidase centers as a function of Cr(TREN) binding (see Supporting Text). The amount of colored Fe(III) intermediate produced in rH-L134P was found to decrease linearly with Cr(TREN) binding, with no intermediate detectable when 48 Cr(TREN) were bound per protein [or 2 Cr(TREN) per subunit]. This finding is consistent with the competitive inhibition of the ferroxidase sites in rH-L134P by Cr(TREN).
Mineralization of iron was measured in all five ferritin–Cr(TREN) complexes (rH, rH-L134P, HoSF, rL, and rL-E2) 2 h after the addition of Fe2+. Unincorporated iron was separated from the ferritins by using native polyacrylamide gels. The ferritin mineral was stained by the formation of Prussian blue (ferriferrocyanide) in gels soaked in acidic solutions of potassium ferrocyanide. Differences in the amount of Prussian blue per protein were quantitated by densitometry of the stained gels. Iron mineralization was inhibited in all proteins tested (Fig. 5). However, the sensitivity to Cr(TREN) stoichiometry varied significantly among the five ferritins, and correlated with the number of functional iron-binding sites present per protein (see Supporting Text). The ferritin rL-E2, lacking ferroxidase sites and with altered nucleation sites (substitution of four COOH side chains by four CH3 side chains), was the most sensitive to Cr(TREN) inhibition, requiring less than one bound Cr(TREN) per subunit (≈21 Cr per protein) for 50% inhibition of iron mineralization. Ferritins containing functional iron nucleation sites (rL and HoSF) required twice as much bound Cr(TREN) to reduce iron mineralization by 50% (≈42 Cr per protein). Finally, ferritins containing functional ferroxidase sites and nucleation sites (rH and rH-L134P) required approximately three times as much bound Cr(TREN) as rL-E2 to achieve 50% inhibition of Fe(III) mineralization (≈60 Cr(TREN) per protein). Although 17% of the subunits in HoSF have functional ferroxidase sites, the protein's sensitivity to 50% inhibition of Fe (III) mineralization by Cr(TREN) is more similar to rL than to rH, presumably because of its higher L subunit content (L/H ≈ 5:1).
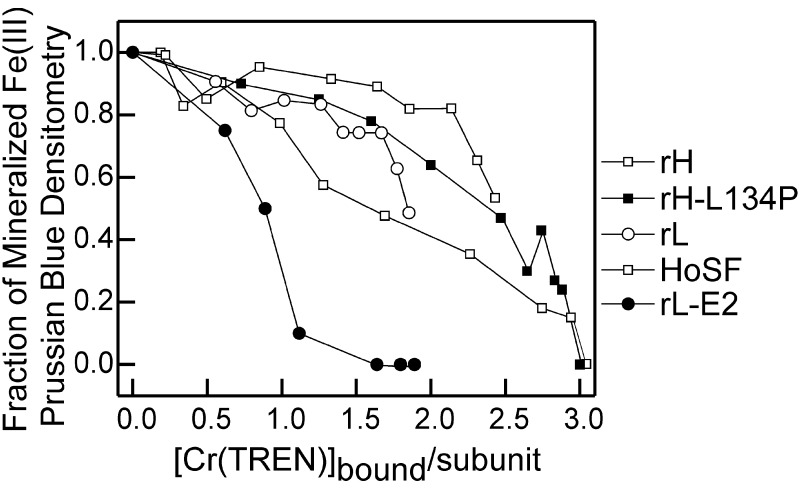
Figure 5.
Inhibition of Fe mineralization in Cr(TREN)–ferritin complexes. Mineralization as a function of [Cr(TREN)] bound per protein subunit is shown. Ferritins (2 μM) were preincubated with Cr(TREN) (0–2.5 mM) for 24 h at 25°C, Fe(II) was added {500 Fe(II) per protein, [Fe] = 1 mM}, and after 2 h incubation, unmineralized Fe was separated from the protein by electrophoresis in native polyacrylamide gels (6%). Mineralization was monitored as the density of Prussian blue formed in protein bands after soaking acidic solutions of potassium ferrocyanide. For each protein, the amount of iron mineralization is shown relative to the iron cores formed in the absence of Cr(TREN). The average of multiple experiments is shown; there was little variation observed in replicate experiments. The number of independent measurements for each type of protein is indicated, with the number of independent protein preparations shown in parenthesis: rH = 3 (1), rH L134P = 4 (3), HoSF = 4 (2), rL = 2 (2), E2 = 2 (1).
Greater than 90% inhibition of Fe(III) mineralization was reached in rL-E2 with only ≈26 bound Cr(TREN) per protein. The structure of rL-E2 is very similar to rL, except at the sites of COOH/CH3 substitution (3), and Cr(TREN) binding to rL-E2 and rL is also very similar (Fig. 3). This result highlights the importance of functional nucleation sites in facilitating Fe(III) core formation of ferritin, and suggests that, as long as Fe(II) entry into the protein cavity occurs and functional nucleation sites are present, an Fe(III) core will be formed. Although the binding of two Cr(TREN) per subunit in rH-L134P completely abolished Fe(II) oxidation at the ferroxidase sites, only at saturating Cr(TREN) stoichiometries was full inhibition of Fe(III) mineralization achieved in HoSF and rH-L134P.
Discussion
Several of the iron-binding sites in ferritin share functional properties with sites in other metal–protein complexes, whereas others are unique to ferritin. Unique sites are the cluster of carboxylate side chains that nucleate the mineral at the interface of two subunits on the inner protein surface/cavity surface and iron-binding sites on the mineral surface. Ferritin sites shared with channel/pore proteins are those for iron ion entry and transport to the mineral core. Ferritin catalytic oxidation sites are shared with a number of non-heme iron oxidases, such as methane monoxygenase, ribonucleotide reductase, and Δ9- (41–43) and oxygenases (44–47). Properties of the Cr(TREN)–ferritin complex indicate that Cr(TREN) binds to carboxylate residues on the surface of the ferritin protein, close to the trigonally symmetric entry pores formed by sets of three subunits: First, the electrophoretic mobility of the Cr(TREN)–ferritin complex changes. Second the UV-vis spectrum (Fig. 2) suggests Cr(TREN) binding to carboxylate or thiolate protein ligands, known to be invariant in ferritin pores and oriented to form a negatively charged path for directing Fe(II) toward the three symmetry-related Glu-130, Glu-130′, and Glu-130" residues that are buried along the pores (Fig. 1). Conservative substitutions of residues along the threefold axes (Asp-127 and Glu-131) dramatically reduce iron uptake or oxidation (44–47) while substitutions surrounding the pore, such as Leu-134, His-114, His-124, Cys-86, Cys-98, and Cys-126, have small effects on iron mineralization (14, 17). In contrast to those conserved substitutions around the pores, binding of Cr(TREN) may sterically hinder Fe(II) entry [because of the large fixed size of the Cr(TREN) molecule], and electrostatically repel incoming Fe(II).
Formation of the Cr(TREN)–ferritin complex occurs with five different types of ferritin that vary in number of internal functional iron sites but share the number of iron entry or pore sites (Table 1). The stoichiometry of Cr(TREN) binding did not vary significantly between proteins containing both ferroxidase and nucleation sites (rH), nucleation sites only (rL), or ferritins lacking both ferroxidase and nucleation sites (rL-E2). Because Cr(TREN) inhibits oxidation of Fe(II) by ferritin (Fig. 4 and Supporting Text) in H-type ferritins rH and rH-L134P (where the reaction is exclusively at the ferroxidase site) and in HoSF (where the reaction occurs on the mineral as well as at the protein ferroxidase site), Cr(TREN) must block a step common to both oxidation pathways.
Cr(TREN) binding inhibited iron mineralization in ferritin even when no active ferroxidase sites were present (Fig. 5; rL and rL-E2) and the protein participated only in entry/translocation and nucleation/chelation. Moreover, Cr(TREN) inhibited mineralization in rL-E2 [the nucleation/chelation site mutant with only the Fe entry/translocation sites functional (3)]. Almost complete inhibition of mineralization occurred in rL-E2 at a stoichiometry of 1.1 Cr(TREN) per subunit. However, significantly more Cr(TREN) was required to block mineralization in the parent protein rL, and more still was needed to block mineralization in rH than rL. Thus, although ferroxidase and nucleations sites change the stoichiometry of Cr(TREN) needed to inhibit mineralization, the decrease in Fe(III) mineralization observed in all proteins must result from a decreased flux of iron into the protein cavity.
Protein-dependent variations in Cr(TREN) binding itself were generally small. Nevertheless, the localized disorder of the polypeptide background around the pores in H-L134P (17), which should facilitate solute access to protein residues, coincided with the highest Cr(TREN) affinity (Fig. 1). The ferroxidase activity in rH-L134P was competitively blocked on binding of two Cr(TREN) per subunit, and the threefold pores were obstructed on binding of an additional equivalent of Cr(TREN), with iron mineralization inhibited by (90%). HoSF, a natural mixture of H and L subunits, behaved more like rH-L134P than to either rH or rL, suggesting that mixture of subunits creates pores more flexible than recombinant proteins of all one subunit type.
The success of the design of Cr(TREN) as a ferrous ion analog/inhibitor is shown by Fe sites blocked by Cr(TREN), and the relative mineralization sensitivity of ferritins with different numbers of iron-binding sites. Although there is some other evidence of binding at another site (C.M.B., S. Petoud, S. M. Cohen, and K.N.R., unpublished data), comparison of Cr(TREN) interactions between sets of wild-type and mutant proteins reinforces the conclusion that Cr(TREN) binds at the surface of the three proteins surrounding the threefold axis pores, obstructing Fe(II) entry; mineralization was inhibited in all five ferritins tested, regardless of the combinations of oxidation sites and nucleation/chelation sites present (Fig. 5). Only interference with an iron-binding site very early in the mineralization process would have such an effect. Because the Cr(TREN) inhibitor has four of the six octahedral coordination sites blocked by an exchange-inert tetraamine ligand, the remaining two sites, which are occupied by OH−/H2O groups, become irreversibly displaced by donor carboxylate/thiolate groups of the ferritin. As a consequence of Cr(TREN)–ferritin binding, ferrous ion uptake through the pore of the protein appears to be completely blocked at saturating concentrations of Cr(TREN).
Supplementary Material
Acknowledgments
We thank Dana Danger, Dr. William Small, Dr. John Fetter, Dr. Hidenori Takagi, and Rene Tipton for the preparation of frog recombinant proteins, and Dr. Julia Brumaghim, Dr. David Hamilton, Emily Dertz, and Kristy Clarke for their generous help with manuscript review. This work was supported by National Institutes of Health Grants AI11744 and DK32999 (to K.N.R.) and DK20251 (to E.C.T.), and a Howard Hughes Institute predoctoral fellowship (to C.M.B.).
Footnotes
Residue numbers are the commonly used scheme for horse L, frog L, and frog H subunits, which can be converted to an alternate scheme, based on the human H sequence, by adding 4.
Residue Cys-126 is not conserved in all ferritins.
The charge of the mononuclear Cr(III) complex at pH 7.4 was determined from the acidity constants obtained from potentiometric titrations and comparison of the Cr(TREN) absorption spectra to that of [Cr(en)2(H2O)2]3+ and other Cr(III) TREN complexes (32, 48) at different pH. The acidity constants are: [Cr(TREN)(H2O)(OH)]2+ + H+ ⇋ [Cr(TREN)(H2O)2]3+ (log K = 4.16); [Cr(TREN)(OH)2]+ + H+ ↼ [Cr(TREN)(H2O)(OH)]2+ (log K = 6.52); Thus at pH 7.0 the predominant species are the monocation [Cr(TREN)(OH)2]+ (75%) and the dication [Cr(TREN)(H2O)(OH)]2+ (25%) in rapid equilibrium. Because binding is much stronger for the dication, Cr(TREN) effectively functions as exclusively as the dication at neutral pH.
The lower % inhibition observed for Cr(TREN)–HoSF at higher Fe(II) concentrations (5–125 Fe per subunit or 120–3,000 Fe per protein; Figure 4, Table 1) may reflect some iron autooxidation reactions outside the protein shell, particularly at high Cr(TREN) stoichiometries of binding. For example, the initial rates of Fe(II) oxidation in HoSF modified with 2.2–2.7 Cr per subunit were comparable to the rates measured for Fe(II) auto-oxidation in the absence of protein (≈20% of the rate of HoSF). Furthermore, at low Fe(II) concentrations (48 Fe per protein), the initial rate of Fe oxidation in HoSF was inhibited by >95% on binding of two Cr(TREN) per subunit, when autooxidation was insignificant. This result suggests that CrTREN inhibits Fe(II) oxidation in HoSF >95% at >2 CrTREN per subunit, and that the % inhibition for 1 Cr per subunit is probably greater than the 67% shown in Fig. 3 and Table 1.
References
- 1.Theil E C, Small G W, He L, Tipton A R, Danger D T. Inorg Chim Acta. 2000;297:242–251. [Google Scholar]
- 2.Harrison P M, Arosio P. Biochim Biophys Acta. 1996;1275:161–203. doi: 10.1016/0005-2728(96)00022-9. [DOI] [PubMed] [Google Scholar]
- 3.Trikha J, Theil E C, Allewell N M. J Mol Biol. 1995;248:948–967. doi: 10.1006/jmbi.1995.0274. [DOI] [PubMed] [Google Scholar]
- 4.Hempstead P D, Yewdall S J, Fernie A R, Lawson D M, Artymiuk P J, Rice D W, Ford G C, Harrison P M. FEBS Lett. 1997;254:207–210. [Google Scholar]
- 5.Orino K, Lehman L, Tsuji Y, Ayaki H, Torti S V, Torti F M. Biochem J. 2001;357:241–247. doi: 10.1042/0264-6021:3570241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cozzi A, Corsi B, Levi S, Santambrogio P, Albertini A, Arosio P. J Biol Chem. 2000;275:25122–25129. doi: 10.1074/jbc.M003797200. [DOI] [PubMed] [Google Scholar]
- 7.Theil E C. In: Handbook of Metalloproteins. Messerschmidt A, Huber R, Poulos T, Wieghardt K, editors. Cichester, U.K.: Wiley; 2000. pp. 771–781. [Google Scholar]
- 8.Kakhlon O, Gruenbaum Y, Cabantchik Z I. Biochem J. 2001;356:311–316. doi: 10.1042/0264-6021:3560311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wardeska J G, Viglione B, Chasteen N D. J Biol Chem. 1986;261:6677–6683. [PubMed] [Google Scholar]
- 10.Harrison P M, Treffry A, Lilley T H. J Inorg Biochem. 1986;27:287–293. doi: 10.1016/0162-0134(86)80068-x. [DOI] [PubMed] [Google Scholar]
- 11.Stefanini S, Desideri A, Vecchini P, Drakenberg T, Chiancone E. Biochemistry. 1989;28:378–382. doi: 10.1021/bi00427a052. [DOI] [PubMed] [Google Scholar]
- 12.Treffry A, Harrison P M, Luzzago A, Cesareni G. FEBS Lett. 1989;247:268–272. doi: 10.1016/0014-5793(89)81350-x. [DOI] [PubMed] [Google Scholar]
- 13.Desideri A, Stefanini S, Polizio F, Petruzzelli R, Chiancone E. FEBS Lett. 1991;287:10–14. doi: 10.1016/0014-5793(91)80004-m. [DOI] [PubMed] [Google Scholar]
- 14.Levi S, Yewdall S J, Harrison P M, Santambrogio P, Cozzi A, Rovida E, Albertini A, Arosio P. Biochem J. 1992;288:591–596. doi: 10.1042/bj2880591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yablonski M J, Theil E C. Biochemistry. 1992;31:9680–9684. doi: 10.1021/bi00155a022. [DOI] [PubMed] [Google Scholar]
- 16.Lee M, Arosio P, Cozzi A, Chasteen N D. Biochemistry. 1994;33:3679–3687. doi: 10.1021/bi00178a026. [DOI] [PubMed] [Google Scholar]
- 17.Takagi H, Shi D, Ha Y, Allewell N M, Theil E C. J Biol Chem. 1998;273:18685–18688. doi: 10.1074/jbc.273.30.18685. [DOI] [PubMed] [Google Scholar]
- 18.Jin W, Takagi H, Pancorbo N M, Theil E C. Biochemistry. 2001;40:7525–7532. doi: 10.1021/bi002509c. [DOI] [PubMed] [Google Scholar]
- 19.Lawson D M, Treffry A, Artymiuk P J, Harrison P M, Yewdall S J, Luzzago A, Cesareni G, Levi S, Arosio P. FEBS Lett. 1989;254:207–210. doi: 10.1016/0014-5793(89)81040-3. [DOI] [PubMed] [Google Scholar]
- 20.Lawson D M, Artymiuk P J, Yewdall S J, Smith J M A, Livingstone J C, Treffry A, Luzzago A, Levi S, Arosio P, Cesareni G, Thomas C D, Shaw W V, Harrison P M. Nature (London) 1991;349:541–544. doi: 10.1038/349541a0. [DOI] [PubMed] [Google Scholar]
- 21.Crichton R R, Herbas A, Chavez-Alba O, Roland F. J Biol Inorg Chem. 1996;1:567–574. [Google Scholar]
- 22.Fetter J, Cohen J, Danger D, Sanders-Loehr J, Theil E C. J Biol Inorg Chem. 1997;2:652–661. [Google Scholar]
- 23.Ha Y, Shi D, Small G W, Theil E C, Allewell N M. J Biol Inorg Chem. 1999;2:243–256. doi: 10.1007/s007750050310. [DOI] [PubMed] [Google Scholar]
- 24.Wade V J, Levi S, Arosio P, Treffry A, Harrison P M, Mann S. J Mol Biol. 1991;221:1443–1452. doi: 10.1016/0022-2836(91)90944-2. [DOI] [PubMed] [Google Scholar]
- 25.Waldo G S, Theil E C. In: Comprehensive Supramolecular Chemistry. Atwood J L, Davies J E D, MacNicol D D, Vögtle F, editors. Vol. 5. Oxford: Pergamon; 1997. pp. 65–89. [Google Scholar]
- 26.Fu D, Libson A, Miercke L J W, Weitzman C, Nollert P, Krucinski J, Stroud R M. Science. 2000;290:481–486. doi: 10.1126/science.290.5491.481. [DOI] [PubMed] [Google Scholar]
- 27.Yang D, Nagayama K. Biochem J. 1995;307:253–246. doi: 10.1042/bj3070253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang X, Chasteen N D. Biophys J. 1996;71:1587–1595. doi: 10.1016/S0006-3495(96)79361-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Treffry A, Hawkins C, Williams J M, Guest J R, Harrison P M. J Biol Inorg Chem. 1996;1:49–60. [Google Scholar]
- 30.Massover W H. Micron. 1993;24:389–437. [Google Scholar]
- 31.Bradford M M. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 32.Zipp S G, Madan S K. Inorg Chem. 1976;15:587–593. [Google Scholar]
- 33.Davies M B. J Inorg Nucl Chem. 1976;38:2251. [Google Scholar]
- 34.Kyuno E, Kanada M, Takana N. Bull Chem Soc Japan. 1967;40:1848–1854. [Google Scholar]
- 35.Weschler C J, Deutsch E. Inorg Chem. 1973;12:2682–2690. [Google Scholar]
- 36.Xu B, Chasteen N D. J Biol Chem. 1991;266:19965–19970. [PubMed] [Google Scholar]
- 37.Waldo G S, Ling J, Sanders-Loehr J, Theil E C. Science. 1993;259:796–798. doi: 10.1126/science.8430332. [DOI] [PubMed] [Google Scholar]
- 38.Yang X, Chasteen N D. Biochem J. 1999;338:615–618. [PMC free article] [PubMed] [Google Scholar]
- 39.Treffry A, Zhao Z, Quail M A, Guest J R, Harrison P M. Biochemistry. 1995;34:15204–15213. doi: 10.1021/bi00046a028. [DOI] [PubMed] [Google Scholar]
- 40.Treffry A, Zhao Z, Quail M A, Guest J R, Harrison P M. FEBS Lett. 1998;432:213–218. doi: 10.1016/s0014-5793(98)00867-9. [DOI] [PubMed] [Google Scholar]
- 41.Pereira A S, Tavares P, Lloyd S G, Danger D, Edmonson D E, Theil E C, Huynh B H. Biochemistry. 1997;36:7917–7927. doi: 10.1021/bi970348f. [DOI] [PubMed] [Google Scholar]
- 42.Pereira A S, Tavares P, Lloyd S G, Danger D, Edmonson D E, Theil E C, Huynh B H. Biochemistry. 1998;37:9871–9876. doi: 10.1021/bi980847w. [DOI] [PubMed] [Google Scholar]
- 43.Hwang J, Krebs C, Huynh B-H, Edmonson D E, Theil E C, Penner-Hahn J E. Science. 2000;287:122–125. doi: 10.1126/science.287.5450.122. [DOI] [PubMed] [Google Scholar]
- 44.Broadwater J A, Ai J, Loehr T M, Sanders-Loehr J, Fox B G. Biochemistry. 1998;37:14664–14671. doi: 10.1021/bi981839i. [DOI] [PubMed] [Google Scholar]
- 45.Broadwater J A, Achim C, Munck E, Fox B G. Biochemistry. 1999;38:12197–12204. doi: 10.1021/bi9914199. [DOI] [PubMed] [Google Scholar]
- 46.Shu L, Nesheim J C, Kauffmann K, Munck E, Lipscomb J D, Que L J. Science. 1997;275:515–518. doi: 10.1126/science.275.5299.515. [DOI] [PubMed] [Google Scholar]
- 47.Rosenzweig A C, Brandstetter H, Whittington D A, Nordlund P, Lippard S J, Frederick C A. Proteins. 1997;29:141–152. [PubMed] [Google Scholar]
- 48.Saliby M J, Sheridan P S, Madan S K. Inorg Chem. 1980;19:1291–1297. [Google Scholar]
- 49.Hamalainen R, Turpeinen U. Acta Chem Scand. 1989;43:15–18. [Google Scholar]
- 50.Laine P, Gourdon A, Launay J-P. Inorg Chem. 1995;34:5138. [Google Scholar]
- 51.Wheeler D E, McCusker J K. Inorg Chem. 1998;37:2296–2307. doi: 10.1021/ic971306i. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.