Abstract
Human scalp electroencephalographic rhythms, indicative of cortical population synchrony, have long been posited to reflect cognitive processing. Although numerous studies employing simultaneous thalamic and cortical electrode recording in nonhuman animals have explored the role of the thalamus in the modulation of cortical rhythms, direct evidence for thalamocortical modulation in human has not, to our knowledge, been obtained. We simultaneously recorded from thalamic and scalp electrodes in one human during performance of a cognitive task and found a spatially widespread, phase-locked, low-frequency rhythm (7–8 Hz) power decrease at thalamus and scalp during semantic memory recall. This low-frequency rhythm power decrease was followed by a spatially specific, phase-locked, fast-rhythm (21–34 Hz) power increase at thalamus and occipital scalp. Such a pattern of thalamocortical activity reflects a plausible neural mechanism underlying semantic memory recall that may underlie other cognitive processes as well.
Electroencephalographic (EEG) fast rhythm (20–60 Hz) power increases have been shown to occur during numerous cognitive tasks, including learning, reading, face perception, posture recognition, and chess playing (1–5). Cortical electrode recording in cat (6–9) and nonhuman primate (10) during visual perception indicates that phase-locked, fast rhythm power can reflect synchronous activity between distinct neuronal populations that may “bind” features into unitary objects. Simultaneous thalamic and cortical electrode recording in cat and thalamic computational modeling indicate that low-frequency (7–14 Hz) and fast cortical rhythms (11) are influenced by spatially widespread (12, 13) and spatially specific (14–16) thalamocortical connections, respectively. Similar to findings in cat, there is convincing evidence that human thalamus interacts with fast cortical rhythms (17, 18).
Although fast rhythms have been postulated to reflect other cognitive functions, the role of such rhythms in semantic recall has not been explored. Recent functional MRI (fMRI) results (19, 20) suggest that the human thalamus is involved during semantic memory recall. We hypothesized that fMRI thalamic activation during semantic recall could reflect a thalamocortical interaction associated with this memory component. To investigate this possibility, we simultaneously recorded from thalamic and scalp electrodes and conducted epoch-based frequency analysis. To our knowledge, this is the first time in human when such a procedure has been conducted during a cognitive process, revealing a phase-locked, thalamocortical fast rhythm during semantic memory recall.
Methods
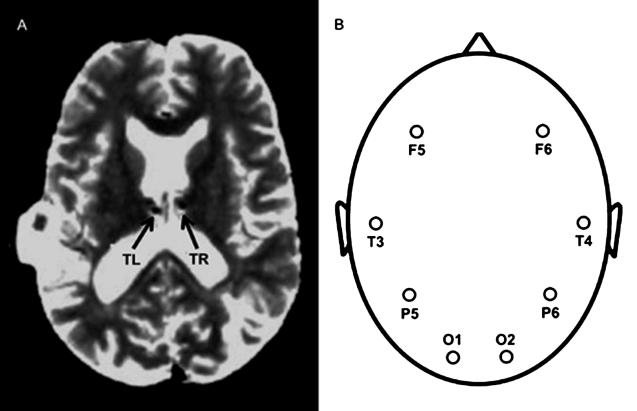
A 44-year-old, right-handed male with a history of intractable seizures had bilateral thalamic electrodes surgically implanted as part of a research protocol to electrically disrupt seizure propagation. Localization of seizure foci in cortex argued in favor of his having normal thalamic function. Each thalamic electrode was tentatively localized to the nucleus medialis (Fig. 1A; ref. 21), which has been implicated in explicit, long-term memory retrieval (22, 23). The anatomic MRI, acquired with depth electrodes in place, posed no danger to the patient (24). Before electrical stimulation, event-related thalamic local field potentials (LFPs) and scalp EEG (Fig. 1B) were recorded.
Figure 1.
Electrode positions used to record thalamic and scalp responses. (A) Anatomic MRI illustrating depth electrodes implanted in left and right thalamus, labeled TL and TR. (B) Scalp electrodes F5, T3, P5, O1, F6, T4, P6, and O2.
The patient was visually presented with 32 word pairs; each pair was presented for 3 s with an intertrial interval of 6 s (the patient was asymptomatic during all testing). For each word pair, the patient was instructed to press the response button if the words combined to activate an object, where half of the word pairs did combine (e.g., the words “desert” and “hump” combine to activate “camel”; a trial culminating in the cognitive process of semantic memory recall), and otherwise was instructed to refrain from responding (e.g., the words “bullets” and “milk” do not activate a third object and thus do not engage this process—nonrecall trials).
LFPs (12, 14–16, 25–30) recorded at thalamic electrodes were amplified 50,000 times. Scalp electrodes were placed according to the international 10–20 convention (31), and voltages were amplified 100,000 times. All electrodes were referenced to scalp electrode Cz (32), bandpass-filtered between 0.1 and 1,000 Hz with a notch filter at 60 Hz, and sampled every 1 ms. Epoch-based Fourier analysis (25) was conducted by using custom software written in matlab (Mathworks, Natick, MA). For each electrode on a trial-by-trial basis, the power spectra and phase within an epoch were obtained by using the fast-Fourier transform and then averaging trials of a given type (i.e., recall and nonrecall). To avoid type I error, and because our aim was to investigate thalamocortical modulation, planned comparisons at scalp electrodes were restricted to frequencies in which there was a significant increase or decrease in difference power spectra in at least one thalamic electrode (P < 0.05, two-tailed t test). In the phase analysis, left and right thalamic responses were very similar within the selected epochs/frequencies (P > 0.6, two-tailed t test); therefore, the left thalamic response phase was chosen arbitrarily for comparison with the phase of each scalp electrode response.
Results
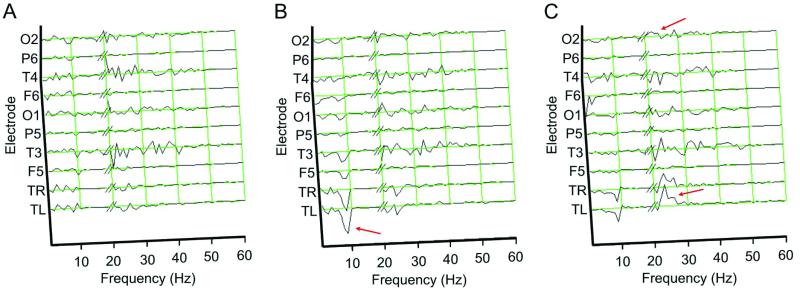
During semantic recall, thalamic power spectra showed little change in amplitude during the 0- to 1-s epoch after stimulus onset (Fig. 2A). In contrast, in the 1- to 2-s response epoch (Fig. 2B), there was a significant drop in low-frequency rhythm power (i.e., 7–8 Hz) at both thalamic electrodes (P < 0.01) and an accompanying, significant, low-frequency rhythm power decrement at all scalp electrodes except P6 (P < 0.05). In the 2- to 3-s epoch (Fig. 2C), there was a significant increase in thalamic fast rhythm power (i.e., 21–34 Hz, P < 0.01), with a concomitant increase at posterior scalp electrode O2 (P < 0.05). No other significant thalamocortical power modulations were observed (all frequencies below 200 Hz were tested).
Figure 2.
Difference power spectra of thalamic and scalp electrode activity. For each electrode within response epochs shown in A (0–1 s), B (1–2 s), and C (2–3 s), the average power spectra of nonrecall trials were subtracted from recall trials. Such a difference is indicative of neuronal frequency components unique to semantic recall. Thalamic and scalp responses >19 Hz were scaled by a factor of 20 and 3, respectively. Green grids indicate zero power difference, and red arrows indicate frequency bands of interest.
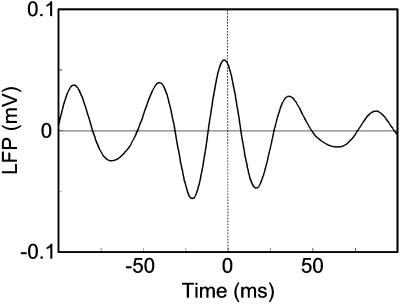
If thalamic and cortical rhythms interact, phase locking of thalamic and scalp responses in epochs with significant changes in power amplitude should occur, as has been shown in cat (12, 14–16, 30–33). Two such epochs/frequencies were selected for phase analysis, based on the results described above: 8 Hz within the 1- to 2-s epoch and 24 Hz within the 2- to 3-s epoch. The phase of scalp responses in the selected epochs/frequencies were similar to the phase of thalamic responses, as assessed statistically (i.e., seven of eight scalp electrodes with P > 0.2, two-tailed t test). To obtain complementary evidence for fast rhythm phase locking, a cross-correlogram (7–9, 11, 15, 16) of LFP responses at TL and O2 within the 2- to 3-s epoch was computed, restricted in bandwidth to significant increases in thalamic fast rhythm power (i.e., 21–34 Hz). Fig. 3 illustrates that the maximum LFP occurred at approximately zero time lag, indicating thalamic and scalp fast rhythms were indeed phase-locked.
Figure 3.
Cross-correlogram computed with LFP responses at electrodes TL and O2 within the 2- to 3-s epoch, restricted to frequencies with a significant increase in thalamic fast rhythm power. The dotted line indicates the expected peak location of phase-locked responses.
Average reaction time for recall trials was 1.57 s. This 1- to 2-s average reaction time range ruled out the possibility that the fast rhythm power increase in the 2- to 3-s epoch during semantic recall was a result of motor response.
Discussion
During semantic recall, a spatially widespread decrease in low-frequency rhythm power was detected. Moreover, as hypothesized, this decrease was followed by an increase in spatially specific fast rhythm power at thalamus and occipital scalp. The decrease in low-frequency rhythm power has previously been shown to occur in human by using scalp EEG during various cognitive tasks (33–37). Human EEG measured directly from the cortical surface with surgically implanted subdural electrode arrays has shown similar modulations of cortical rhythms during movement (38, 39) (i.e., a spatially widespread decrease in low-frequency rhythm power with more focal increase in fast rhythm power). Recently published recordings from monkey cerebral cortex during a spatial attention task has also shown a decrease in low-frequency rhythm power and increase in fast rhythm power (30). Such modulations are similar to the low-frequency rhythm power decrease measured with simultaneous thalamic depth and scalp electrodes in cat (12).
The low-frequency rhythms in cat appear to be controlled by means of spatially widespread, inhibitory (i.e., GABAergic; refs. 40 and 41) projections from the thalamic reticular nucleus (RE) to thalamocortical (TC) cells in other thalamic nuclei and mediate cyclic activity by rebound excitation (12, 27, 28, 41, 42). Spatially widespread connections from cortex to RE also have proven necessary for low-frequency synchronization (26, 29, 41). In contrast, high-frequency thalamocortical rhythms in cat have been proposed mediated by spatially specific, monosynaptic excitatory (e.g., glutamatergic) cortico–thalamo–cortical pathways that do not necessarily engage RE (15). Our findings in human during semantic memory recall are suggestive of this described neural circuitry in cat.
Both fast rhythm power increases in cat (6–9) and posterior scalp potential increases in human (43, 44) have been localized to occipital cortex during visual perception. Thus, the fast rhythm increase at electrode O2 during semantic recall may reflect object visualization (as indicated by fMRI activation in visual areas with similar procedures; ref. 19). The relative difficulty of recall trials may have led to recruitment of posterior visual areas, resulting in activation of electrode O2, as has been argued to occur during difficult perceptual learning tasks (45). An alternative but complementary hypothesis is that the increase in O2 fast rhythm power during recall was a result of task-specific reliance upon a visual representation system, as opposed to a language representation system alone (46).
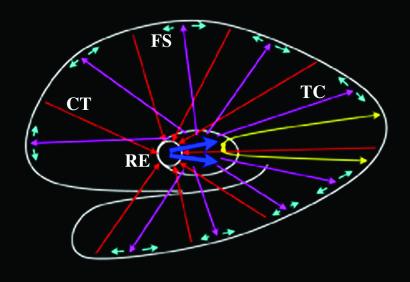
Fig. 4 illustrates a plausible thalamocortical model, based on the present data and numerous animal studies, that may underlie semantic recall. Before recall trial onset, spatially widespread, low-frequency synchronization is elicited by interactions between cortex, RE, and TC cells. Excitatory afferents from cortex to RE maintain global, low-frequency synchronization (26, 29, 41). Afferents from RE cyclically inhibit TC cells, which, in turn, rebound in volleys of excitation (12, 27, 28, 41, 42). During each volley, TC afferents make excitatory synapses with both cortical cells (41) and cortical interneurons (47–49), the latter of which result in local cortical inhibition (50) likely by means of fast-spiking (FS) GABAergic cells (51). In this model, low-frequency synchronization may functionally represent a tonic state of cortical inhibition. Indeed, an increase in EEG low-frequency rhythm power has been postulated to represent inhibition of cortical processing (52) during semantic memory (34). Conversely, the low-frequency rhythm power decrease during the semantic memory task in the present study may represent global cortical disinhibition (which also implies a global cortical inhibition associated with low-frequency power during the preceding epoch). The spatially specific fast rhythm burst, subserved by monosynaptic excitatory cortico–thalamo–cortical connections via the body of the thalamus (15), may mediate feature binding during recall.
Figure 4.
Thalamocortical model of fast and low-frequency rhythm synchronization. A near midsagittal view of the cortical mantle is shown as the outermost white trace whereas the outer boundary of the thalamus, also in white, is shown near the center of the figure. The RE is also shown within the thalamus. Spatially specific excitatory neurons, shown in yellow, subserve fast rhythm synchronization and make monosynaptic connections via the body of the thalamus. The remaining circuitry subserves low-frequency rhythm synchronization. Spatially widespread excitatory corticothalamic (CT) afferents, shown as red arrows, project to RE. Thick blue arrows indicate inhibitory afferents from RE to TC neurons. Spatially widespread excitatory TC neurons, shown as magenta arrows, project to cerebral cortex and activate fast-spiking (FS) inhibitory cells, shown in cyan.
In the present study, the spatially widespread, thalamocortical, low-frequency rhythm power decrement occurred earlier than the more focal, thalamocortical, fast rhythm power increase. The increase in fast rhythm power appears to be associated, in this instance, with aspects of recall in semantic memory and, as such, reflects a plausible mechanism underlying this process. Moreover, the same overall pattern of cortical rhythmic activity (i.e., a decrease in low-frequency rhythm power and increase in fast rhythm power) has been reported during selective attention (30) and voluntary movement (38, 39) in nonhuman primate and human. Such similarities across species and disparate tasks may reflect a common thalamocortical mechanism subserving certain cognitive functions.
Acknowledgments
We thank V. B. Mountcastle for helpful discussions and invaluable comments on the manuscript, J. Segal for assistance with MRI data collection, B. Weber for hardware support, J. Schwarzbach for assistance with stimulus coding, and D. Zee and S. Yantis for insightful comments on the manuscript. S.D.S. is supported by postdoctoral Training Grant MH19971 in perceptual and cognitive neuroscience from the National Institute of Mental Health.
Abbreviations
- EEG
electroencephalography
- fMRI
functional MRI
- LFP
local field potential
- RE
reticular nucleus
- TC
thalamocortical
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Miltner W H R, Braun C, Arnold M, Witte H, Taub E. Nature (London) 1999;397:434–436. doi: 10.1038/17126. [DOI] [PubMed] [Google Scholar]
- 2.Pulvermuller F, Lutzenberger W, Preissl H, Birbaumer N. NeuroReport. 1995;6:2059–2064. doi: 10.1097/00001756-199510010-00025. [DOI] [PubMed] [Google Scholar]
- 3.Rodriguez E, George N, Lachaux J, Martinerie J, Renault B, Varela F J. Nature (London) 1999;397:430–433. doi: 10.1038/17120. [DOI] [PubMed] [Google Scholar]
- 4.Slobounov S, Tutwiler R, Slobounova E, Rearich M, Ray W. Cognit Brain Res. 2000;9:177–192. doi: 10.1016/s0926-6410(99)00055-5. [DOI] [PubMed] [Google Scholar]
- 5.Amidzic O, Riehle H J, Fehr T, Wienbruch C, Elbert T. Nature (London) 2001;412:603. doi: 10.1038/35088119. [DOI] [PubMed] [Google Scholar]
- 6.Gray C M, Singer W. Proc Natl Acad Sci USA. 1989;86:1698–1702. doi: 10.1073/pnas.86.5.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gray C M, Konig P, Engel A K, Singer W. Nature (London) 1989;338:334–337. doi: 10.1038/338334a0. [DOI] [PubMed] [Google Scholar]
- 8.Singer W, Gray C M. Annu Rev Neurosci. 1995;18:555–586. doi: 10.1146/annurev.ne.18.030195.003011. [DOI] [PubMed] [Google Scholar]
- 9.Fries P, Roelfsema P R, Engel A K, Konig P, Singer W. Proc Natl Acad Sci USA. 1997;94:12699–12704. doi: 10.1073/pnas.94.23.12699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kreiter A K, Singer W. J Neurosci. 1996;16:2381–2396. doi: 10.1523/JNEUROSCI.16-07-02381.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Steriade M. Cereb Cortex. 1997;7:583–604. doi: 10.1093/cercor/7.6.583. [DOI] [PubMed] [Google Scholar]
- 12.Contreras D, Steriade M. J Neurosci. 1995;15:604–622. doi: 10.1523/JNEUROSCI.15-01-00604.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang X J, Golomb D, Rinzel J. Proc Natl Acad Sci USA. 1995;92:5577–5581. doi: 10.1073/pnas.92.12.5577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Steriade M, Curro D R, Pare D, Oakson G. Proc Natl Acad Sci USA. 1991;88:4396–4400. doi: 10.1073/pnas.88.10.4396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Steriade M, Contreras D, Amzica F, Timofeev I. J Neurosci. 1996;16:2788–2808. doi: 10.1523/JNEUROSCI.16-08-02788.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Amzica F, Neckelmann D, Steriade M. Proc Natl Acad Sci USA. 1997;94:1985–1989. doi: 10.1073/pnas.94.5.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ribary U, Ioannides A A, Singh K D, Hasson R, Bolton J P R, Lado F, Mogilner A, Llinas R. Proc Natl Acad Sci USA. 1991;88:11037–11041. doi: 10.1073/pnas.88.24.11037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Llinas R R, Ribary U, Jeanmonod D, Kronberg E, Mitra P P. Proc Natl Acad Sci USA. 1999;96:15222–15227. doi: 10.1073/pnas.96.26.15222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kraut M A, Kremen S, Segal J B, Calhoun V, Moo L R, Hart J. J Cognit Neurosci. 2002;14:24–36. doi: 10.1162/089892902317205294. [DOI] [PubMed] [Google Scholar]
- 20.Kraut M A, Kremen S, Moo L R, Segal J B, Calhoun V, Hart J. J Cognit Neurosci. 2002;14:37–47. doi: 10.1162/089892902317205302. [DOI] [PubMed] [Google Scholar]
- 21.Engelborghs S, Marien P, Martin J-J, De Deyn P P. Acta Neurol Belg. 1998;98:252–265. [PubMed] [Google Scholar]
- 22.Aggleton J P, Mishkin M. J Comp Neurol. 1984;222:56–68. doi: 10.1002/cne.902220106. [DOI] [PubMed] [Google Scholar]
- 23.Squire L R, Zola-Morgan S. Trends Neurosci. 1988;11:170–175. doi: 10.1016/0166-2236(88)90144-0. [DOI] [PubMed] [Google Scholar]
- 24.Tronnier V M, Staubert A, Hahnel S, Sarem-Aslani A. Neurosurgery. 1999;44:118–126. doi: 10.1097/00006123-199901000-00073. [DOI] [PubMed] [Google Scholar]
- 25.Logothetis N K, Pauls J, Augath M, Trinath T, Oeltermann A. Nature (London) 2001;412:150–157. doi: 10.1038/35084005. [DOI] [PubMed] [Google Scholar]
- 26.Contreras D, Destexhe A, Sejnowski T J, Steriade M. Science. 1996;274:771–774. doi: 10.1126/science.274.5288.771. [DOI] [PubMed] [Google Scholar]
- 27.Destexhe A, Contreras D, Steriade M. J Neurophysiol. 1998;79:999–1016. doi: 10.1152/jn.1998.79.2.999. [DOI] [PubMed] [Google Scholar]
- 28.Grenier F, Timofeev I, Steriade M. Proc Natl Acad Sci USA. 1998;95:13929–13934. doi: 10.1073/pnas.95.23.13929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Destexhe A, Contreras D, Steriade M. Neuroscience. 1999;92:427–443. doi: 10.1016/s0306-4522(99)00024-x. [DOI] [PubMed] [Google Scholar]
- 30.Fries P, Reynolds J H, Rorie A E, Desimone R. Science. 2001;291:1560–1563. doi: 10.1126/science.1055465. [DOI] [PubMed] [Google Scholar]
- 31.Jasper H H. Electroencephalograph Clin Neurophysiol. 1958;10:371–375. [PubMed] [Google Scholar]
- 32.Sharbrough F W. In: Current Practice of Clinical Electroencephalography. 2nd Ed. Daly D D, Pedley T A, editors. New York: Raven; 1990. pp. 29–49. [Google Scholar]
- 33.Klimesch W. Brain Res Rev. 1999;29:169–195. doi: 10.1016/s0165-0173(98)00056-3. [DOI] [PubMed] [Google Scholar]
- 34.Klimesch W, Doppelmayr M, Schwaiger J, Auinger P, Winkler T. Cognit Brain Res. 1999;7:493–501. doi: 10.1016/s0926-6410(98)00056-1. [DOI] [PubMed] [Google Scholar]
- 35.Klimesch W, Schimke H, Pfurtscheller G. Brain Topogr. 1993;5:241–251. doi: 10.1007/BF01128991. [DOI] [PubMed] [Google Scholar]
- 36.Klimesch W. Int J Psychophysiol. 1996;24:61–100. doi: 10.1016/s0167-8760(96)00057-8. [DOI] [PubMed] [Google Scholar]
- 37.Schier M A. Int J Psychophysiol. 2000;37:155–162. doi: 10.1016/s0167-8760(00)00079-9. [DOI] [PubMed] [Google Scholar]
- 38.Crone N E, Miglioretti D L, Gordon B, Sieracki J M, Wilson M T, Uematsu S, Lesser R P. Brain. 1998;121:2271–2299. doi: 10.1093/brain/121.12.2271. [DOI] [PubMed] [Google Scholar]
- 39.Crone N E, Miglioretti D L, Gordon B, Lesser R P. Brain. 1998;121:2301–2315. doi: 10.1093/brain/121.12.2301. [DOI] [PubMed] [Google Scholar]
- 40.Huntsman M M, Porcello D M, Homanics G E, DeLorey T M, Huguenard J R. Science. 1999;283:541–543. doi: 10.1126/science.283.5401.541. [DOI] [PubMed] [Google Scholar]
- 41.Steriade M. Proc Natl Acad Sci USA. 2001;98:3625–3627. doi: 10.1073/pnas.071051998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Golshani P, Liu X, Jones E. Proc Natl Acad Sci USA. 2001;98:4172–4177. doi: 10.1073/pnas.061013698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Slotnick S D, Klein S A, Carney T, Sutter E E. Clin Neurophysiol. 2001;112:1349–1356. doi: 10.1016/s1388-2457(01)00561-2. [DOI] [PubMed] [Google Scholar]
- 44.Slotnick S D, Klein S A, Carney T, Sutter E E, Dastmalchi S. Clin Neurophysiol. 1999;110:1793–1800. doi: 10.1016/s1388-2457(99)00135-2. [DOI] [PubMed] [Google Scholar]
- 45.Ahissar M, Hochstein S. Nature (London) 1997;387:401–406. doi: 10.1038/387401a0. [DOI] [PubMed] [Google Scholar]
- 46.Hart J, Gordon B. Nature (London) 1992;359:60–64. doi: 10.1038/359060a0. [DOI] [PubMed] [Google Scholar]
- 47.Toyama T, Matsunami K, Ohno T, Tokashiki S. Brain Res. 1974;14:518–520. doi: 10.1016/0006-8993(69)90128-0. [DOI] [PubMed] [Google Scholar]
- 48.Jones E G. In: The Organization of the Cerebral Cortex. Schmitt F O, Worden F G, Adelman G, Dennis S G, editors. Cambridge, MA: MIT Press; 1981. pp. 199–234. [Google Scholar]
- 49.Jones E G. The Thalamus. New York: Plenum; 1985. [Google Scholar]
- 50.Contreras D, Destexhe A, Steriade M. J Neurophysiol. 1997;78:335–350. doi: 10.1152/jn.1997.78.1.335. [DOI] [PubMed] [Google Scholar]
- 51.Galarreta M, Hestrin S. Science. 2001;292:2295–2299. doi: 10.1126/science.1061395. [DOI] [PubMed] [Google Scholar]
- 52.Pfurtscheller G, Stancak A, Neuper C. Int J Psychophysiol. 1996;24:39–46. doi: 10.1016/s0167-8760(96)00066-9. [DOI] [PubMed] [Google Scholar]