Abstract
Aiming to facilitate the analysis of human genetic variations in the context of disease susceptibility and varied drug response, we have developed a genotyping method that entails incorporation of a chemically labile nucleotide by PCR followed by specific chemical cleavage of the resulting amplicon at the modified bases. The identity of the cleaved fragments determines the genotype of the DNA. This method, termed Incorporation and Complete Chemical Cleavage, utilizes modified nucleotides 7-deaza-7-nitro-dATP, 7-deaza-7-nitro-dGTP, 5-hydroxy-dCTP, and 5-hydroxy-dUTP, which have increased chemical reactivity but are able to form standard Watson–Crick base pairs. Thus one analog is substituted for the corresponding nucleotide during PCR, generating amplicons that contain nucleotide analogs at each occurrence of the selected base throughout the target DNA except for the primer sequences. Subsequent treatment with an oxidant followed by an organic base results in chemical cleavage at each site of modification, which produces fragments of different lengths and/or molecular weights that reflect the genotype of the original DNA sample at the site of interest. This incorporation and cleavage chemistry are widely applicable to many existing nucleic acid analysis platforms, including gel electrophoresis and mass spectrometry. By combining DNA amplification and analog incorporation into one step, this strategy eliminates preamplification, DNA-strand separation, primer extension, and purification procedures associated with traditional chain-termination chemistry and therefore presents significant advantages in terms of speed, cost, and simplicity of genotyping.
Inherited genetic differences contribute to human phenotypic diversity, including variation in disease susceptibility and drug response (1, 2). Genetic studies of such traits, which include genome-wide screens of DNA polymorphisms as well as candidate gene-based approaches, require the genotyping of ever larger numbers of polymorphisms in ever larger number of subjects. Recently the focus of human genome research has turned to genome-wide discovery of single-nucleotide polymorphisms (SNPs), resulting in a repertoire of several million SNPs. These developments highlight the need for rapid, accurate, and economical genotyping technologies.
Many genotyping methods have been developed during the past decade. Some methods are based on differential hybridization of matched versus mismatched probe–target heteroduplexes [e.g., microarrays (3), Taqman (4), and molecular beacon (5)], whereas others, such as primer extension (6), template-directed ligation (7), and flap endonuclease cleavage (8), use enzyme specificity. None of the available methods is optimally suited to the broad range of genotyping projects underway (9). The limitations of hybridization-based methods are well documented and arise largely from their reliance on single-base discrimination (10). Their performance is also strongly influenced by the sequences neighboring a SNP and by interference from secondary structures, which might be favored by one or the other allele (11). The specificity of enzymatic reactions is usually much higher than that of differential hybridization, and a popular approach uses polymerase primer extension with dideoxy terminators (12). The most commonly used methods for genotype readout are based either on fluorescence or mass spectrometry (MS). Fluorescence readout has a higher sensitivity but often relies on secondary reporter systems for detection (7, 8). In contrast, MS readout has the advantage of directly detecting fragments containing the original DNA sequence information (13) and thereby potentially reduces false positive and false negative results.
Here we describe a polymerase-based genotyping strategy, termed Incorporation and Complete Chemical Cleavage (ICCC), that is compatible with both MS and fluorescence detection platforms. ICCC takes advantage of the structural similarity and the differential chemical reactivity of modified versus naturally occurring nucleotides. The modified nucleotides are incorporated into DNA during PCR amplification and are subsequently cleaved to afford oligonucleotide fragments indicative of the genotypes of the original genomic DNA samples. Chemical cleavage conditions are designed to ensure that the amplicon is cleaved specifically and completely at the modified nucleotides while leaving unmodified nucleotides intact. The PCR primers and modified nucleotides are selected so that the cleavage products from each allele generate oligonucleotides of different sizes or molecular weights (MWs) that reveal the genotypes of each DNA sample. Successful genotyping depends on two critical features of the modified nucleotides: (i) their complete incorporation in place of the corresponding native nucleotide; and (ii) their specific cleavage by chemical treatment to near completion. The described ICCC strategy is simple and straightforward, requiring only one enzymatic reaction followed by in situ chemical reactions. Because it involves less sample manipulation than other available genotyping methods, ICCC reduces the likelihood of human and instrument error. In addition, it is compatible with many existing DNA analysis instruments and provides maximum flexibility from target selection to platform choice. Further, the MS-based applications of this method can be designed to provide information about both DNA strands, providing a level of internal confirmation not achievable by other genotyping methods.
Materials and Methods
Synthesis of Modified Nucleotides.
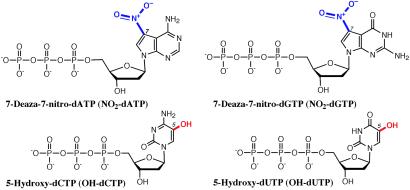
Nucleotide analogs 5-hydroxy-dCTP and 5-hydroxy-dUTP were synthesized from dCTP and dUTP (Sigma–Aldrich), respectively, according to published methods (14). 7-Deaza-7-nitro-dATP was prepared from 7-deaza-2′-deoxyadenosine (Chem-Genes, Ashland, MA) by using a modified procedure based on a previous publication (15). Details of the synthesis of 7-deaza-7-nitro-dGTP from 6-chloro-7-deazaguanine (Chem-Genes) will be discussed elsewhere. These analogs (shown in Fig. 1) may be purchased from Waters as part of the NuCleave Genotyping kit.
Fig 1.
The chemical structures of modified nucleotides used in ICCC genotyping. The modified groups are highlighted.
PCR Incorporation.
Plasmids containing the human transferrin receptor (TR) gene with either an A or G at position 424, or a mixture of both, were used as templates for PCR amplification. Primers used in this study (Invitrogen) and the amplified sequences are listed in Table 1. Each 20-μl PCR contains 10 ng of plasmid DNA, 0.5–2 units of AmpliTaq Gold polymerase (Applied Biosystems), 20 pmols of each primer (32P-labeled at the 5′end where applicable), 0.2 mM of dNTPs (one modified and three unmodified, or four unmodified as control), 2 mM MgCl2, and 1 × buffer supplied by the manufacturer. After heat activation of AmpliTaq Gold at 95°C for 12 min, PCR was run by using an MJ Research PTC 200 thermocycler (Waltham, MA) for 50 cycles (94°C, 30 s; 55°C, 30 s; 72°C, 120 s), followed by a final extension at 72°C for 10 min. The products (1.5 μl each) were analyzed on a 10% nondenaturing polyacrylamide gel (1 × TBE, 90 mM Tris/90 mM boric acid/2 mM EDTA, pH 8.0) and stained with a 1:1 mixture of ethidium bromide/SYBR green I (Molecular Probes). When 32P-labeled primers were used, an autoradiogram was also obtained.
Table 1.
PCR primers and amplified human TR sequences around polymorphic site 424A/G
| Modified nucleotide | PCR primers and corresponding amplicons of human TR | Length, bp |
|---|---|---|
| A: | Primer 1: 23 nt/7,050.9 Da | |
| 7-deaza-7-nitro-dATP | 5′-GAAACTGGACAGCACAGACTTCACC(A/G)GCACCATCAAGCTGCTGAATGAAAATTCATATGTCCCTCGTGAG | |
| 3′-CTTTGACCTGTCGTGTCTGAAGTGG(T/C)CGTGGTAGTTCGACGACTTACTTTTAAGTATACAGGGAGCACTC | 71 | |
| Primer 2: 22 nt/6,758.7 Da | ||
| G: | Primer 1: 23 nt/7,050.9 Da | |
| 7-deaza-7-nitro-dGTP | 5′-GAAACTGGACAGCACAGACTTCACC(A/G)GCACCATCAAGCTGCTGAATGAAAATTCATATGTCCCTCGTGAG | |
| 3′-CTTTGACCTGTCGTGTCTGAAGTGG(T/C)CGTGGTAGTTCGACGACTTACTTTTAAGTATACAGGGAGCACTC | 71 | |
| Primer 2: 22 nt/6,758.7 Da | ||
| C: | Primer 3: 21 nt/6,519.5 Da | |
| 5-hydroxy-dCTP | 5′-CCTGAAGAGAAAGTTGTCGGAGAAACTGGACAGCACAGACTTCACC(A/G)GCACCATCAAGCTGCTGAA | |
| 3′-GGACAACTCTTTCAACAGCCTCTTTGACCTGTCGTGTCTGAAGTGG(T/C)CGTGGTAGTTCGACGACTT | 66 | |
| Primer 4: 19 nt/5,835.0 Da | ||
| T: | Primer 3: 21 nt/6,519.5 Da | |
| 5-hydroxy-dUTP | 5′-CCTGAAGAGAAAGTTGTCGGAGAAACTGGACAGCACAGACTTCACC(A/G)GCACCATCAAGCTGCTGAATG | |
| 3′-GGACAACTCTTTCAACAGCCTCTTTGACCTGTCGTGTCTGAAGTGG(T/C)CGTGGTAGTTCGACGACTTAC | 68 | |
| Primer 5: 20 nt/6,148.2 Da |
SNPs are shown within parentheses. Primers are underlined, with name, length, and MW listed. Each duplex represents PCR products of three genotypes: 424 A/A, 424 A/G, and 424 G/G.
Chemical Cleavage and PAGE Analysis.
All chemicals were purchased from Sigma–Aldrich. PCR products (8 μl) were oxidized by adding 0.5 μl of 10 mM KMnO4 and incubating at room temperature for 2–5 min (where applicable). To initiate cleavage, 4 μl of pyrrolidine, 1 μl of 0.1 M EDTA, pH 8, and H2O to 30 μl were added, and the reaction mixtures were incubated at 95°C for 1 hr. The reaction mixtures were then purified by using spin columns of Sephadex G25 resin in 1 × TE, pH 8, and separated on a 15% denaturing polyacrylamide gel (1 × TBE). An 8 to 32-nt oligo ladder (Invitrogen) was used as reference. The gel was analyzed by exposure to Kodak BIOMAX MR film and densitometry by using the image gauge Ver. 3.0 from Fuji.
Chemical Cleavage and MS Analysis.
For selective oxidation, each PCR sample (15 μl) was mixed with 1 μl of 10 mM aqueous KMnO4 and incubated at room temperature for 2–5 min. To initiate cleavage, 3 μl of aqueous 7.5 M 3-pyrrolidinol, 40 mM EDTA, pH 8, and 35 mM DTT were added, and samples were heated at 98°C for 1 hr. The cleaved DNA samples were purified by vacuum filtration through a 384-well sample preparation plate containing 5 mg of polymeric sorbent (Waters) per well. Each well was washed with 2 × 90 μl of 60% (vol/vol) aqueous isopropanol and 3 × 90 μl of 1 M triethylammonium acetate (TEAA), pH 7. Each cleavage reaction mixture was diluted with 270 μl of 0.33 M TEAA, pH 7, and loaded (3 × 90 μl) into each well. After rinsing with 90 μl of 2.25 M TEAA, pH 7.65, and 3 × 90 μl of 0.1 M TEAA, pH 7, the 384-well plate was reassembled on the vacuum manifold along with a collection plate. Each well was eluted with 60% (vol/vol) aqueous isopropanol (60 μl), and the collected eluates were lyophilized by using a Speedvac (Savant). Each sample was resuspended in 3 μl of a 3-hydroxypicolinic acid-based matrix solution (16), deposited on a 384 × 600-μm AnchorChip target plate (Bruker Daltonics, Billerica, MA), and allowed to air dry. The samples were analyzed on a Bruker Daltonics Biflex III matrix-assisted desorption/ionization–time-of-flight (MALDI-TOF) mass spectrometer equipped with a 337-nm nitrogen laser and a 1.2-m linear flight path in negative ion mode. The spectra for primary fragments (high MW) were obtained by using a 19-kV accelerating voltage with a 17.3-kV extraction voltage and medium time delay, and those for internal fragments (low MW) were acquired by using a 20-kV accelerating voltage with an 18.33-kV extraction voltage and medium time delay.
High-Throughput MS Analysis.
Individual human lymphocyte cell cultures were obtained from Coriell Cell Repositories (Camden, NJ), and the corresponding genomic DNAs were extracted by using a genomic DNA isolation kit (Qiagen, Valencia, CA). ICCC PCR reactions were performed with NO2-dATP and d(CGT)TPs by using Pfu (exo−) polymerase (Stratagene) and related buffer solutions (detailed conditions are published as supporting information on the PNAS web site, www.pnas.org). Chemical cleavage and sample preparation for MALDI-TOF analysis were all done according to the manufacturer's recommended protocol (NuCleave Genotyping Kit, Waters), either manually or by using a MicroLab 4200 robotic workstation with MPH-96 fluid handling head (Hamilton), with similar results.
Results and Discussion
Genotyping Strategy.
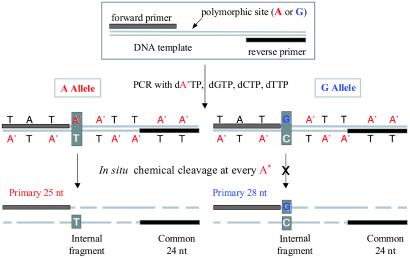
The two key elements of the ICCC method, polymerase incorporation and chemical cleavage, are illustrated in Fig. 2. During PCR amplification of a target sequence containing the SNP of interest, one of the four nucleotides used in PCR is replaced by its modified analog, which is chemically more reactive than the naturally occurring nucleotide. The identity of the expected SNPs guides the selection of the modified nucleotide (e.g., either modified dA*TP or modified dG*TP is used when an A/G polymorphism is anticipated). Fig. 2 illustrates the use of dA*TP in such a situation. The forward PCR primer is located 5′ of the SNP, so that the first incorporation of dA*TP is at the SNP site. The resulting amplicon contains A* at each occurrence of A outside of the primers. Because of the increased reactivity of the modified nucleotide, complete cleavage may be elicited selectively at A* sites to generate fragments of various sizes. Included in this set of fragments are the entire forward or reverse primer sequences, because these fragments lack modified nucleotides. Although the reverse primer-containing fragment does not contain SNP information and is identical for both alleles, the length of the forward primer-containing fragment depends on the genotype of the SNP. In the example of Fig. 2, this fragment is shorter for the A than for the G allele, because the A allele is cleaved at the SNP site (25-nt fragment), whereas the G allele is cleaved at the next A* (28-nt fragment). The differences in length and MW between these two primary products can be detected and used to determine the genotype of the original DNA sample. The other fragment that carries genotype-specific information is the internal fragment on the opposite strand, which contains T or C. MS analysis of this fragment can be used either to supplement the information obtained from the primary fragments or as an independent means for genotype identification. For the latter use, the modified nucleotide is selected to allow the production of an internal fragment that contains the SNP base and possess a unique molecular weight for MS analysis. Utilization of internal fragments for genotyping readout also allows greater flexibility in primer selection, leading to improved success rates for PCR amplification.
Fig 2.
Schematic of the ICCC genotyping strategy. A* represents a chemically modified analog of A.
Modified Nucleotides.
The best-known examples of chemical cleavage at modified nucleotides are Maxam–Gilbert sequencing, which involves base-specific chemical cleavage (17), and the Eckstein sequencing method, which relies on cleavage of alkylated phosphorothioates (18). However, neither of these approaches leads to near complete cleavage at the modified sites, which is an essential feature of ICCC genotyping.
The success of the ICCC strategy depends on the availability of chemically modified nucleotides that can be incorporated by DNA polymerases to form standard Watson–Crick base pairs. Furthermore, these modified nucleotides must be stable enough to survive multiple cycles of PCR amplification and yet sufficiently labile to allow for highly specific, complete cleavage under chemical conditions tolerated by the native bases. Verdine and coworkers (15, 19) used base modified nucleotides in a template-directed interference (TDI) footprinting method to analyze protein–DNA interactions. Many of the requirements for the TDI footprinting analogs coincide with the needs of the genotyping strategy described here: the analogs must be able to form base pairs and be incorporated by DNA polymerase, and they must contain chemical modifications that can lead to DNA-strand scission under conditions that do not lead to cleavage of normal DNA (15, 19). However, TDI footprinting involves only low-level statistical incorporation of an analog, whereas ICCC method requires complete substitution of a normal base by the analog, a much more demanding operation. Therefore, two TDI analogs, 5-hydroxy-dUTP and 7-deaza-7-nitro-dATP (15, 19), as well as 5-hydroxy-dCTP and 7-deaza-7-nitro-dGTP (20), which have similar structural and electronic characteristics, were developed for use in the ICCC genotyping platform (Fig. 1).
PCR Incorporation.
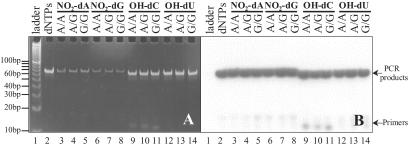
To establish the feasibility of using these analogs for the ICCC genotyping strategy, we used a model system to test the three major steps of the process: PCR amplification using modified nucleotides, chemical cleavage, and product analysis. In this system, two plasmids containing the human TR gene were used as PCR templates, each of which contains a naturally occurring SNP at position 424 (A or G) (21). Each of the four nucleotide analogs was assessed for its ability to genotype this SNP. The primers and amplicons for PCR are listed in Table 1. Primers were selected to flank the SNP and to generate amplicons of similar length. In addition, the length and position of the primers were designed to ensure that the cleavage products could be distinguished from each other and from unextended primers, thus providing genotype information on the original DNA template. For the sake of simplicity, a minimum number of primers was used in this study. Specifically, to ensure that the first modified base is at a polymorphic site, Primer 1 was used for both the NO2-dATP and -dGTP reactions, Primer 4 was used for the OH-dCTP reaction, and Primer 5 was used for the OH-dUTP reaction. Polymerases with low exonuclease activity (e.g., AmpliTaq Gold) were used for amplification to avoid complications that might arise from base removal at the 3′ end of the primer and subsequent incorporation of modified nucleotides into the primer sequence. After 5′ radiolabeling of all primers, 13 PCR reactions, representing one control and three genotypes for each modified nucleotide, were carried out by using standard PCR conditions and identical concentrations of modified and unmodified nucleotides. Gel electrophoresis followed by autoradiography (Fig. 3B) demonstrated efficient PCR incorporation of each modified nucleotide to produce amplicons of the expected lengths with similar product intensities and very low quantities of unextended primers. A comparison of Fig. 3 A and B also revealed that the dye-staining efficiency of PCR products containing NO2-dA or -dG was much lower than those containing OH-dC, OH-dU, or regular dNTPs, reflecting different fluorescent properties of these dye–DNA complexes because of modifications within DNA.
Fig 3.
Nondenaturing PAGE (10%) analysis of PCR products using modified nucleotides. (A) Gel stained with 1:1 mixture of ethidium bromide and SYBR green I dyes. (B) An autoradiogram of the gel shown in A.
Chemical Cleavage.
Confirmation of successful incorporation of the modified nucleotides was provided by selective chemical cleavage at the modified bases. Each PCR mixture containing a radiolabeled amplicon was divided into two portions and was treated with either pyrrolidine alone or with KMnO4 followed by pyrrolidine. The products expected from complete cleavage at each modified base for each DNA product are listed in Table 2. After cleavage, only the primary and common fragments are visible by autoradiography, because only they contain the 5′ end labels of each primer. Complete cleavage of DNA containing NO2-dA and -dG was achieved by treatment with aqueous pyrrolidine at 95°C (lanes 3–8 of Fig. 4A), which afforded only partial cleavage of DNA containing OH-dC or -dU and generated ladders of oligonucleotides of various lengths (lanes 9–14 of Fig. 4A). When oxidation with KMnO4 was performed before pyrrolidine treatment, the OH-dC and -dU samples were also cleaved to completion because of the increased lability of the corresponding oxidative products (Fig. 4B). To unify the cleavage procedure for all four analogs, we incorporated the KMnO4 oxidation step into all of the cleavage reactions. Excess pyrrolidine was added to quench unused KMnO4, presumably through formation of pyrrolidine-N-oxide and Mn(II), and to catalyze DNA-strand scission at modified nucleotides. To carry out cleavage reactions in situ, it was important to add EDTA to the cleavage reaction mixtures to quench divalent metal ions, which would promote nonspecific cleavage of the DNA backbone at high pH and elevated temperatures. Under the described one-pot incorporation–cleavage conditions, satisfactory cleavage was achieved for all four analogs (lanes 3–14 of Fig. 4B), whereas the control DNA sample remained intact (lane 2 of Fig. 4 A and B).
Table 2.
Major cleavage products are shown 5′→3′, with p representing phosphate groups at the 5′ and/or 3′ ends of the fragment
| Primary information fragment (length/MW) | Internal fragment (length/MW) | Common fragment | |
|---|---|---|---|
| NO2-dA:A allele | GAAACTGGACAGCACAGACTTCACCp(25 nt/7,709.3 Da) | pTGGTGCTGGTGp(11 nt/3,579.3 Da) | (24 nt/7,432.1 Da) |
| G allele | GAAACTGGACAGCACAGACTTCACCGGCp(28 nt/8,656.9 Da) | pTGGTGCCGGTGp(11 nt/3,564.3 Da) | |
| NO2-dG:A allele | GAAACTGGACAGCACAGACTTCACCAp(26 nt/8,022.5 Da) | too short to be useful | (29 nt/8,956.1 Da) |
| G allele | GAAACTGGACAGCACAGACTTCACCp(25 nt/7,709.3 Da) | too short to be useful | |
| OH-dC:A allele | TTCAGCAGCTTGATGGTGCTGGTGAAGTp(28 nt/8,770.9 Da) | too short to be useful | (25 nt/7,868.4 Da) |
| G allele | TTCAGCAGCTTGATGGTGCp(19 nt/5,915.0 Da) | pGGTGAAGTp(8 nt/2,649.7 Da) | |
| OH-dU:A allele | CATTCAGCAGCTTGATGGTGCp(21 nt/6,517.4 Da) | pCACCAGCACCAp(11 nt/3,415.3 Da) | (26 nt/8,157.6 Da) |
| G allele | CATTCAGCAGCTTGATGGTGCCGGp(24 nt/7,465.0 Da) | pCACCGGCACCAp(11 nt/3,431.3 Da) |
Segments from the primers are underlined.
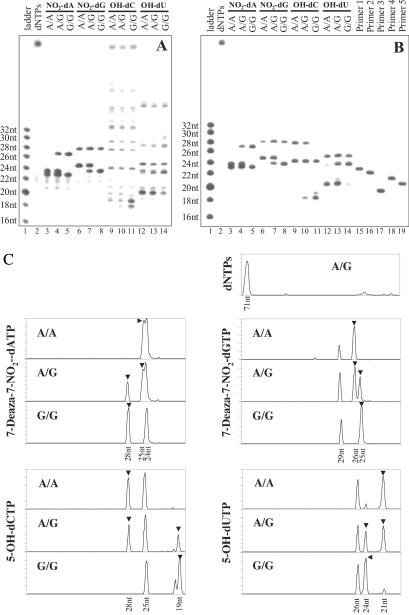
Fig 4.
PAGE analysis of cleavage products from PCR samples shown in Fig. 3. (A) An autoradiogram of cleavage products from pyrrolidine treatment alone. (B) An autoradiogram of cleavage products from chemical reactions with KMnO4 followed by pyrrolidine. (C) Profile presentation of B. ▾ points to genotype calling peaks.
As illustrated in Fig. 2, the genotypes of the original DNA templates may be derived from these cleavage products. A comparison of the expected primary information fragments listed in Table 2 with results in Fig. 4B revealed an excellent correspondence. For example, the NO2-dA products observed in Fig. 4B (lanes 3–5) concurred with the lengths of the anticipated cleavage products. The A/A homozygous template produced a primary fragment of 25 nt and a common fragment of 24 nt; the A/G heterozygous sample generated primary fragments of 25 and 28 nt, as well as a common fragment of 24 nt; the G/G homozygote yielded a primary fragment of 28 nt and a common fragment of 24 nt. A similar correlation was also observed with the other three analogs. Although the common fragments do not provide any genotype information, they may be used as internal references. The autoradiogram shown in Fig. 4B was converted by densitometry analysis into a profile representation (Fig. 4C), a common form of output used by fluorescence-based DNA analysis instruments such as the Applied Biosystems (ABI) 377 and ABI 3700 DNA sequencers. Because commercial dye-labeled primers are stable under current chemical cleavage conditions (O. Charlat, J.O., and J.L.W., unpublished results), we expect the ICCC strategy to be compatible with fluorescence detection.
MS Analysis.
The chemical cleavage products may also be distinguished by their mass differences by using MALDI-TOF MS. Because the sensitivity and accuracy of MALDI-TOF MS can be severely compromised by the presence of nonvolatile contaminants, their removal before MS analysis was essential. We have developed a high-throughput desalting method for MS sample preparation by using a simple solid-phase extraction procedure (16). For purification of chemically cleaved DNA fragments from reaction mixtures containing PCR buffers and cleavage reagents, cleavage and solid-phase extraction protocols were optimized in tandem. As a result, the cleavage reagent pyrrolidine was replaced by 3-pyrrolidinol, a slightly more hydrophilic and much less volatile secondary amine. Although its increased hydrophilicity makes it easier to remove during reversed-phase purification, its lower volatility eliminates the organic amine odor and helps to maintain a constant pH throughout the process.
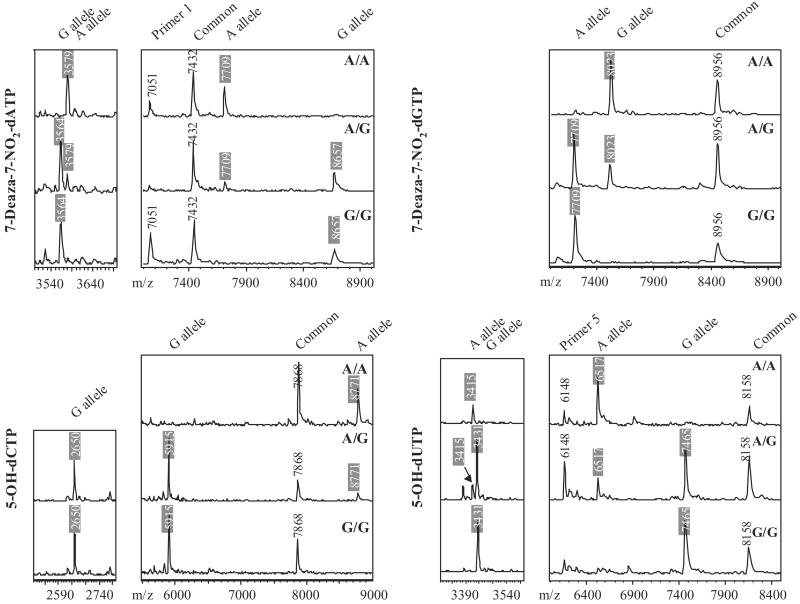
Using the model system described above, PCR samples were prepared by using each of the three TR genotypes in combination with each of the four modified nucleotides (Table 1). On the basis of the calculated MWs of the expected major cleavage fragments listed in Table 2, genotypes can be determined by MW differences between primary fragments or internal fragments. In general, the mass difference between primary fragments is greater than the mass of one nucleotide (≈300 Da) and is easily resolved by MALDI-TOF MS even under suboptimal detection conditions. In contrast, the MW difference between internal fragments is the MW difference between the polymorphic bases contained within the fragments, which depends on the specific base substitution and ranges from 9 to 40 Da. But less material is required for MS analysis of internal fragments, because their lower MWs yield increased MS signal compared with higher MW fragments. An additional benefit of using internal fragments is that the design of PCR primers is extremely flexible, because the internal fragments are independent of the termini of the amplicon. Ideally, both sets of fragments can be used to confirm the genotype calling. Fig. 5 shows the MS data obtained from the 12 ICCC samples. Perfect agreement between the expected and the observed MWs was observed for each sample. Common fragments, as well as unextended primers, were observed and used as MW controls. Examining the MS data for incorporation of NO2-dATP (Fig. 5), the internal fragment for the A allele, pTGGTGCTGGTGp, was observed in both A/A and A/G samples (the 3,579-Da peak in the low MW panel), whereas the pTGGTGCCGGTGp fragment for the G allele was detected in both A/G and G/G samples (the 3,564-Da peak). In the high MW region, the 25-nt fragment corresponding to the A allele was observed (7,709 Da) in both A/A and A/G samples, whereas the 8,657-Da peak corresponding to 28 nt, and the G allele was present in both A/G and G/G samples. In addition, 7,432- and 7,051-Da peaks were observed and correspond to the common fragment and primer 1, respectively. These results demonstrate the excellent flexibility of the ICCC genotyping strategy, with multiple experimental designs available for each SNP. In our model system, all four nucleotide analogs can be used for primary fragment analysis, and five of eight internal fragments of TR424 A:G SNP are informative (Table 2).
Fig 5.
MALDI-TOF mass spectra of chemical cleavage products from treating 12 PCR products listed in Table 1 with KMnO4 followed by 3-pyrrolidinol. Genotype calling fragments are indicated by shaded MWs.
Application of the ICCC Genotyping Method.
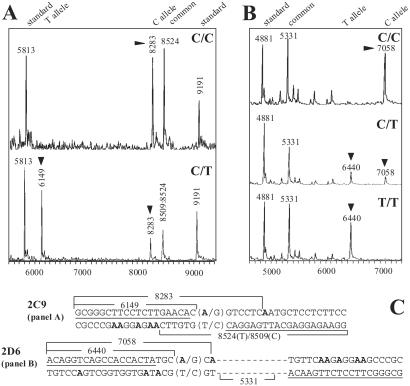
To evaluate the general applicability and performance of this MS-based genotyping procedure, we designed ICCC assays for clinically important SNPs from the drug metabolizing cytochromes P450 (CYP)2C9 and -2D6 and ran assays in a high-throughput format. Using a panel of previously sequenced DNAs representing 32 individuals with known CYP2D6 and CYP2C9 genotypes, we observed an average call rate in excess of 90% with no miscalls. Representative spectra from each assay for available genotypes are shown in Fig. 6 A and B). The sequences surrounding the 2C9 and 2D6 SNPs and the expected diagnostic fragments are shown in Fig. 6C. These results indicate that ICCC can be successfully adapted to serve as a high-throughput genotyping method.
Fig 6.
Representative, high-throughput cytochromes P450 2C9 (A) and 2D6 (B) genotyping results for samples with different genotypes. ▾ points to allele-diagnostic mass peaks. C shows the sequences of the PCR amplicons and cleavage fragments (see supporting information on the PNAS web site for additional sequence and mass information).
Conclusion
We have developed a simple genotyping strategy that is applicable to both fluorescence and mass-based analytical platforms. We chose to develop the MS platform because of its unparalleled accuracy and potential for high-throughput analysis, which are vital for analyzing clinical samples and correlating genetic variations with drug response. Compared with other MS-based genotyping methods that use dideoxynucleotides, the ICCC strategy described here provides great flexibility and the unique opportunity to generate two useful sets of genotype-specific DNA fragments within a single assay, enabling the possibility of internal confirmation of genotype assignment. ICCC, which generates primer-derived fragments with large mass differences, is potentially more robust and more amenable to high-throughput genotyping analysis than single-nucleotide extension methods, which demand greater mass resolution to detect single-nucleotide substitutions in oligonucleotides of ≈20 nt. ICCC also permits the analysis of the shorter internal DNA fragments, which improves detection sensitivity and accuracy but is not available with dideoxynucleotide-based methods. Finally, by combining genomic DNA amplification and sample fragmentation into a unified single-tube process, this method reduces the amount of sample manipulation and thus minimizes the opportunity for error. To address future genotyping needs, we are developing multiplexed MS assays to increase throughput and hope to apply the principle of incorporation-cleavage to additional genotyping platforms.
Supplementary Material
Acknowledgments
We thank Tracy Zimmermann, Ann Ferentz, Chuck Allerson, and Marilyn Gosse for critical reading of the manuscript. We are grateful for contributions to this project by Bing Wang, Lyne Breault, Valerie Stone, Olga Charlat, and Anne Bailey at various stages, and to Alexander Ernst and Michael Storek for generously sharing materials and experimental data before publication.
Abbreviations
ICCC, Incorporation and Complete Chemical Cleavage
KMnO4, potassium permanganate
MALDI-TOF, matrix-assisted desorption/ionization–time-of-flight
MW, molecular weight
SNP, single-nucleotide polymorphism
TR, transferrin receptor
References
- 1.Housman D. & Ledley, F. D. (1998) Nat. Biotechnol. 16, 492-493. [DOI] [PubMed] [Google Scholar]
- 2.Risch N. J. (2000) Nature (London) 405, 847-856. [DOI] [PubMed] [Google Scholar]
- 3.Wang D. G., Fan, J. B., Siao, C. J., Berno, A., Young, P., Sapolsky, R., Ghandour, G., Perkins, N., Winchester, E., Spencer, J., et al. (1998) Science 280, 1077-1082. [DOI] [PubMed] [Google Scholar]
- 4.Livak K. J., Marmaro, J. & Todd, J. A. (1995) Nat. Genet. 9, 341-342. [DOI] [PubMed] [Google Scholar]
- 5.Tyagi S., Marras, S. A. E. & Kramer, F. R. (2000) Nat. Biotechnol. 18, 1191-1196. [DOI] [PubMed] [Google Scholar]
- 6.Syvanen A. C. (1999) Hum. Mutat. 13, 1-10. [DOI] [PubMed] [Google Scholar]
- 7.Faruqi A. F., Hosono, S., Driscoll, M. D., Dean, F. B., Alsmadi, O., Bandaru, R., Kumar, G., Grimwade, B., Zong, Q., Sun, Z., et al. (2001) BMC Genomics 2, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hall J. G., Eis, P. S., Law, S. M., Reynaldo, L. P., Prudent, J. R., Marshall, D. J., Allawi, H. T., Mast, A. L., Dahlberg, J. E., Kwiatkowski, R. W., et al. (2000) Proc. Natl. Acad. Sci. USA 97, 8272-8277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chicurel M. (2001) Nature (London) 412, 580-582. [DOI] [PubMed] [Google Scholar]
- 10.Pastinen T., Kurg, A., Metspalu, A., Peltonen, L. & Syvanen, A. C. (1997) Genome Res. 7, 606-614. [DOI] [PubMed] [Google Scholar]
- 11.Southern E., Mir, K. & Shchepinov, M. (1999) Nat. Genet. 21, 5-9. [DOI] [PubMed] [Google Scholar]
- 12.Sanger F., Nicklen, S. & Coulson, A. R. (1977) Proc. Natl. Acad. Sci. USA 74, 5463-5467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buetow K. H., Edmonson, M., MacDonald, R., Clifford, R., Yip, P., Kelley, J., Little, D. P., Strausberg, R., Koester, H., Cantor, C. R., et al. (2001) Proc. Natl. Acad. Sci. USA 98, 581-584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Purmal A. A., Kow, Y. W. & Wallace, S. S. (1994) Nucleic Acids Res. 22, 72-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Min C. H., Cushing, T. D. & Verdine, G. L. (1996) J. Am. Chem. Soc. 118, 6116-6120. [Google Scholar]
- 16.Gilar M., Belenky, A. & Wang, B. H. (2001) J. Chromatogr. A 921, 3-13. [DOI] [PubMed] [Google Scholar]
- 17.Maxam A. M. & Gilbert, W. (1980) Methods Enzymol. 65, 499-560. [DOI] [PubMed] [Google Scholar]
- 18.Nakamaye K. L., Gish, G., Eckstein, F. & Vosberg, H. P. (1988) Nucleic Acids Res. 16, 9947-9959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mascarenas J. L., Hayashibara, K. C. & Verdine, G. L. (1993) J. Am. Chem. Soc. 115, 373-374. [Google Scholar]
- 20.Storek M. J., Ernst, A. & Verdine, G. L. (2002) Nat. Biotechnol. 20, 183-186. [DOI] [PubMed] [Google Scholar]
- 21.Tsuchihashi Z., Hansen, S. L., Quintana, L., Kronmal, G. S., Mapa, F. A., Feder, J. N. & Wolff, R. K. (1998) Blood Cells Mol. Dis. 24, 317-321. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.