Abstract
A statistical thermodynamic approach is used to analyze the various contributions to the free energy change associated with the insertion of proteins and protein fragments into lipid bilayers. The partition coefficient that determines the equilibrium distribution of proteins between the membrane and the solution is expressed as the ratio between the partition functions of the protein in the two phases. It is shown that when all of the relevant degrees of freedom (i.e., those that change their character upon insertion into the membrane) can be treated classically, the partition coefficient is fully determined by the ratio of the configurational integrals and thus does not involve any mass-dependent factors, a conclusion that is also valid for related processes such as protein adsorption on a membrane surface or substrate binding to proteins. The partition coefficient, and hence the transfer free energy, depend only on the potential energy of the protein in the membrane. Expressing this potential as a sum of a "static" term, corresponding to the equilibrium (minimal free energy) configuration of the protein in the membrane, and a "dynamical" term representing fluctuations around the equilibrium configuration, we show that the static term contains the "solvation" and "lipid perturbation" contributions to the transfer free energy. The dynamical term is responsible for the "immobilization" free energy, reflecting the loss of translational and rotational entropy of the protein upon incorporation into the membrane. Based on a recent molecular theory of lipid-protein interactions, the lipid perturbation and immobilization contributions are then expressed in terms of the elastic deformation free energy resulting from the perturbation of the lipid environment by the foreign (protein) inclusion. The model is formulated for cylindrically shaped proteins, and numerical estimates are given for the insertion of an alpha-helical peptide into a lipid bilayer. The immobilization free energy is shown to be considerably smaller than in previous estimates of this quantity, and the origin of the difference is discussed in detail.
Full text
PDF
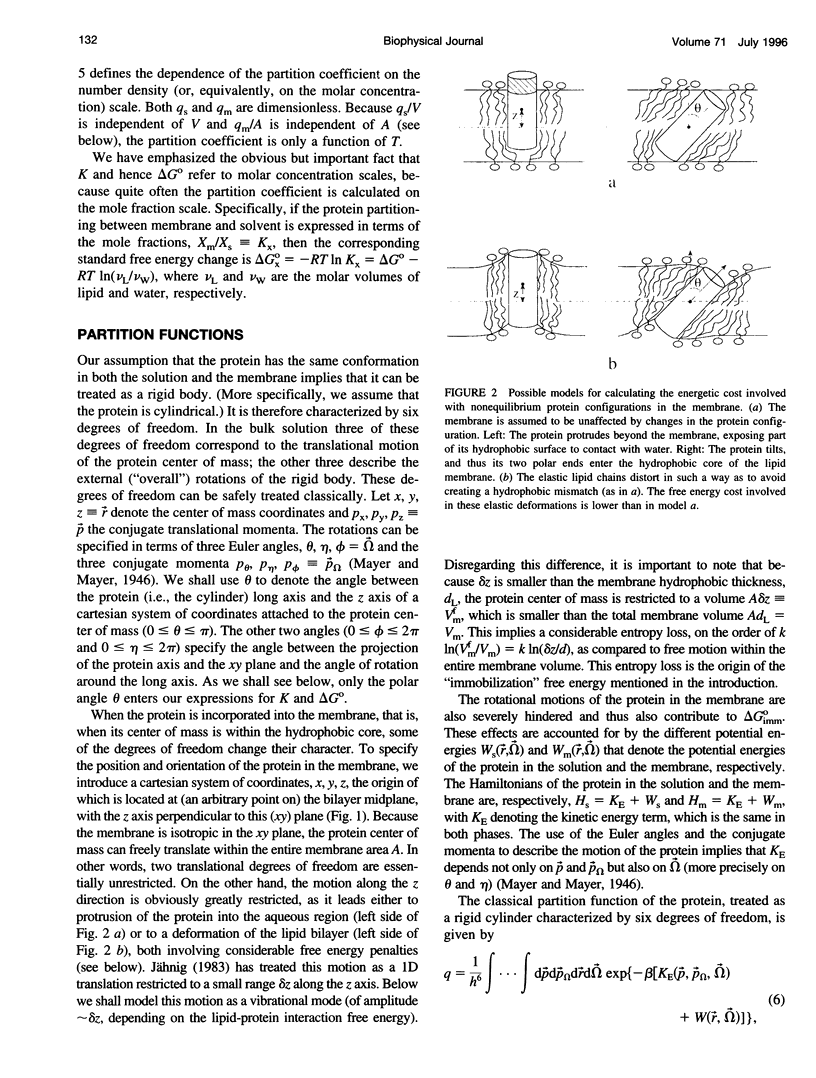





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ben-Tal N., Ben-Shaul A., Nicholls A., Honig B. Free-energy determinants of alpha-helix insertion into lipid bilayers. Biophys J. 1996 Apr;70(4):1803–1812. doi: 10.1016/S0006-3495(96)79744-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwyer J. D., Bloomfield V. A. Binding of multivalent ligands to mobile receptors in membranes. Biopolymers. 1981 Nov;20(11):2323–2336. doi: 10.1002/bip.1981.360201104. [DOI] [PubMed] [Google Scholar]
- Engelman D. M., Steitz T. A., Goldman A. Identifying nonpolar transbilayer helices in amino acid sequences of membrane proteins. Annu Rev Biophys Biophys Chem. 1986;15:321–353. doi: 10.1146/annurev.bb.15.060186.001541. [DOI] [PubMed] [Google Scholar]
- Engelman D. M., Steitz T. A. The spontaneous insertion of proteins into and across membranes: the helical hairpin hypothesis. Cell. 1981 Feb;23(2):411–422. doi: 10.1016/0092-8674(81)90136-7. [DOI] [PubMed] [Google Scholar]
- Fattal D. R., Ben-Shaul A. A molecular model for lipid-protein interaction in membranes: the role of hydrophobic mismatch. Biophys J. 1993 Nov;65(5):1795–1809. doi: 10.1016/S0006-3495(93)81249-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein A. V., Janin J. The price of lost freedom: entropy of bimolecular complex formation. Protein Eng. 1989 Oct;3(1):1–3. doi: 10.1093/protein/3.1.1. [DOI] [PubMed] [Google Scholar]
- Jacobs R. E., White S. H. The nature of the hydrophobic binding of small peptides at the bilayer interface: implications for the insertion of transbilayer helices. Biochemistry. 1989 Apr 18;28(8):3421–3437. doi: 10.1021/bi00434a042. [DOI] [PubMed] [Google Scholar]
- Janin J., Chothia C. Role of hydrophobicity in the binding of coenzymes. Appendix. Translational and rotational contribution to the free energy of dissociation. Biochemistry. 1978 Jul 25;17(15):2943–2948. doi: 10.1021/bi00608a001. [DOI] [PubMed] [Google Scholar]
- Jähnig F. Thermodynamics and kinetics of protein incorporation into membranes. Proc Natl Acad Sci U S A. 1983 Jun;80(12):3691–3695. doi: 10.1073/pnas.80.12.3691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leto T. L., Holloway P. W. Mechanism of cytochrome b5 binding to phosphatidylcholine vesicles. J Biol Chem. 1979 Jun 25;254(12):5015–5019. [PubMed] [Google Scholar]
- Milik M., Skolnick J. Insertion of peptide chains into lipid membranes: an off-lattice Monte Carlo dynamics model. Proteins. 1993 Jan;15(1):10–25. doi: 10.1002/prot.340150104. [DOI] [PubMed] [Google Scholar]
- Moll T. S., Thompson T. E. Semisynthetic proteins: model systems for the study of the insertion of hydrophobic peptides into preformed lipid bilayers. Biochemistry. 1994 Dec 27;33(51):15469–15482. doi: 10.1021/bi00255a029. [DOI] [PubMed] [Google Scholar]
- Mouritsen O. G., Bloom M. Mattress model of lipid-protein interactions in membranes. Biophys J. 1984 Aug;46(2):141–153. doi: 10.1016/S0006-3495(84)84007-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shai Y. Molecular recognition between membrane-spanning polypeptides. Trends Biochem Sci. 1995 Nov;20(11):460–464. doi: 10.1016/s0968-0004(00)89101-x. [DOI] [PubMed] [Google Scholar]
- Vogel H. Incorporation of melittin into phosphatidylcholine bilayers. Study of binding and conformational changes. FEBS Lett. 1981 Nov 2;134(1):37–42. doi: 10.1016/0014-5793(81)80545-5. [DOI] [PubMed] [Google Scholar]