ABSTRACT
Proliferation of Vibrio spp. in aquatic ecosystems is associated with climate change and, concomitantly, increased incidence of vibriosis. They are autochthonous to aquatic environments globally, but traditional metagenomic methods for detecting and typing pathogenic Vibrio spp. are challenged by their presence in relatively low abundance and ability to persist in a viable but nonculturable state. In the study reported here, hybridization capture sequencing (HCS) was employed to profile low-abundance Vibrio spp. in environmental samples. The HCS panel targeted a family of molecular chaperones (CPN60) specific to 69 Vibrio spp. and 162 Vibrio-specific virulence factors. This approach was evaluated in parallel with traditional whole-community shotgun sequencing in a metagenomic analysis of water and oyster samples collected from the Chesapeake Bay. In addition, Vibrio parahaemolyticus and Vibrio vulnificus strains isolated from the samples were subjected to whole-genome sequencing to determine the genetic characteristics of pathogenic Vibrio spp. circulating in an aquatic environment. HCS, employed to determine the incidence and characterization of specific Vibrio spp., yielded significantly greater metagenomic insight, notably a variety of other Vibrio spp., including detection of Vibrio cholerae, Vibrio fluvialis, and Vibrio aestuarianus, in addition to Vibrio parahaemolyticus and Vibrio vulnificus, and also important virulence factors not detectable using traditional molecular methods. Thus, pathogenic Vibrio spp. in aquatic ecosystems may be far more common than currently understood. It is concluded that environmental surveillance should include HCS, a valuable tool for the detection and characterization of pathogenic agents in aquatic ecosystems, notably vibrios.
IMPORTANCE
The increasing prevalence of pathogenic Vibrio spp. in aquatic ecosystems, driven by climate change, is closely linked to a rise in cholera and vibriosis cases, emphasizing the need for improved environmental surveillance. Vibrios are naturally occurring in aquatic environments globally, but traditional metagenomic methods for detecting and typing pathogenic Vibrio spp. are challenged by their presence in relatively low abundance and ability to persist in a viable but nonculturable state. In the study reported here, hybridization capture sequencing was employed to profile low-abundance Vibrio spp. in metagenomic samples, namely water and oysters collected from the Chesapeake Bay. This approach was evaluated in parallel with traditional whole-community shotgun sequencing and whole-genome sequencing of Vibrio parahaemolyticus and Vibrio vulnificus strains isolated from the samples. Results suggest pathogenic Vibrio spp. in aquatic ecosystems may be far more common than currently understood, when multiple methods are considered for environmental surveillance.
KEYWORDS: metagenomics, hybridization capture, targeted enrichment, whole-genome sequencing, next generation sequencing, microbiome, Vibrio, virulence
INTRODUCTION
Members of the genus Vibrio are autochthonous to the aquatic environment and play an important role in carbon and nitrogen cycling as well as other significant biogeochemical processes (1, 2). While Vibrio spp. are present in larger numbers in coastal waters, their abundance is strongly influenced by environmental factors, notably temperature and salinity (3). Furthermore, Vibrio spp. are commensals and/or symbionts of aquatic invertebrates, namely crustaceans, zooplankton, and bivalves, all of which harbor these bacteria (3–6). Vibrio spp. have an established mutualistic relationship with zooplankton, namely copepods, a major host of these bacteria and considered a vector of Vibrio cholerae (5, 7, 8).
Vibrio spp. concentrate in oysters and other filter-feeding shellfish, which are often consumed raw, thereby exposing consumers to potentially infective doses of pathogenic agents (6, 9, 10). The Centers for Disease Control and Prevention (CDC) considers infections associated with any species of the family Vibrionaceae as reportable through the Cholera and Other Vibrio Illness Surveillance (COVIS) system (11). Historically, most infections in humans have been associated with only a few Vibrio spp., of which V. cholerae, V. parahaemolyticus, and V. vulnificus are significant (3, 11–13). Yet, there has been an increasing number of reports of vibriosis in humans and marine animals globally, with pathogenic strains of Vibrio alginolyticus and Vibrio fluvialis dramatically more relevant (3, 11, 13, 14). In the USA, the majority of reported Vibrio spp. infections have been foodborne (12). However, wound infections caused by V. vulnificus and related vibrios are rapidly outpacing foodborne infections in the USA (3, 11, 14, 15), corroborated by the increase in Vibrio spp. infections reported in Florida during 1992–2022 (15) and in Maryland during 2006–2019 (14). Concerningly, both global and regional climate variability are impacting the emergence and prevalence of these disease-causing agents, resulting in a trend of increased incidence of Vibrio spp. infections, which can be expected to expand further (8, 16, 17). Thus, routine monitoring and predictive intelligence models to assess and forecast the risk of vibriosis are critical to public health.
Traditionally, Vibrio spp. have been detected and characterized by using culture-dependent methods. A recent culture-based investigation of V. parahaemolyticus and V. vulnificus in the Chesapeake Bay, carried out between 2009 and 2022, reaffirmed specific environmental predictors for these bacteria and documented their population increase and extended seasonality, notably increased numbers during the fall months of the year (4). However, since the vast majority of microorganisms present in the environment have not been successfully cultured (18), relying solely on culture-based techniques would fail to detect a significant portion of microbial diversity, including Vibrio spp. that can enter a viable but nonculturable (VBNC) state (19). Molecular methods of detection, e.g., polymerase chain reaction (PCR) and loop-mediated isothermal amplification (LAMP), have been successfully employed for the identification of Vibrio spp. in both environmental and clinical settings (15, 20–25). However, due to the complex nature of the matrices that Vibrio spp. inhabit, e.g., water, sediment, and/or crustaceans and bivalves, even these molecular-based methods are not sufficient to assess the totality of their presence (26). The methods are also limited in genetic profiling of samples since they detect only one or a few markers in a single assay. Whole-genome sequencing (WGS) provides valuable insight into the phylogenetic diversity of Vibrio spp. (27, 28) but requires preparation of DNA from isolates in pure culture, hence being dependent on successful culture. Metagenomics obviates the need for culturing microorganisms of interest by utilizing the genetic material of a sample to identify composition, thus allowing detailed comparison and exploration of microbial communities (29).
Metagenomics currently includes a variety of approaches for genomic profiling of the microbiota (Fig. S1), directly by whole-community shotgun metagenomic sequencing and targeted using amplicon sequencing and hybridization capture sequencing (HCS) (29, 30). In addition to 16S rRNA gene sequencing, other PCR amplification protocols have been successfully employed for detecting Vibrio spp., including assays targeting specific markers such as the vibriobactin utilization (viuB) protein-coding gene for V. cholerae (31), and the heat shock protein 60 (HSP60), commonly referred to as chaperonins, CPN60, or GroEL, for broader Vibrio genus identification (32). Multiplex PCR approaches have allowed for the simultaneous detection of multiple targets within a single reaction (33, 34), and amplicon sequencing has been used in smaller prokaryotic genomes, namely viruses (35). However, these PCR-based methods are limited in scalability since they generally target only a small number of loci per assay and may miss genetically diverse or novel strains due to primer bias or low abundance in complex samples. Furthermore, PCR multiplexing is constrained by primer compatibility, making it impractical to screen for many diverse targets simultaneously.
Considering these various methods for detection and characterization, shotgun metagenomics is attractive in terms of determining relative abundance of members of the microbiome and their functional genes, including virulence and antimicrobial resistance determinants. Shotgun metagenomic sequencing has been successful in the detection of Vibrio spp. in clinical settings, namely determining pathogenic strains in the stool of cholera (36) and vibriosis patients (37). Vibrio spp. detection in wastewater employing shotgun metagenomics has also been successful (38, 39), and a few studies detected pathogenic vibrios in surface water (15, 40, 41). However, those studies showed limited genetic profile characterization of Vibrio spp. present in the samples. Hence, shotgun metagenomic sequencing for the identification of Vibrio spp., especially pathogenic strains, in environmental samples is limited since they are present in low abundance, thereby underestimating risk.
HCS amplifies specific genomic regions of interest, targeted prior to sequencing, to increase detection of low-abundance targets in complex samples. HCS employs microarray technologies for sequencing, whereby a fragmented shotgun library is selectively enriched by hybridization of nucleic acid fragments (DNA or RNA) representing multiple genomic targets. Generally, probes designed for HCS (ca. 80 bp–120 bp) are longer than for PCR (ca. 15 bp–20 bp), allowing for targets to be amplified even if mutations have occurred in the binding area. A major benefit of HCS over PCR amplicon sequencing is that it is not restricted by the overall size or number of targets; hence, there is less interference between capture probe sets, as with PCR primers.
Vezzulli et al. (42) employed HCS for direct genotyping and metagenomic analysis of low-abundance V. cholerae DNA in complex environmental samples based on biotinylated baits for enrichment of V. cholerae metagenomic DNA via hybridization (43). Additional hybridization capture bait sets have been designed for Vibrio spp. to study pathobiota of oysters (44) as well as 16S rRNA (45) and antimicrobial resistance genes (46). These studies concluded that without targeted sequencing, it would not have been possible to detect the broad set of Vibrio subvariants and associated biomarkers, as traditional methods lack the necessary scalability and resolution.
Building on previous work (42, 44–46), the objective of the current study was to provide accurate analysis of the composition of Vibrio communities in complex samples by development of HCS that allows determination of Vibrio spp. diversity, detection of pathogenic subspecies, and carriage of clinically relevant virulence factors (VFs). We describe HCS targeting 69 Vibrio spp. and 162 virulence markers. A comparison of this method with traditional shotgun metagenomics used for microbiome profiling of water and oyster samples collected from the Chesapeake Bay in 2019 is provided. With the addition of WGS analysis of V. vulnificus and V. parahaemolyticus isolates, it is concluded that by incorporating HCS as a complement to culture and related molecular techniques, enhanced characterization and improved understanding of Vibrio spp. in complex samples are achieved, providing valuable information for risk assessment.
MATERIALS AND METHODS
Site description and sample collection
Methods employed for sample collection and processing have been described in detail previously (4, 15), and a summary of methods relative to this study is provided here. Between June and October of 2019, water and oyster (Eastern oyster [Crassostrea virginica]) were collected at six stations in the Chesapeake Bay, Maryland, USA. Sampling event details and station abbreviations are shown in Table 1.
TABLE 1.
Sampling location information
| Station | Abbrev | Collection date (yr-mo-day) | Latitude | Longitude | Temp (°C) | Salinity (ppt) |
pH | Conductivity (mS/cm) |
Optical dissolved oxygen (mg/L) |
Total Chl a (mg/L) |
Pheophytin (mg/L) |
Active Chl a (mg/L) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chester River | CHE | 2019-06-03 | 39.085980 | −76.164233 | 25.2 | 3.7 | 7.32 | 6816 | 8.0 | 21.76 | 9.27 | 16.59 |
| St. Mary’s River | SMR | 2019-07-02 | 38.145361 | −76.437833 | 26.6 | 9.1 | 8.6 | 15700 | 8.3 | 20.72 | 4.02 | 18.46 |
| Manokin River (Tangier Sound) |
MAN | 2019-07-16 | 38.109861 | −75.869111 | 29.7 | 12.5 | 8.03 | 21030 | 6.8 | 15.59 | 3.18 | 13.80 |
| Broad Creek | BRO | 2019-08-19 | 38.747250 | −76.248167 | 29.2 | 8.8 | 8.2 | NDa | 6.5 | 16.49 | 6.79 | 12.70 |
| Wicomico River | WIC | 2019-09-10 | 38.277139 | −76.826333 | 25.6 | 11.3 | 8.4 | ND | ND | 16.88 | 3.82 | 14.73 |
| Miles River | MIL | 2019-10-24 | 38.842556 | −76.232417 | 16.8 | 14.2 | 8.1 | 23440 | 9.7 | 12.05 | 2.75 | 10.50 |
ND, not determined.
During each sampling event, environmental parameters, including water temperature, pH, dissolved oxygen (DO), salinity, conductivity, and total dissolved solids, were measured 0.3 m below the surface using a handheld water probe (Eureka, TX). Water (12 L) was collected 0.3 m below the surface using a Van Dorn water sampler (WildCo, NY) and stored in a sterile Nalgene carboy (Thermo Fisher Scientific, Waltham, MA). Up to 30 oysters were collected by dredging and stored in clean double-zipper freezer bags. Samples were transported to the laboratory in a cooler with ice, and the temperature of the coolers was monitored using a single-trip temperature alert indicator (LogTag Recorders, Auckland, New Zealand). The temperature of the water samples was recorded upon arrival at the laboratory, and samples were stored at 4°C until processing (<4 h).
Sample processing
Water (250 mL) was filtered using 25 mm glass microfiber filters (Cytiva, MA), and concentrations of chlorophyll-a (total and active) and pheophytin were measured in acetone extracts on a Shimadzu UV 2401PC spectrophotometer, following procedures recommended by University of Maryland Center for Environmental Science for fluorometric detection of chlorophyll-a in waters of fresh/estuarine/coastal areas (47). Chlorophyll-a analysis was performed in duplicate, and averages are presented.
Water and oyster samples were treated following methods outlined in the Bacteriological Analytical Manual for food sampling/preparation of sample homogenate (48) and Vibrio (49). From each sampling event, 250 mL of water was concentrated using syringe filtration with 0.22 µm pore size Sterivex filter units (Millipore Sigma, MA). Oysters were rinsed and scrubbed under running deionized water to remove debris from the shell and opened using a sterile shucking knife. Oyster tissue in an equal volume of phosphate-buffered saline (pH 7.4) was homogenized in a sterile blender for 90 s. Filter units and homogenized oyster tissue (500 µL) were stored at −80°C in DNA/RNA Shield Stabilization Solution (Zymo Research, CA).
Isolation of V. parahaemolyticus and V. vulnificus
Water and oyster samples were respectively inoculated using alkaline peptone water (APW; 1% peptone, 1% NaCl [pH 8.5]). Briefly, unfiltered water (1 L) and homogenized oyster tissue (10 g) were resuspended in APW (10×) and incubated for 16 h at 37°C with moderate aeration (orbit diameter 2.5 cm × 30 rpm). A loopful of pellicle from each APW-enriched sample was respectively subcultured on selective agar media, including Vibrio-specific chromogenic agar (CHROMagar, France), thiosulfate citrate bile-salts sucrose agar (Oxoid, Canada), and M190 V. vulnificus agar (50), and incubated up to 18 h at 37°C. Presumptive Vibrio spp. colonies were purified on Luria-Bertani agar (Difco, NY) and maintained under standard bacteriological conditions for Vibrio spp. (51). Confirmation and identification of Vibrio spp. were done using established molecular assays, as detailed below.
Nucleic acid preparation
Genomic DNA was prepared from homogenized oyster tissue and pure culture isolates grown under standard conditions (Luria-Bertani broth with aeration at 37°C for 16 h), using the DNeasy Blood and Tissue Kit (Qiagen, Germany). Total DNA was isolated from the microbial biomass collected on Sterivex filter units, using the ZymoBIOMICS DNA Miniprep Kit (Zymo Research, CA) with modifications recommended previously (52). DNA extracts were further purified using the DNA Clean and Concentrator Kit (Zymo Research, CA).
Preparation of mock community
To prepare the mock community, DNA was prepared from pure cultures of 15 representative Vibrio spp. type strains (V. cholerae O39 AM-19226, V. cholerae O1 classical O395, V. cholerae O1 El Tor N16961, V. cholerae O139 MO10, V. vulnificus [vcgE] ATCC 27562, V. vulnificus [vcgC] ATCC 29307, V. parahaemolyticus O3:K6 VP-NY4, V. parahaemolyticus ATCC 17802, V. alginolyticus ATCC 17749, Vibrio mimicus ATCC 33653, Vibrio furnissii ATCC 35016, V. fluvialis ATCC 33809, Vibrio harveyi ATCC 14126, Vibrio aestuarianus ATCC 35048, and Aliivibrio [Vibrio] fischeri ATCC 25918), using the DNeasy Blood and Tissue Kit (Qiagen, Germany). Double-stranded DNA concentrations were measured using the NanoDrop ND-1000 Spectrophotometer (Thermo Fisher, MA), and genomic DNA from each strain was normalized with nuclease-free water to equal concentration (1,000 ng) and combined. Pooled mock community DNA was purified using the DNA Clean and Concentrator Kit (Zymo Research, CA).
Polymerase chain reaction
PCR methods have previously been established for detection and characterization of Vibrio spp. Primer sequences, amplicon sizes, and thermal cycler conditions are detailed in Table 2. Amplified products were fractionated by electrophoresis through 1.5% (wt/vol) agarose gel along with a 100 bp molecular weight marker (HyperLadder; BioLine, NJ) and visualized using SafeGLO Pre-Stain (BioLink, CA). For quality control, a no template control (NTC) consisting of nuclease-free water and positive/negative controls was included with each reaction.
TABLE 2.
Primers, PCR parameters, and reference strains used in this study
| Description (reference) | Oligonucleotide name | Sequence (5´−3´) | PCR product size (bp) | Annealing temp (°C) | Reference |
|---|---|---|---|---|---|
| Vibrio genus 16S rRNA | 567F | GGCGTAAAGCGCATGCAGGT | 120 | 55 | (21) |
| 680R | GAAATTCTACCCCCCTCTACAG | ||||
| toxR of V. parahaemolyticus (Vp), V. cholerae (Vc), and V. vulnificus (Vv) | UtoxF | GASTTTGTTTGGCGYGARCAAGGTT | (53) | ||
| Vp-toxR | GGTTCAACGATTGCGTCAGAAG | 297 (Vp) | 60 | ||
| Vc-toxR | GGTTAGCAACGATGCGTAAG | 640 (Vc) | 55 | ||
| Vv-toxR | AACGGAACTTAGACTCCGAC | 435 (Vv) | 55 | ||
| Total and hemolysin-producing V. parahaemolyticus via tlh, tdh, and trh | L-tl | AAAGCGGATTATGCAGAAGCACTG | 450 (tlh) | 58 | (20) |
| R-tl | GCTACTTTCTAGCATTTTCTCTGC | ||||
| L-tdh | GTAAAGGTCTCTGACTTTTGGAC | 269 (tdh) | 58 | ||
| R-tdh | TGGAATAGAACCTTCATCTTCACC | ||||
| L-trh | TTGGCTTCGATATTTTCAGTA | 500 (trh) | 58 | ||
| R-trh | CATAACAAACATATGCCCATTTCCG | ||||
| V. vulnificus hemolysin vvhA | F-vvh | TTCCAACTTCAAACCGAACTATGAC | 205 | 58 | (54) |
| R-vvh | ATTCCAGTCGATGCGAATACGTTG | ||||
| V. vulnificus biotype 1 virulence-correlated gene vcgC/E | F-vcgC | AGCTGCCGATAGCGATCT | 97 (vcgC) | 57 | (55) |
| R-vcgC | TGAGCTAACGCGAGTAGTGAG | ||||
| F-vcgE | CTCAGAAAGGCTCAATTGAC | 199 (vcgE) | 57 | ||
| F-vcgE | GATTAACGCTGTAAGGCCG | ||||
| V. vulnificus pilA | VvpAF32 | TGGCTGCTGTTGCTATTC | 217 | 60 | (56) |
| VvpAR2 | GGTCCACCACTAGTACCAAC | ||||
| V. vulnificus rtxA1 | ERMGST1 | CGGGATCCTATGGCGTGAACGGCGAAG | 1,440 | 68 | (57) |
| ERMGST2 | CGGGATCCAGCAGCCACAAGCGATTC | ||||
| Toxigenic V. cholerae via ctxA, rfb-O1, and rfb-O139 | O139F2 | AGCCTCTTTATTACGGGTGG | 449 (O139) | 55 | (58) |
| O139R2 | GTCAAACCCGATCGTAAAGG | ||||
| O1F2 | GTTTCACTGAACAGATGGG | 192 (O1) | 55 | ||
| O1R2-2 | GGTCATCTGTAAGTACAAC | ||||
| VCT1 | ACAGAGTGAGTACTTTGACC | 308 (ctxA) | 55 | ||
| VCT2 | ATACCATCCATATATTTGGGAG |
Next-generation sequencing (NGS)
Samples analyzed by WGS include seven isolates (V. parahaemolyticus, n = 3; V. vulnificus n = 4) from environmental water samples collected in 2019 (4) and identified by PCR as pathogenic V. parahaemolyticus ([Vp-toxR+ and tlh+] and [tdh+ and/or trh+]) or V. vulnificus ([Vv-toxR+, vvhA+, and vcgE+] and [pilA+ and/or rtxA+]). Microbiome analysis included Sterivex concentrated water and homogenized oyster tissue from each of the six sampling events during 2019 (12 total samples, consisting of six water and six oyster samples), along with a mock community sample (positive control) and NTC consisting of nuclease-free water processed using the DNeasy Blood and Tissue Kit (Qiagen, Germany). WGS and shotgun metagenomic libraries were prepared using the NEBNext Ultra II FS Library Prep Kit for Illumina sequencing (New England Biolabs, MA). For HCS, target enrichment was performed in four-plex on an aliquot of each of the libraries prepared for shotgun metagenomic sequencing, using the xGen Hybridization Capture of DNA Libraries kit (IDT, IA) with custom probes, described below.
Double-stranded DNA concentration was measured using the Qubit 3.0 fluorometer (Thermo Fisher, MA) and confirmation of library size was achieved using High Sensitivity D1000 ScreenTape Assay (Agilent Technologies, CA). NGS was done using the NextSeq 2000 System (Illumina, CA) with 150 bp paired-end reads. WGS and shotgun metagenomics were performed targeting 40M paired read clusters, while HCS was done targeting 10M. To avoid laboratory contamination of test samples, all analyses, including DNA extraction, amplification, and library preparation, were carried out in a separate Good Laboratory Practice-accredited and Current Good Manufacturing Practice-compliant laboratory (EzBiome Inc., MD) using a dedicated set of reagents and consumables. While DNA extractions for NTCs and mock community positive controls were performed separately from test samples, DNA amplification and library preparation were conducted simultaneously to ensure proper sequencing controls.
Probe design
Molecular chaperones are present in plastids, mitochondria, and cytoplasm of eukaryotes, bacteria, and archaea. Group I chaperonins (CPN60, also known as HSP60 or GroEL) are a diverse family of molecular chaperones specific to bacteria and have proved useful for differentiating closely related taxa and are commonly employed as targets for detection and identification of species, including vibrios (32, 59–61). An investigation of CPN60 sequences from bacterial and eukaryotic species (62) led to the discovery of a conserved region (~550 bp–570 bp) termed “universal target” (UT) to delineate closely related taxa and provide greater phylogenetic resolution than 16S rRNA, by providing a higher evolutionary rate of CPN60 (63–66). To this end, a reference database was created by downloading all nonredundant nucleotide sequences for the CPN60 UT (one sequence per species, type strain preferred) from the manually curated chaperonin database (67). Members of the genus Vibrio were extracted (n = 70) and aligned using the CLUSTALW algorithm (68), and evolutionary history was inferred using the maximum-likelihood method and Tamura-Nei Model (69) in MEGA 11 v.11.0.10 (70). To ensure phylogenetic resolution in the reference database, indistinguishable reference sequences were omitted. Two nodes (Vibrio agarivorans and Vibrio sagamiensis) were indistinguishable, hence combined, i.e., V. agarivorans/V. sagamiensis, resulting in 69 unique CPN60 UT sequences. In addition to the CPN60 sequences, relevant genomic markers (a total of 162 genes) commonly used for Vibrio spp. detection and identification, along with specific virulence factors, were manually curated from public repositories, namely Virulence Factor Database (79 genes) (71) and NCBI Pathogen Detection Reference Gene Catalog (83 genes) (72). CPN60 UT sequences and additional genomic targets were evaluated using CD-HIT-EST v.4.8.1 (73) to eliminate identical sequences in the reference database. The resulting targets comprised 231 unique reference sequences (~281 kbp) (Table S1).
Custom probes (120 bp DNA oligonucleotide sequences) were designed commercially by Integrated DNA Technologies xGen Pool Design Service (IDT, IA). The initial design contained 2,450 unique probes (2,553 probes pre-duplicate removal) (Table S2). Coverage of the original probe design was evaluated by mapping probes back to the reference database using Bowtie2 v.2.4.1 (74) with the “--very-sensitive” option. To make the HCS panel more accessible and cost-effective, the overall number of probes was reduced based on similarity of original target sequences by removing predicted functionally redundant probes, using a multi-strain algorithm (IDT, IA). The output of the multi-strain design contained 1,779 unique probes, considered functionally equivalent to a 1× tiling design (Table S3). Probe sequences generated using the multi-strain algorithm were analyzed for potential homology with other taxa using Kraken2 v.2.1.3 (75) and Bracken (76) with the PlusPF database, which includes reference sequences of archaeal, bacterial, viral, plasmid, protozoan, fungal, human, and vector origin, and visualized using Krona Tools (77).
Resulting probe sequences of multi-strain design were synthesized with 5´ biotin modification using xGen Minimal Residual Disease Research Hybridization Panel Tool (IDT, IA). The xGen Universal Blockers (IDT, IA) were employed to prevent off-target fragments from annealing to intended target sequence via adapter-to-adapter hybridization. HCS libraries were prepared using 500 ng from each of the 12 sequencing libraries prepared for shotgun metagenomics.
Preprocessing of sequencing reads
Read quality was assessed using FastQC v.0.12.1 (78) and MultiQC v.1.11 (79). Adapter sequences were removed, and low-quality bases were trimmed using fastp v.0.23.4, with options for base correction, polyG tail trimming, and low-complexity filtering enabled (80). While unintended human DNA contamination in sequencing read libraries was not expected, it has been shown to be a major problem in genomics, resulting in false identification of spurious proteins (81). Accordingly, potential reads originating from human DNA were identified and removed from all libraries using Bowtie2 (74) by mapping reads against the Human Telomere-to-Telomere Consortium CHM13 Project reference genome (GCA_009914755.4). For microbiome samples, Bowtie2 was used to remove potential reads originating from oyster DNA by mapping reads against reference genomes of the Eastern oyster (Crassostrea virginica: GCA_002022765.4) and Pacific oyster (Magallana gigas: GCA_963853765.1). To minimize the impact of background contamination on microbiome data integrity, notably in low microbial biomass samples (82), we employed a conservative approach to identify and remove potential contaminating reads (Fig. S2). Sequencing reads from NTCs were first assembled into contigs using the metagenomics workflow from Bioinformatics Tools (bit) v.1.9.4 (83). Sample read libraries were then mapped to these NTC-derived contigs using Bowtie2 (74), and any matching reads were excluded from downstream analyses.
Comparative genomics of Vibrio spp. isolates
Processed sequencing read libraries of purified culture isolates were assembled into contigs using Unicycler v.0.5.0, a SPAdes-based optimizer for bacterial genome assemblies (84). Contigs less than 500 bp were discarded. Assembly statistics, completeness, and genome quality were assessed using the Quality Assessment tool for Genome Assemblies v.5.2.0 (85) and Benchmarking Universal Single-Copy Orthologs (BUSCO) tool v.5.7.1 (86) with 1,445 BUSCOs from the “vibrionales_odb10” data set. Draft genome assemblies were annotated with Prokka v.1.14.6 (87), using all complete reference genomes of the genus Vibrio (n = 67) and all complete genomes of V. cholerae (n = 109), V. vulnificus (n = 27), and V. parahaemolyticus (n = 113), from RefSeq as guide, accessed 4 June 2024. Presence of antimicrobial resistance genes and virulence factors was assessed using the Short, Better Representative Extract Dataset (ShortBRED) algorithm (88) and BLAST (89), as described below. Multilocus sequence types (MLSTs) were predicted using the MLST software program (90) with the Public Database for Molecular Typing and Microbial Gene Diversity (PubMLST) database (91). Isolates identified as V. parahaemolyticus were subjected to serotyping by profiling serogroup-specific genes based on WGS data, using VPsero (92).
Phylogenomic tree reconstruction
Genomic data in public repositories vary widely in quality, including differences in genome size, number of contigs, assembly status (i.e., complete, chromosome, scaffold, contig), and N50 values. These inconsistencies may introduce bias during whole-genome alignment, as higher quality and more complete genomes may disproportionately influence phylogenetic analyses. To mitigate this issue, genome-level evolutionary relationships among the Vibrio spp. were inferred using GToTree v.1.8.6 (93), which constructs phylogenies based on a core genome approach using single copy genes (SCGs). First, a local database was built from CDSs of (n = 233) representative Vibrio spp. genomes present in the Genome Taxonomy Database (GTDB) v.220 (94). Using the “gtt-gen-SCG-HMMs” command within GToTree, all protein family (Pfam) Hidden Markov Model (HMM) profiles were downloaded from the Pfam database, released 28 May 2024 (95), and HMMs with an average coverage of <50% of the underlying protein sequence were removed. The filtered Pfam HMM profiles were searched against all coding sequences from the representative Vibrio spp. database using HMMER v.3.4 (96), specifically “hmmsearch” with the “-cut_ga” flag. Pfams with exactly one hit in at least 90% of the representative Vibrio spp. genomes were retained as the SCG set for the genus Vibrio (n = 355 targets). For building phylogenetic trees, CDSs were retrieved for input genomes from public repositories or called with Prodigal v.2.6.3 (97) when CDSs were not available. The Vibrio-specific SCGs were identified using “hmmsearch” as mentioned, and spurious gene hits were filtered based on the fraction of target genes detected. Each gene set was aligned with MUSCLE v.5.1 (98), and automated trimming was done with TrimAl v.1.4.15 (99). The trimmed gene sets were concatenated for each genome, and phylogenetic trees were built using FastTree v.2.1.11 (100), employing the maximum-likelihood Jones-Taylor-Thornton model with the default number of rate categories (CAT). The resulting trees were viewed with iTOL v.6 (101). Using this approach, the genomic relatedness of seven Vibrio spp. isolates from this study was evaluated among the 233 representative Vibrio spp. from GTDB (Table S4). These isolates were further classified with existing lineages of V. parahaemolyticus (n = 259) (Table S5) and V. vulnificus (n = 118) (Table S6).
Metagenomic community profiling
A total of 12 samples (both water and oyster from six sampling events) and controls (mock community positive control and NTC) were selected for metagenomic community profiling, which included both shotgun metagenomic sequencing and HCS.
Whole-community microbiome profiling
Metagenomic sequencing reads were profiled using KMCP v.0.9.4 (102) with multiple prebuilt databases from the KMCP software suite, including GTDB v.214 (94) (bacteria and archaea), RefSeq (fungi), and GenBank (viruses), using “single-end” mode for higher sensitivity, per recommendations of the KMCP documentation. The search results were merged, and profiling was done with flags for “--mode 3” and “--no-amb-corr.” Resulting KMCP scores, i.e., 90th percentile of query coverage (matched kmers/query kmers), were filtered, and taxa with a score <100 were labeled “unclassified.” Taxonomic profiles were visualized using Phylosmith v.1.0.6 (103) and presented as relative sequencing read abundance.
Depth of the sequencing read libraries was normalized to the sample with the minimum number of sequencing reads >10,000 using the “rarefy_even_depth” function of phyloseq v.1.46 (104); four oyster samples (two each from HCS and shotgun metagenomics) were omitted because they contained fewer reads than the simulation threshold. Alpha diversity metrics were obtained using phyloseq’s “estimate_richness” function. The observed (number of taxa), Chao1 (richness), and inverse Simpson’s diversity index (richness under uniform evenness) were visualized using the “plot_richness” function of phyloseq (104).
Gene profiling for CPN60 diversity
Processed sequencing read libraries of metagenomic samples were assembled into contigs using the workflow for metagenomics from Bioinformatics Tools (bit) v.1.9.4 (83). To assign taxonomy to the CPN60 sequences, a custom BLAST (89) database was built using the full nucleotide sequences of all group I reference taxa present in the chaperonin database, accessed 19 August 2024 (67). Metagenomic contigs were mapped against the CPN60 database using blastn v.2.16.0 (89) with an e-value threshold of 1E − 180. Taxonomy was assigned initially at the species level for positive hits with ≥90% identity and ≥30% query coverage. To confirm taxonomic identity, the evolutionary relationships of identified CPN60 sequences were explored among Vibrio spp. reference gene sequences present in the chaperonin database (67). Briefly, nucleotide sequences coding for CPN60 were extracted from metagenomic contigs (n = 10) (Table S7) and aligned with reference gene sequences (n = 21) (Table S8) using Clustal Omega v.1.2.4 (105), and evolutionary history was inferred using the neighbor-joining method (106) in MEGA 11 v.11.0.10 (70) with 1,000 bootstrap replications. Resulting trees were visualized using iTOL v.6 (101).
Detection of antimicrobial resistance and virulence determinants
Presence of antimicrobial resistance genes and virulence determinants was determined for both Vibrio spp. isolates and metagenomic samples using ShortBRED v.0.9.5 (88) and blastx v.2.16.0 (89). Briefly, the ShortBRED pipeline consists of two components: (i) a method that reduces reference proteins of interest to short, highly representative amino acid sequences, i.e., “markers” and (ii) a search step that maps reads to these markers to quantify relative abundance of their associated proteins (88). Pre-computed ShortBRED markers were employed for resistance-related determinants targeting the Comprehensive Antibiotic Resistance Database (CARD) 2017 (107), and custom markers were designed to target virulence factors. Genes from the Virulence Factor Database (VFDB) 2025 (71), supplemented with those used in probe design of the HCS panel (Table S1), served as the proteins of interest for identifying marker families using “shortbred_identify.py” with the “--clustid 0.95” option. The Comprehensive and Non-redundant UniProt Reference Clusters (Uniref90) database (108), accessed 27 February 2024, was used as the reference proteins to help rule out non-unique regions of the proteins of interest. ShortBRED will record a hit to a marker if the resulting alignment has at least 95% identity and is at least as long as the minimum of the marker length or 95% of the read length. Gene abundances were normalized in reads per kilobase per million mapped reads (RPKM) using “shortbred_quantify.py.” That is, genes detected in a sample were normalized to the total number of reads in a sample and the gene length. In addition, assembled contigs were mapped against BLAST databases, built using protein sequences of the antimicrobial resistance and virulence determinants, using blastx (89) with an e-value threshold of 1E − 180. Genes were considered to be present only if detected by both methods.
RESULTS
Environmental parameters and PCR of Vibrio spp.
Between June and October of 2019, samples were collected from six stations (Fig. 1A). A summary of environmental parameters recorded during each sampling event is presented in Table 1. Temperature did not vary significantly across stations at the time of collection but increased with seasonality as expected, with the warmest temperatures observed during July and August. In general, lower salinity concentrations were recorded at stations located in the upper bay, namely CHE, MIL, and BRO, compared to those in the lower bay.
Fig 1.
Area of study and PCR characterization. (A) Map of sampling locations in the Chesapeake Bay, Maryland, where water and oyster samples were collected between June and October 2019. (B) Heatmap showing detection (presence/absence) of Vibrio genus- and species-specific biomarkers, including virulence-associated genes, in environmental water and oyster samples as well as pure culture isolates (V. parahaemolyticus and V. vulnificus).
Detection of genetic markers targeting the genus Vibrio, V. parahaemolyticus, V. vulnificus, and V. cholerae, as well as important virulence factors, was achieved using PCR. Reaction parameters are shown in Table 2 and results in Fig. 1B. Presence of the genus Vibrio (16S rRNA) was detected in all samples. V. vulnificus (toxR and/or vvhA) was detected at all stations, V. parahaemolyticus (toxR and/or tlh) was detected at all stations except those from CHE, and V. cholerae (toxR) at all stations except MAN. Pathogenic biomarkers for V. parahaemolyticus (tdh and trh) and V. cholerae (rfb-O1, rfb-O139, and ctxA) were not detected in the environmental samples. However, V. parahaemolyticus isolates recovered from these stations were shown to carry tdh/trh. Of the three V. parahaemolyticus isolates, all carried trh, and two carried both trh and tdh. Of the four V. vulnificus isolates, all encoded the environmental vcgE biotype, three encoded rtxA, and one encoded pilA.
Microbiome analysis
Validation on a microbial mock community
Rather than sequencing all genetic material in a sample, as done in traditional shotgun metagenomic sequencing, targeted HCS selectively amplifies specific genomic targets of interest prior to sequencing. To this aim, a targeted HCS protocol was developed for analysis of Vibrio spp. diversity and detection of primary virulence factors. A total of 1,779 probes were designed (Table S3) to target 69 Vibrio spp. and 162 virulence determinants (Table S1). To assess specificity, probe sequences were profiled using Kraken2 (75) and Bracken (76) against the PlusPF database, curated to include archaea, bacteria, viruses, plasmids, protozoa, fungi, human, and vectors. The results showed minimal homology with non-target taxa, with >99% of probe sequences identified as originating from Vibrio (Fig. S3), indicating that probes were appropriately designed for the intended application.
Hybridization capture sequencing and shotgun metagenomics were then used to profile a synthetic metagenome composed of equal DNA concentrations from 15 representative Vibrio spp. type strains. These included four strains of V. cholerae, two strains each of V. vulnificus and V. parahaemolyticus, and one strain each of V. alginolyticus, Vibrio mimicus, Vibrio furnissii, V. fluvialis, Vibrio harveyi, Vibrio aestuarianus, and Aliivibrio (Vibrio) fischeri.
To calculate the proportion of reads corresponding to target genes, Bowtie2 (74) was used to map processed sequencing reads against the genes included in the HCS panel design (Table S1). The results demonstrated that HCS significantly enriched target genes, with approximately 96% of reads mapping to panel targets (94% corresponding to virulence determinants and 1.6% to Vibrio-specific cpn gene sequences). In contrast, shotgun metagenomics yielded less than 2% of the reads carrying target genes (1% virulence determinates and 0.8% cpn sequences). These findings indicate that HCS effectively enhances the detection of target genes compared to traditional shotgun metagenomics.
K-mer-based metagenomic profiling was performed on control samples using the KMCP algorithm with whole-genome reference databases (Fig. S4). Among the 10 representative Vibrio spp., 9 were correctly identified by shotgun metagenomics and 8 by HCS. In the latter case, V. alginolyticus was identified as Vibrio diabolicus, which is a taxonomic synonym for V. alginolyticus. V. fischeri was not detected using either method. Since the mock community was prepared with equal DNA concentrations for each strain, V. cholerae was expected to be the most abundant, followed by V. parahaemolyticus and V. vulnificus with the remaining species appearing at lower abundances. Shotgun sequencing most closely matched this expected profile, while HCS showed more than 60% relative sequencing read abundance profiled as V. cholerae. Importantly, no taxa were detected in the NTC samples for either HCS or shotgun metagenomics. Furthermore, all taxa identified in the mock community were classified as members of the genus Vibrio, indicating no detectable cross-contamination among samples.
Community microbiome profiles employing KMCP
Twelve samples, including water and oyster collected during six sampling events, were selected for microbiome analysis based on results from PCR and ability to recover Vibrio spp. isolates from the area. Subsequently, all samples were subjected to whole-community shotgun metagenomics and targeted HCS (Fig. 2). Measures of alpha diversity are shown in Fig. 2A. Overall, a greater number of microbial species were detected using shotgun metagenomic sequencing compared to HCS (Fig. S5). However, no significant differences were observed for alpha diversity indices between sequencing methods. Although it is worth noting that differences were observed between sample types, with overall alpha diversity significantly higher in water samples, compared to oyster, for both sequencing methods.
Fig 2.
Microbial community composition based on k-mer alignment to whole-genome reference databases. (A) Alpha diversity metrics (observed, Chao1, and inverse Simpson indices) at the species level, comparing water and oyster microbiome profiled by hybridization capture sequencing and shotgun metagenomics. Violin plots are grouped by sample type and colored by sample site. (B) Stacked bar plots showing the relative sequence read abundance (%) of dominant bacterial and archaeal phyla across control, water, and oyster samples. (C) Stacked bar plots showing relative abundance (%) of the top 20 most abundant genera. (D) Heatmap of log-transformed relative abundances, log(RA%), of Vibrio spp. detected across samples and controls, highlighting improved detection sensitivity of hybridization capture sequencing compared to shotgun metagenomics. (E) Heatmap of log-transformed relative abundances, log(RA%), of archaeal, algal, viral, and phage taxa, showing broader taxonomic diversity captured by shotgun metagenomics in water samples. No archaeal, algal, viral, or phage taxa were found in oyster samples; these taxa were combined for each sequencing method (hybridization capture and shotgun metagenomics) to aid visualization. Taxonomic profiling was performed using KMCP with reference databases from GTDB, RefSeq, and GenBank.
Community microbiome profiles were evaluated using the KMCP algorithm with whole-genome reference databases. Bacterial phyla detected were similar via both sequencing methods, i.e., HCS and shotgun metagenomics (Fig. 2B). Cyanobacteriota were dominant in oyster samples and also profiled in water samples. Pseudomonadota (formerly Proteobacteria) were most abundant in water samples and frequent in oyster samples profiled by HCS but not detected by shotgun metagenomics. Bacteria of the phyla Actinobacteria, Bacteroidota, and Verrucomicrobiota were detected in all water samples, regardless of sequencing method. A greater number of genera were profiled in water samples (Fig. 2C), compared to oyster. However, it is worth noting that for oyster samples, the majority of sequencing reads were removed during preprocessing as being profiled as Crassostrea virginica (Fig. S2). Of the reads profiled in oyster samples, Synechococcus was dominant, while Acidimicrobium was common in water samples. Members of the genus Vibrio profiled to species are shown in Fig. 2D. Employing HCS, Vibrio spp. were detected in five of six stations, including five water and four oyster samples. In comparison, Vibrio was detected in only one water sample and not in oyster samples, employing shotgun metagenomic sequencing. For HCS samples, V. cholerae was the most commonly detected species, followed by V. parahaemolyticus, V. vulnificus, V. fluvialis, and V. aestuarianus. While HCS showed increased sensitivity in detecting target taxa, namely members of the genus Vibrio, shotgun metagenomics proved best in detecting archaea and viruses not included in the HCS panel (Fig. 2E). Archaea and viruses were not detected in any of the oyster samples. Of those profiled in water samples, pathogenic viruses known to infect humans were not detected, with bacteriophages the majority, namely those specific to Synechococcus bacteria.
Taxonomic diversity of CPN60
Gene profiling of the CPN60 marker was used to assess the diversity of Vibrio spp. within the metagenomic samples (Fig. 3). The taxonomic assignment of CPN60 sequences revealed the presence of multiple taxa, with water samples showing a greater diversity compared to oyster samples (Fig. 3A). Although shotgun metagenomics detected a higher overall number of taxa, Vibrio spp. were exclusively detected using HCS. Notably, V. aestuarianus, V. vulnificus, and V. cholerae were identified using HCS, whereas Aphanothece spp. and Cyanobium spp. were predominantly detected in shotgun metagenomic samples.
Fig 3.
CPN60 diversity and phylogenetic analysis of recovered sequences. (A) Taxonomic assignment of CPN60 sequences from metagenomic-assembled contigs across control, water, and oyster samples, using both hybridization capture and shotgun metagenomics. Yellow indicates detection of CPN60 sequences assigned to each taxon; blue indicates not detected. Taxa include Vibrio species, primarily detected by hybridization capture sequencing, as well as non-Vibrio microbial taxa detected by shotgun metagenomics. (B) Neighbor-joining phylogenetic tree of Vibrio spp. CPN60 nucleotide sequences recovered from metagenomics (blue text, starred) and reference sequences (black text) from the chaperonin database. Red dots represent bootstrap support at key nodes. Tree illustrates the phylogenetic placement of environmental sequences among known Vibrio species, confirming species-level assignments and revealing diversity among recovered CPN60 sequences.
Phylogenetic analysis of the recovered CPN60 sequences (Fig. 3B) confirmed the taxonomic assignments of Vibrio spp., with the recovered sequences clustering within the expected clades, indicating consistency between environmental isolates and known Vibrio lineages. For all Vibrio-specific CPN60 assignments, except for V. vulnificus recovered from a water sample collected at BRO, the identified species were also detected using k-mer-based whole-genome profiling (Fig. 2D). These results highlight the utility of CPN60 as a robust taxonomic marker for profiling bacterial diversity in complex environmental samples.
Capturing antimicrobial resistance and virulence determinants
Antimicrobial resistance genes and virulence factors were identified from metagenomic samples using ShortBRED (88) and blastx (89). The detected antimicrobial resistance genes (ARGs), categorized by resistance mechanism, are shown in Fig. 4A. Genes related to antibiotic efflux (e.g., oleC, AcrF, CpxR, and Yojl), target alteration (e.g., rpsL, fabL, EF-Tu, vanR, gyrB, and PmrF), and target protection (tetW) were predominantly found in water samples processed via shotgun metagenomics, whereas HCS detected fewer ARGs overall. Notably, no ARGs were detected in oyster samples.
Fig 4.
Detection and quantification of antimicrobial resistance and virulence factor-associated genes using ShortBRED. (A) Heatmap showing detection and quantification of antimicrobial resistance genes from the CARD, stratified by resistance mechanism. Color scale indicates log-transformed reads per kilobase per million mapped reads, log(RPKMs). (B) Bar plot showing the total count of virulence factor genes detected in each sample, grouped by virulence category based on annotations from the VFDB and probe targets included in the HCS panel. (C) Heatmap showing detection and quantification of virulence factors, including genes from VFDB and custom probe targets, across different sample types and sequencing methods. Virulence genes are grouped by genus (top) and functional category (bottom). Color scale represents log-transformed RPKMs, and VF categories are color-coded in the legend to indicate functional roles.
The distribution of detected VFs across sample types is shown in Fig. 4B and C. Overall, a greater number of VF genes were identified in water samples compared to oyster samples, with HCS consistently detecting more VFs than shotgun metagenomics. The detected VFs spanned several functional categories, including adherence, exoenzymes, biofilm formation, and competitive advantage, with genes characteristic of Vibrio spp. being particularly prevalent. Among samples profiled using HCS, several biomarkers for species detection and identification were identified, including toxR (genus Vibrio), tlh (V. parahaemolyticus), vvhA (V. vulnificus), and ompW (V. cholerae).
However, important determinants associated with clinical V. cholerae (ctxA and rfb-O1) and V. parahaemolyticus (tdh and trh) were not detected, despite the presence of the latter in WGS isolates from the sampling areas. Other VFs, such as those related to type III secretion system (T3SS) (type 1) and type VI secretion system (T6SS), exotoxins (rtxA, chxAIII, toxA, rtxA, rtxB, and hlyA), and exoenzymes (hflk and colA), were commonly identified. Additionally, genes associated with V. cholerae conjugative elements, namely, Vibrio seventh pandemic island II (VSP-II), were detected in most water samples.
It is also worth noting that the detection of non-Vibrio VFs was more frequently detected in samples analyzed via shotgun metagenomics, which aligns with previous observations from this study. This indicates that while HCS is highly efficient for detecting targets of interest, shotgun metagenomics captures a broader range of background microbial diversity, including ARGs and VFs.
Comparative genomics of Vibrio spp. isolates employing WGS
The seven Vibrio spp. isolates were subjected to WGS, and comparative genomics with 233 representative Vibrio spp. genomes present in the GTDB (94) was performed. A search for homologous genes returned 355 SCGs (ca. 8.05 × 104 amino acids in length) shared among the genus Vibrio. Within the Vibrionaceae phylogeny, each species formed coherent clusters in taxonomic subclades (Fig. 5A). Four isolates obtained in this study formed a distinct cluster with representative V. vulnificus strains. Three isolates clustered with representative V. parahaemolyticus, the latter forming a subclade within the V. harveyi group that also included V. alginolyticus, V. diabolicus, V. campbellii, V. owensii, and V. harveyi.
Fig 5.
Phylogenomic relationships of Vibrionaceae isolates and characterization of antimicrobial resistance and virulence factor-associated genes using ShortBRED. (A) Maximum-likelihood phylogenetic tree of Vibrionaceae constructed using single-copy core genes identified by GToTree. Isolates recovered in this study are marked with red stars, and taxonomic groups of interest (V. parahaemolyticus and V. vulnificus) are highlighted in orange and green, respectively. (B) Heatmaps showing detection of VFs in Vibrio spp. isolates. VFs are grouped by functional category according to the VFDB and probe targets included in the HCS panel, with color-coding for functional role. (C) Detection of antimicrobial resistance genes from the CARD database, grouped by resistance mechanism. Yellow indicates the presence, and blue indicates the absence of the target gene or protein family.
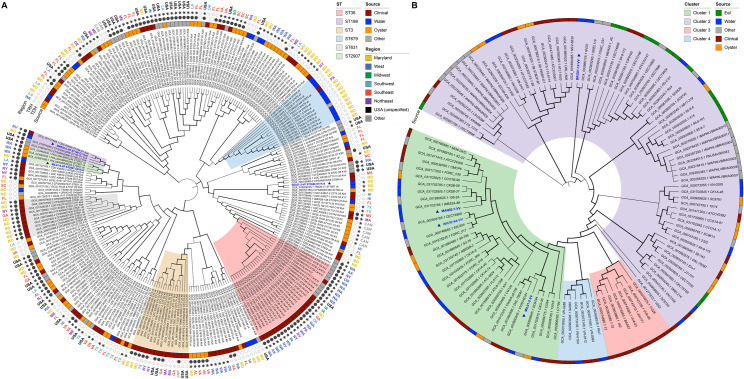
Using a homologous search of the Vibrio SCG set, phylogenomic trees were also built for V. parahaemolyticus (Fig. 6A) and V. vulnificus (Fig. 6B). Using alleles for dnaE, gyrB, recA, dtdS, pntA, pyrC, and tnaA, the three V. parahaemolyticus isolates were each assigned to different MLST profiles (ST199, ST2907, and ST3669). None of the isolates were assigned to clonal complexes (Table S11). Based on the phylogeny of the Vibrio SCG set for V. parahaemolyticus, well-defined clades for sequence types primarily of clinical origin were observed, including ST3, ST36, and ST631. While V. parahaemolyticus isolates from this study encoded tdh/trh, they clustered most closely with other environmental isolates. WIC01-5 clustered closely with an isolate recovered previously from Tangier Sound, Maryland, while MIL01-6 clustered with oyster isolates from Washington and SMR02-8 with a clinical isolate from Nevada. Of the four V. vulnificus isolates, WIC02-6A was assigned to ST286, and the remaining isolates were not assigned to an established MLST profile, using alleles for glp, gyrB, mdh, metG, purM, dtdS, lysA, pntA, pyrC, and tnaA (Table S12). Following Vibrio SCG phylogeny, V. vulnificus strains clustered into four distinct groups (C1 to C4). C1 and C2 comprised most of the genomic diversity, while isolates within C3 and C4 were more closely related to each other. Three isolates were phylogenetically joined in C1 and one in C2. While both clinical and environmental isolates were present in distally related clades, isolates from this study paired most closely with those of environmental origin.
Fig 6.
Phylogenetic relationships of Vibrio species isolates recovered in this study. Maximum-likelihood phylogenetic tree of (A) Vibrio parahaemolyticus (n = 259 genomes) and (B) Vibrio vulnificus (n = 118 genomes), constructed using single-copy core genes identified by GToTree. Isolates recovered in this study are highlighted in blue and denoted with triangles. Trees were rooted and visualized using iTOL. Clustering illustrates the genomic relatedness of environmental isolates from this study relative to globally distributed reference strains, supporting lineage classification and potential virulence associations.
Genetic characterization of Vibrio isolates
Profiling Vibrio spp. isolates using ShortBRED (88) and blastx (89) yielded important information concerning the presence of VFs (Fig. 5B) and ARGs (Fig. 5C). Species identity was supported by the presence of species-specific virulence regulator toxR in all isolates. In addition, VF genes associated with adherence, exotoxin production, and exoenzyme activity were commonly detected across isolates.
Among the V. parahaemolyticus isolates, the virulence markers tlh and the T3SS (type 1) were consistently present. Consistent with PCR results (Fig. 1B), two V. parahaemolyticus isolates carried the thermostable direct hemolysin (tdh) gene, and all encoded the tdh-related (trh) gene. In addition, one V. parahaemolyticus isolate carried impG/vasA, suggesting the presence of the T6SS.
All V. vulnificus isolates encoded the hemolysin gene vvhA and RTX toxin. In addition, genes associated with iron acquisition, such as vcgE, vulnibactin (viuB), and pilA, were detected, along with the V. vulnificus homolog of tlh. Two V. vulnificus isolates also encoded the type IV secretion system, while one isolate carried T6SS.
Regarding antimicrobial resistance, all V. parahaemolyticus isolates carried ARGs (Fig. 5C), notably genes conferring resistance to tetracycline (tet35) and penicillin (CARB-23). Additionally, two V. parahaemolyticus isolates harbored the multidrug resistance gene CRP, indicating potential resistance to multiple antibiotic classes. In contrast, no ARGs were detected in the V. vulnificus isolates.
These findings indicate that V. parahaemolyticus isolates in this study possess a greater potential for antimicrobial resistance compared to V. vulnificus isolates. However, all isolates in this study harbored a broad range of VFs, highlighting the potential for diverse pathogenic mechanisms within environmental Vibrio populations in the Chesapeake Bay.
DISCUSSION
Vibrio spp. are an integral part of the microbial flora in aquatic environments globally, particularly within crustaceans and zooplankton. Environmental factors influence the proliferation of Vibrio spp. and their transmission through food and water. Climate parameters, in particular, have been linked to the increased proliferation of pathogenic Vibrio spp., correlating with a rise in the number of infections caused by these bacteria globally (3, 4, 8, 15, 109–114).
Given the ecological significance of Vibrio spp., including their commensal and mutualistic interactions with multicellular hosts, controlling human infections caused by these bacteria requires accurate surveillance and effective public health interventions. Early warning systems that identify conditions conducive to growth and proliferation of pathogenic strains in the environment are essential for mitigating infection risks. Development of such systems will require a multidisciplinary integration approach, integrating knowledge of environmental factors that promote the persistence of pathogenic agents with sociological and behavioral factors facilitating human interactions with those pathogens.
Surveillance of the incidence and virulence profiles of pathogens in the environment is crucial for informing and training predictive models. Historically, detecting vibrios in the environment has been challenging due to their ability to enter a VBNC state, which complicates detection using traditional culture-based methods and can lead to underestimation of risk. Advanced molecular techniques offer a more detailed understanding of Vibrio spp. population dynamics, providing valuable data that can be incorporated into predictive models to enhance early warning systems.
Investigation of pathogenic Vibrio spp. has been ongoing in the Chesapeake Bay since the 1960s (6–8). Studies have shown that Vibrio spp. thrive in warm water with moderate salinity. However, detecting and typing Vibrio spp. have been done primarily for V. cholerae, V. parahaemolyticus, and more recently V. vulnificus. Recent assessment of the microbial community composition of blue crabs and water from Maryland coastal regions confirmed frequent detection of both V. parahaemolyticus and V. vulnificus by employing real-time PCR (115). However, detection of Vibrio spp. was infrequent when employing 16s rRNA amplicon sequencing. Furthermore, other important biomarkers could not be identified. Shotgun metagenomic sequencing provided a method for studying the total genetic material of a sample and proved useful in identifying pathogenic Vibrio spp., especially in clinical samples, including cholera (36) and vibriosis patients (37). However, when shotgun metagenomics is applied to environmental samples, the number of reads profiled as Vibrio spp. is often not sufficient for highly specialized applications, such as comparative analyses or threat assessment and attribution (15). A primary reason is that pathogenic agents frequently may be present only at low levels relative to the total microbial population of a given sample, particularly in shotgun metagenomic analysis of oyster samples, since sequencing read libraries are dominated by the host DNA, thereby complicating metagenomic profiling (116).
In this study, an alternative method for Vibrio spp. detection and characterization has been devised by creating targeted HCS assays of 69 Vibrio spp. and 162 virulence markers (Table S1). To confirm its value, the method was employed, along with traditional culture WGS and whole-community shotgun metagenomic sequencing, to profile the microbiome of water and oyster samples collected from the Chesapeake Bay.
Employing k-mer alignment to whole-genome reference databases (Fig. 2), HCS captured a greater diversity of Vibrio spp. compared to shotgun metagenomics. The probes designed for the enrichment panel, which included both CPN60 and Vibrio-specific biomarkers, enhanced homologous genomic regions of Vibrio spp. as well as the intended targets. One unexpected finding was the lack of significant differences in alpha diversity between the two sequencing methods (Fig. 2A). It was anticipated that preferential enrichment of Vibrio spp. targets would reduce overall diversity in HCS samples. However, this outcome may be due to the inherent nature of HCS, which does not fully deplete non-target sequences but instead increases the abundance of specific targets while still capturing background diversity from abundant taxa. Additionally, HCS increased the number of sequencing reads suitable for taxonomic profiling in oyster samples, which were predominantly composed of Synechococcus and Vibrio, consistent with previous studies (117). In contrast, shotgun sequencing detected a greater number of non-target taxa, notably viruses. This indicates that while HCS improved the sensitivity of Vibrio detection, it does not completely exclude non-target organisms, resulting in diversity estimates comparable to those obtained through shotgun sequencing.
Metagenomic analysis of a mock community (Fig. S4) revealed that shotgun sequencing most accurately reflected the expected relative abundance profiles, while HCS showed a bias toward V. cholerae. This bias was anticipated, as many of the probes were specifically designed to target well-characterized virulence factors in V. cholerae, particularly the Affertcholeramvirus CTXφ. Although HCS increased the detection sensitivity of Vibrio spp., this inherent bias indicates that the method is not currently suitable for accurate quantification due to targeted enrichment.
Among the 10 representative Vibrio spp. included in the mock community, 9 were correctly identified using shotgun metagenomics, and 8 were detected using HCS, based on k-mer alignment and whole-genome databases. These observations were further supported by the taxonomic assignment of CPN60 sequences recovered from metagenomic contigs, which proved to be more accurate at profiling the mock community. Notably, V. alginolyticus was identified as V. diabolicus/Vibrio sp. Ex25, which are taxonomic synonyms for V. alginolyticus (118). In addition, V. fischeri was not detected using either method, likely due to the absence of a high-quality genome sequence for Aliivibrio (Vibrio) fischeri ATCC 25918 in public repositories, resulting in its incomplete representation in the reference databases.
Due to the study design, the limit of detection could be estimated only at approximately 0.6% relative sequencing read abundance for species detection. However, previous research reported HCS targeting the 16S rRNA gene achieved a much lower limit of detection, around 0.00006% relative abundance (119). Further validation studies are needed to assess the sensitivity of the HCS approach developed here under different environmental contexts, since the lack of detection of certain genes may result from either natural environmental variability or methodological limitations.
Use of the CPN60 marker in metagenomic analysis provided valuable insight into the diversity of Vibrio spp. within environmental samples, highlighting the effectiveness of HCS in detecting specific taxa. Findings of CPN60 profiling of metagenomic contigs revealed that, while shotgun metagenomics identified a higher overall number of taxa, Vibrio spp. were exclusively detected using HCS (Fig. 3). Among the Vibrio spp. identified, V. aestuarianus, V. vulnificus, and V. cholerae were detected using HCS, while other non-Vibrio taxa such as Aphanothece spp. and Cyanobium spp. were predominantly observed with shotgun sequencing. This discrepancy between methods can be attributed to the targeted nature of HCS, compared to shotgun metagenomics which provides a broader snapshot of microbial diversity. In addition, phylogenetic analysis of the recovered CPN60 sequences further validated taxonomic assignments (Fig. 3B). Notably, all Vibrio-specific CPN60 assignments, except for a V. vulnificus sequence, were also detected using k-mer-based whole-genome profiling (Fig. 2D). These findings demonstrate the utility of CPN60 as a reliable marker for Vibrio spp. profiling, particularly when combined with HCS for enhanced detection sensitivity.
Comprehensive metagenomic analysis revealed ARGs and VFs were more commonly detected in water samples compared to oyster samples, with HCS generally identifying more VFs while shotgun metagenomics detected more ARGs (Fig. 4). This difference can be attributed to the design of the HCS probes, which specifically targeted Vibrio VFs. Other targeted capture protocols developed for ARGs, e.g., (46), will be considered in our future studies to improve antimicrobial resistance profiling in complex samples.
The identified VFs encompassed a range of functions, including adherence, exoenzymes, biofilm formation, and competitive advantage, with Vibrio-specific markers such as toxR, tlh, vvhA, and ompW being prevalent in samples analyzed using HCS. However, the primary VFs of V. parahaemolyticus (tdh and trh) were not detected using HCS, despite being present in WGS isolates from the sampling areas. Similarly, tlh and vvhA were detected more commonly by PCR (Fig. 1B) than by metagenomic methods. Consistent with previous findings, non-Vibrio VFs were more frequently detected using shotgun metagenomics compared to HCS.
V. parahaemolyticus and V. vulnificus isolates were recovered from water samples collected during a comprehensive study done during 2019 in the Chesapeake Bay (4) and subjected to WGS for phylogenomic analysis (Fig. 5 and 6). Phylogenetic clustering revealed multiple clonal populations circulating simultaneously, rather than all isolates from the same region clustering together. This pattern aligns with previous analyses of Vibrionaceae (120), V. parahaemolyticus (121–123), and V. vulnificus (124, 125). Among the V. parahaemolyticus isolates, two carried both tdh and trh, while one carried only trh. Notably, the pandemic complex (ST3, O3:K6) and prevalent clinical strains in North America, e.g., ST36 (O4:K12) and ST631, were not detected. However, one isolate profiled as ST199 had been previously reported from clinical cases in the USA (121) and China (126). V. vulnificus isolates classified into clusters C1 and C2, which have been reported elsewhere (124). All V. vulnificus isolates encoded the environmental biotype of the virulence-correlated gene (vcgE), although recent evidence suggests that biotype alone is not a reliable predictor of virulence potential (127, 128). Compared to V. parahaemolyticus, fewer V. vulnificus isolates from the Chesapeake Bay have been sequenced, with most available genomes originating from clinical sources. The genetic diversity observed in this study suggests that the Chesapeake Bay may serve as a reservoir of Vibrio genetic variability, highlighting the need for further genomic investigations.
While traditional culture-based methods proved useful for recovery of pathogenic V. parahaemolyticus and V. vulnificus from the Chesapeake Bay, it should be noted that the majority of V. parahaemolyticus isolates recovered from the Chesapeake Bay did not encode tdh and/or trh (4, 129). Hence, while pathogenic Vibrio spp. are clearly present in the Chesapeake Bay, culture isolation-based studies do not reflect the complex dynamics of microbial communities accurately. In contrast, culture-independent molecular methods, namely shotgun metagenomics and HCS, effectively profile the microbiome, allowing more accurate detection and characterization of Vibrio spp.
Challenges and future directions
Currently, PCR is routinely employed for biomarker detection and has proven both practical and useful for infectious disease diagnostics and for some aspects of environmental surveillance by circumventing issues associated with culturing. In this study, PCR detected V. cholerae, V. parahaemolyticus, and V. vulnificus (Fig. 1B). However, PCR is limited in its ability to genetically profile samples since it only targets one or a few markers at a time. Except for a single water sample, whole-community shotgun metagenomic sequencing, performed at the depth used in this study, was insufficient to profile sequencing reads to members of the genus (Fig. 2D). In contrast, the HCS method demonstrated the presence of Vibrio spp. and revealed that carriage of Vibrio-specific VFs was more common than previously recognized. As expected, shotgun metagenomics of both water and oyster samples identified a wider range of species and other taxa, notably viruses, that were not detected by HCS.
The most time-consuming aspect of this study was the initial design and optimization of the HCS panel. Now that the panel has been developed, incorporating it into traditional NGS workflows adds approximately 1 additional day. Despite this added time, the method offers significant advantages by enabling detection of a broader range of target species and genes without the need to screen each target individually. The probe targets were specifically designed to keep the total number of probes low (less than 2,000, i.e., the minimum panel design available for most commercial suppliers), thereby reducing costs and making the method more accessible while still achieving a broad depth of targets.
Clearly, HCS can be further enhanced in probe design. Although HCS presently takes longer to perform than other sequencing methods (Fig. S1), it achieves much greater analytical sensitivity using fewer sequencing reads, significantly reducing sequencing cost when the targets of interest are known (130). With panels developed for Vibrio pathobiota of oysters (44), as well as 16S rRNA (45) and antimicrobial resistance genes (46), hybridization capture bait sets can be scaled rapidly. Additionally, selective culture enrichment using APW facilitates the identification of diverse elements and amplification of Vibrio spp. (15, 22, 131). Hence, HCS coupled with enrichment offers a promising area for further improvement.
One unexpected finding was the absence of tdh and trh, important V. parahaemolyticus VFs, in HCS data, despite their presence in WGS samples (Fig. 1B). This discrepancy suggests that while HCS improves detection sensitivity for specific targets, it may still miss certain critical markers. Thus, PCR, shotgun metagenomic sequencing, and HCS should be considered complementary rather than interchangeable methods of comprehensive environmental surveillance.
Although this study reports results for a small number of samples without duplication, the results clearly reveal the presence of pathogenic Vibrio spp. V. cholerae, V. parahaemolyticus, V. vulnificus, V. fluvialis, and V. aestuarianus, all of which are known causative agents of vibriosis, notably reported cases in Maryland (14). Additional studies in progress will characterize microbial communities in addition to Vibrio to provide a baseline of pathogenic agents present in water and oyster samples. Seasons of the year need to be studied as well to shed light on shifts in Vibrio populations annually and illuminate the spectra of microbiomes relative to changing environmental conditions. Predictive models will benefit from the inclusion of data from WGS, shotgun metagenomics, and HCS, particularly for pathogen surveillance, evolutionary analysis, and tracking the emergence of VFs and antibiotic resistance, as well as source attribution and outbreak monitoring.
ACKNOWLEDGMENTS
This research was supported in part by an appointment to the Intelligence Community Postdoctoral Research Fellowship Program at University of Maryland, College Park, administered by Oak Ridge Institute for Science and Education (ORISE) through an interagency agreement between the US Department of Energy (DOE) and the Office of the Director of National Intelligence (ODNI) awarded to K.D.B. and R.R.C. Further support was provided by the National Science Foundation (OCE1839171 and CCF1918749), National Institute of Environmental Health Sciences, National Institutes of Health (R01ES030317A), and the National Aeronautics and Space Administration (80NSSC20K0814 and 80NSSC22K1044), awarded to A.H., A.S.J., and R.R.C. This work was also funded in part under agreement no. HSHQDC-15-C-00064 awarded to Battelle National Biodefense Institute (BNBI) by the Department of Homeland Security (DHS) Science and Technology Directorate (S&T) for the management and operation of the National Biodefense Analysis and Countermeasures Center (NBACC), a Federally Funded Research and Development Center. The views and conclusions contained in this document are those of the authors and should not be interpreted as necessarily representing the official policies, either expressed or implied, of DHS or the US Government. DHS does not endorse any products or commercial services mentioned in this presentation. In no event shall DHS, ORISE, DOE, ODNI, BNBI, or NBACC have any responsibility or liability for any use, misuse, inability to use, or reliance upon the information contained herein. In addition, no warranty of fitness for a particular purpose, merchantability, accuracy, or adequacy is provided regarding the contents of this document.
Contributor Information
Rita R. Colwell, Email: rcolwell@umd.edu.
Deborah Hinton, National Institutes of Health, Bethesda, Maryland, USA.
DATA AVAILABILITY
Sequencing data generated for all samples included in this study have been deposited in the NCBI Sequence Read Archive database under BioProject ID PRJNA1188529. Probe sequences for the custom Vibrio spp. hybridization panel (Tables S1 through S3), cpn60 sequences recovered from metagenomic assembled contigs (Table S7), and accession numbers for metagenomic sequencing libraries (Table S9) and draft genome assemblies (Table S10) are provided in the supplemental material.
SUPPLEMENTAL MATERIAL
The following material is available online at https://doi.org/10.1128/mbio.00516-25.
Figures S1 to S5 and Tables S1 to S12.
ASM does not own the copyrights to Supplemental Material that may be linked to, or accessed through, an article. The authors have granted ASM a non-exclusive, world-wide license to publish the Supplemental Material files. Please contact the corresponding author directly for reuse.
REFERENCES
- 1. Joseph SW, Colwell RR, Kaper JB. 1982. Vibrio parahaemolyticus and related halophilic vibrios. CRC Crit Rev Microbiol 10:77–124. doi: 10.3109/10408418209113506 [DOI] [PubMed] [Google Scholar]
- 2. Le Roux F, Blokesch M. 2018. Eco-evolutionary dynamics linked to horizontal gene transfer in vibrios. Annu Rev Microbiol 72:89–110. doi: 10.1146/annurev-micro-090817-062148 [DOI] [PubMed] [Google Scholar]
- 3. Brumfield K.D, Usmani M, Chen KM, Gangwar M, Jutla AS, Huq A, Colwell RR. 2021. Environmental parameters associated with incidence and transmission of pathogenic Vibrio spp. Environ Microbiol 23:7314–7340. doi: 10.1111/1462-2920.15716 [DOI] [PubMed] [Google Scholar]
- 4. Brumfield KD, Chen AJ, Gangwar M, Usmani M, Hasan NA, Jutla AS, Huq A, Colwell RR. 2023. Environmental factors influencing occurrence of Vibrio parahaemolyticus and Vibrio vulnificus. Appl Environ Microbiol 89:e0030723. doi: 10.1128/aem.00307-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Huq A, Small EB, West PA, Huq MI, Rahman R, Colwell RR. 1983. Ecological relationships between Vibrio cholerae and planktonic crustacean copepods. Appl Environ Microbiol 45:275–283. doi: 10.1128/aem.45.1.275-283.1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lovelace T, Tubiash H, Colwell R. 1968. Quantitative and qualitative commensal bacterial flora of Crassostrea virginica in Chesapeake Bay. Proc Natl Shellfish Assoc 58:82–87. [Google Scholar]
- 7. Colwell RR, Kaper J, Joseph SW. 1977. Vibrio cholerae, Vibrio parahaemolyticus, and other vibrios: occurrence and distribution in Chesapeake Bay. Science 198:394–396. doi: 10.1126/science.198.4315.394 [DOI] [PubMed] [Google Scholar]
- 8. Colwell RR. 1996. Global climate and infectious disease: the cholera paradigm. Science 274:2025–2031. doi: 10.1126/science.274.5295.2025 [DOI] [PubMed] [Google Scholar]
- 9. Berk SG, Colwell RR. 1981. Transfer of mercury through a marine microbial food web. J Exp Mar Biol Ecol 52:157–172. doi: 10.1016/0022-0981(81)90034-4 [DOI] [Google Scholar]
- 10. Froelich BA, Noble RT. 2016. Vibrio bacteria in raw oysters: managing risks to human health . Phil Trans R Soc B 371:20150209. doi: 10.1098/rstb.2015.0209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. CDC . 2023. Cholera and Other Vibrio Illness Surveillance (COVIS). Available from: https://www.cdc.gov/vibrio/surveillance.html
- 12. Newton A, Kendall M, Vugia DJ, Henao OL, Mahon BE. 2012. Increasing rates of vibriosis in the United States, 1996-2010: review of surveillance data from 2 systems. Clin Infect Dis 54:S391–S395. doi: 10.1093/cid/cis243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Baker-Austin C, Oliver JD, Alam M, Ali A, Waldor MK, Qadri F, Martinez-Urtaza J. 2018. Vibrio spp. infections. Nat Rev Dis Primers 4:1–19. doi: 10.1038/s41572-018-0005-8 [DOI] [PubMed] [Google Scholar]
- 14. Morgado ME, Brumfield KD, Clifford M, Michelle BM, Colwell RR, Sapkota AR. 2023. Increased incidence of vibriosis in. Environ Res 244:117940. doi: 10.1016/j.envres.2023.117940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Brumfield KD, Usmani M, Santiago S, Singh K, Gangwar M, Hasan NA, Netherland M Jr, Deliz K, Angelini C, Beatty NL, Huq A, Jutla AS, Colwell RR. 2023. Genomic diversity of Vibrio spp. and metagenomic analysis of pathogens in Florida Gulf coastal waters following Hurricane Ian. MBio 14:e0147623. doi: 10.1128/mbio.01476-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lobitz B, Beck L, Huq A, Wood B, Fuchs G, Faruque ASG, Colwell R. 2000. Climate and infectious disease: use of remote sensing for detection of Vibrio cholerae by indirect measurement. Proc Natl Acad Sci USA 97:1438–1443. doi: 10.1073/pnas.97.4.1438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jayakumar JM, Martinez-Urtaza J, Brumfield KD, Jutla AS, Colwell RR, Cordero OX, Almagro-Moreno S. 2024. Climate change and Vibrio vulnificus dynamics: a blueprint for infectious diseases. PLoS Pathog 20:e1012767. doi: 10.1371/journal.ppat.1012767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sleator RD, Shortall C, Hill C. 2008. Metagenomics. Lett Appl Microbiol 47:361–366. doi: 10.1111/j.1472-765X.2008.02444.x [DOI] [PubMed] [Google Scholar]
- 19. Colwell RR, Brayton PR, Grimes DJ, Roszak DB, Huq SA, Palmer LM. 1985. Viable butnon-culturable Vibrio cholerae and related pathogens in the environment: implications for release of genetically engineered microorganisms. Nat Biotechnol 3:817–820. doi: 10.1038/nbt0985-817 [DOI] [Google Scholar]
- 20. Bej AK, Patterson DP, Brasher CW, Vickery MCL, Jones DD, Kaysner CA. 1999. Detection of total and hemolysin-producing Vibrio parahaemolyticus in shellfish using multiplex PCR amplification of tl, tdh and trh. J Microbiol Methods 36:215–225. doi: 10.1016/s0167-7012(99)00037-8 [DOI] [PubMed] [Google Scholar]
- 21. Thompson JR, Randa MA, Marcelino LA, Tomita-Mitchell A, Lim E, Polz MF. 2004. Diversity and dynamics of a north atlantic coastal Vibrio community. Appl Environ Microbiol 70:4103–4110. doi: 10.1128/AEM.70.7.4103-4110.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Huq A, Haley BJ, Taviani E, Chen A, Hasan NA, Colwell RR. 2012. Detection, isolation, and identification of Vibrio cholerae from the environment. Curr Protoc Microbiol Chapter 6:mc06a05s26. doi: 10.1002/9780471729259.mc06a05s26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kim HJ, Ryu JO, Lee SY, Kim ES, Kim HY. 2015. Multiplex PCR for detection of the Vibrio genus and five pathogenic Vibrio species with primer sets designed using comparative genomics. BMC Microbiol 15:239. doi: 10.1186/s12866-015-0577-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ahmed R, Rafiquzaman SM, Hossain MT, Lee JM, Kong IS. 2016. Species-specific detection of Vibrio alginolyticus in shellfish and shrimp by real-time PCR using the groEL gene. Aquacult Int 24:157–170. doi: 10.1007/s10499-015-9916-5 [DOI] [Google Scholar]
- 25. Chamanrokh P, Colwell RR, Huq A. 2022. Loop-mediated isothermal amplification (LAMP) assay for rapid detection of viable but non-culturable Vibrio cholerae O1. Can J Microbiol 68:103–110. doi: 10.1139/cjm-2021-0142 [DOI] [PubMed] [Google Scholar]
- 26. Alaeddini R. 2012. Forensic implications of PCR inhibition--A review. Forensic Sci Int Genet 6:297–305. doi: 10.1016/j.fsigen.2011.08.006 [DOI] [PubMed] [Google Scholar]
- 27. Chun J, Grim CJ, Hasan NA, Lee JH, Choi SY, Haley BJ, Taviani E, Jeon Y-S, Kim DW, Lee J-H, Brettin TS, Bruce DC, Challacombe JF, Detter JC, Han CS, Munk AC, Chertkov O, Meincke L, Saunders E, Walters RA, Huq A, Nair GB, Colwell RR. 2009. Comparative genomics reveals mechanism for short-term and long-term clonal transitions in pandemic Vibrio cholerae . Proc Natl Acad Sci USA 106:15442–15447. doi: 10.1073/pnas.0907787106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Martinez-Urtaza J, van Aerle R, Abanto M, Haendiges J, Myers RA, Trinanes J, Baker-Austin C, Gonzalez-Escalona N. 2017. Genomic variation and evolution of Vibrio parahaemolyticus ST36 over the course of a transcontinental epidemic expansion. MBio 8:e01425-17. doi: 10.1128/mBio.01425-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Brumfield KD, Huq A, Colwell RR, Olds JL, Leddy MB. 2020. Microbial resolution of whole genome shotgun and 16S amplicon metagenomic sequencing using publicly available NEON data. PLOS ONE 15:e0228899. doi: 10.1371/journal.pone.0228899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Brumfield KD, Hasan NA. 2024. Human gut microbiome: metagenomic insights and prospectus. J Appl Microbiomics 1 [Google Scholar]
- 31. Kirchberger PC, Orata FD, Nasreen T, Kauffman KM, Tarr CL, Case RJ, Polz MF, Boucher YF. 2020. Culture-independent tracking of Vibrio cholerae lineages reveals complex spatiotemporal dynamics in a natural population. Environ Microbiol 22:4244–4256. doi: 10.1111/1462-2920.14921 [DOI] [PubMed] [Google Scholar]
- 32. Jesser KJ, Noble RT. 2018. Characterizing the ecology in the neuse river estuary, North Carolina, characterized by next-generation amplicon sequencing of the gene encoding heat shock protein 60 (hsp60) next-generation amplicon sequencing. Appl Environ Microbiol 84:e00333-18. doi: 10.1128/AEM.00333-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Panicker G, Call DR, Krug MJ, Bej AK. 2004. Detection of pathogenic Vibrio spp. in shellfish by using multiplex PCR and DNA microarrays. Appl Environ Microbiol 70:7436–7444. doi: 10.1128/AEM.70.12.7436-7444.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sarengaowa A, Hu W, Feng K, Jiang A, Xiu Z, Lao Y, Li Y, Long Y. 2019. An in situ-synthesized gene chip for the detection of food-borne pathogens on fresh-cut cantaloupe and lettuce. Front Microbiol 10:3089. doi: 10.3389/fmicb.2019.03089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Itokawa K, Sekizuka T, Hashino M, Tanaka R, Kuroda M. 2020. Disentangling primer interactions improves SARS-CoV-2 genome sequencing by multiplex tiling PCR. PLoS One 15:e0239403. doi: 10.1371/journal.pone.0239403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chen J, Byun H, Liu R, Jung IJ, Pu Q, Zhu CY, Tanchoco E, Alavi S, Degnan PH, Ma AT, Roggiani M, Beld J, Goulian M, Hsiao A, Zhu J. 2022. A commensal-encoded genotoxin drives restriction of Vibrio cholerae colonization and host gut microbiome remodeling. Proc Natl Acad Sci USA 119:e2121180119. doi: 10.1073/pnas.2121180119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Li Y, Zhang S, Li J, Chen M, He M, Wang Y, Zhang Y, Jing H, Ma H, Li Y, Zhao L, Zhao H, Kan B, Pang B. 2019. Application of digital PCR and next generation sequencing in the etiology investigation of a foodborne disease outbreak caused by Vibrio parahaemolyticus. Food Microbiol 84:103233. doi: 10.1016/j.fm.2019.05.017 [DOI] [PubMed] [Google Scholar]
- 38. Brumfield KD, Leddy M, Usmani M, Cotruvo JA, Tien CT, Dorsey S, Graubics K, Fanelli B, Zhou I, Registe N, Dadlani M, Wimalarante M, Jinasena D, Abayagunawardena R, Withanachchi C, Huq A, Jutla A, Colwell RR. 2022. Microbiome analysis for wastewater surveillance during COVID-19. MBio 13:e00591-22. doi: 10.1128/mbio.00591-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yasir M. 2020. Analysis of microbial communities and pathogen detection in domestic sewage using metagenomic sequencing. Diversity (Basel) 13:6. doi: 10.3390/d13010006 [DOI] [Google Scholar]
- 40. Brumfield KD, Cotruvo JA, Shanks OC, Sivaganesan M, Hey J, Hasan NA, Huq A, Colwell RR, Leddy MB. 2021. Metagenomic sequencing and quantitative real-time PCR for fecal pollution assessment in an urban watershed. Front Water 3:626849. doi: 10.3389/frwa.2021.626849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Roy MA, Arnaud JM, Jasmin PM, Hamner S, Hasan NA, Colwell RR, Ford TE. 2018. A metagenomic approach to evaluating surface water quality in Haiti. Int J Environ Res Public Health 15:2211. doi: 10.3390/ijerph15102211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Vezzulli L, Grande C, Tassistro G, Brettar I, Höfle MG, Pereira RPA, Mushi D, Pallavicini A, Vassallo P, Pruzzo C. 2017. Whole-genome enrichment provides deep insights into vibrio cholerae metagenome from an african river. Microb Ecol 73:734–738. doi: 10.1007/s00248-016-0902-x [DOI] [PubMed] [Google Scholar]
- 43. Gnirke A, Melnikov A, Maguire J, Rogov P, LeProust EM, Brockman W, Fennell T, Giannoukos G, Fisher S, Russ C, Gabriel S, Jaffe DB, Lander ES, Nusbaum C. 2009. Solution hybrid selection with ultra-long oligonucleotides for massively parallel targeted sequencing. Nat Biotechnol 27:182–189. doi: 10.1038/nbt.1523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lasa A, di Cesare A, Tassistro G, Borello A, Gualdi S, Furones D, Carrasco N, Cheslett D, Brechon A, Paillard C, Bidault A, Pernet F, Canesi L, Edomi P, Pallavicini A, Pruzzo C, Vezzulli L. 2019. Dynamics of the Pacific oyster pathobiota during mortality episodes in Europe assessed by 16S rRNA gene profiling and a new target enrichment next‐generation sequencing strategy. Environ Microbiol 21:4548–4562. doi: 10.1111/1462-2920.14750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Beaudry MS, Wang J, Kieran TJ, Thomas J, Bayona-Vásquez NJ, Gao B, Devault A, Brunelle B, Lu K, Wang JS, Rhodes OE, Glenn TC. 2021. Improved microbial community characterization of 16S rRNA via metagenome hybridization capture enrichment. Front Microbiol 12:644662. doi: 10.3389/fmicb.2021.644662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Guitor AK, Raphenya AR, Klunk J, Kuch M, Alcock B, Surette MG, McArthur AG, Poinar HN, Wright GD. 2019. Capturing the resistome: a targeted capture method to reveal antibiotic resistance determinants in metagenomes. Antimicrob Agents Chemother 64:e01324-19. doi: 10.1128/AAC.01324-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. UMCES . 2022. Standard Operating Procedures for Fluorometric Determination of Chlorophyll α in Waters and Sediments of Fresh/Estuarine/Coastal Areas. Available from: https://www.umces.edu/sites/default/files/Chlorophyll%20Fluoromtretic%20Method%202022-1.pdf
- 48. Andrews WH, Hammack TS. 2019. BAM Chapter 1: Food sampling/preparation of sample homogenate. Bacteriol Anal Man. https://www.fda.gov/food/laboratory-methods-food/bam-chapter-1-food-samplingpreparation-sample-homogenate.
- 49. Kaysner C, DePaola A, Jones J. 2019. BAM Chapter 9: Vibrio. Bacteriol Anal Man. https://www.fda.gov/food/laboratory-methods-food/bam-chapter-9-vibrio.
- 50. FDA . 2017. BAM Media M190: Vibrio Vulnificus Agar (VVA). Available from: https://www.fda.gov/food/laboratory-methods-food/bam-media-m190-vibrio-vulnificus-agar-vva
- 51. Brumfield KD, Carignan BM, Ray JN, Jumpre PE, Son MS. 2017. Laboratory techniques used to maintain and differentiate biotypes of Vibrio cholerae clinical and environmental isolates. J Vis Exp 123:55760. doi: 10.3791/55760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Brumfield KD, Hasan NA, Leddy MB, Cotruvo JA, Rashed SM, Colwell RR, Huq A. 2020. A comparative analysis of drinking water employing metagenomics. PLoS ONE 15:e0231210. doi: 10.1371/journal.pone.0231210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Bauer A, Rørvik LM. 2007. A novel multiplex PCR for the identification of Vibrio parahaemolyticus, Vibrio cholerae and Vibrio vulnificus. Lett Appl Microbiol 45:371–375. doi: 10.1111/j.1472-765X.2007.02195.x [DOI] [PubMed] [Google Scholar]
- 54. Panicker G, Myers ML, Bej AK. 2004. Rapid detection of Vibrio vulnificus in shellfish and Gulf of Mexico water by real-time PCR. Appl Environ Microbiol 70:498–507. doi: 10.1128/AEM.70.1.498-507.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Warner EB, Oliver JD. 2008. Multiplex PCR assay for detection and simultaneous differentiation of genotypes of Vibrio vulnificus biotype 1. Foodborne Pathog Dis 5:691–693. doi: 10.1089/fpd.2008.0120 [DOI] [PubMed] [Google Scholar]
- 56. Paranjpye RN, Strom MS. 2005. A Vibrio vulnificus type IV pilin contributes to biofilm formation, adherence to epithelial cells, and virulence. Infect Immun 73:1411–1422. doi: 10.1128/IAI.73.3.1411-1422.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Kim YR, Lee SE, Kook H, Yeom JA, Na HS, Kim SY, Chung SS, Choy HE, Rhee JH. 2008. Vibrio vulnificus RTX toxin kills host cells only after contact of the bacteria with host cells. Cell Microbiol 10:848–862. doi: 10.1111/j.1462-5822.2007.01088.x [DOI] [PubMed] [Google Scholar]
- 58. Hoshino K, Yamasaki S, Mukhopadhyay AK, Chakraborty S, Basu A, Bhattacharya SK, Nair GB, Shimada T, Takeda Y. 1998. Development and evaluation of a multiplex PCR assay for rapid detection of toxigenic Vibrio cholerae O1 and O139 . FEMS Immunol Med Microbiol 20:201–207. doi: 10.1111/j.1574-695X.1998.tb01128.x [DOI] [PubMed] [Google Scholar]
- 59. Hossain MT, Kim EY, Kim YR, Kim DG, Kong IS. 2013. Development of a groEL gene-based species-specific multiplex polymerase chain reaction assay for simultaneous detection of Vibrio cholerae, Vibrio parahaemolyticus and Vibrio vulnificus. J Appl Microbiol 114:448–456. doi: 10.1111/jam.12056 [DOI] [PubMed] [Google Scholar]
- 60. King WL, Siboni N, Kahlke T, Green TJ, Labbate M, Seymour JR. 2019. A new high throughput sequencing assay for characterizing the diversity of natural Vibrio communities and its application to a Pacific oyster mortality event. Front Microbiol 10:2907. doi: 10.3389/fmicb.2019.02907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Silvester R, Alexander D, Antony AC, Hatha M. 2017. GroEL PCR- RFLP – An efficient tool to discriminate closely related pathogenic Vibrio species. Microb Pathog 105:196–200. doi: 10.1016/j.micpath.2017.02.029 [DOI] [PubMed] [Google Scholar]
- 62. Goh SH, Potter S, Wood JO, Hemmingsen SM, Reynolds RP, Chow AW. 1996. HSP60 gene sequences as universal targets for microbial species identification: studies with coagulase-negative staphylococci. J Clin Microbiol 34:818–823. doi: 10.1128/jcm.34.4.818-823.1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Brousseau R, Hill JE, Préfontaine G, Goh SH, Harel J, Hemmingsen SM. 2001. Streptococcus suis serotypes characterized by analysis of chaperonin 60 gene sequences. Appl Environ Microbiol 67:4828–4833. doi: 10.1128/AEM.67.10.4828-4833.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Guglielmotti DM, Pujato SA, Quiberoni A, Suárez VB. 2022. Hsp60 gene as a reliable target for taxonomical identification and discrimination of Leuconostoc species of dairy origin. Int Dairy J 126:105227. doi: 10.1016/j.idairyj.2021.105227 [DOI] [Google Scholar]
- 65. Marston EL, Sumner JW, Regnery RL. 1999. Evaluation of intraspecies genetic variation within the 60 kDa heat-shock protein gene (groEL) of Bartonella species. Int J Syst Evol Microbiol 49:1015–1023. doi: 10.1099/00207713-49-3-1015 [DOI] [PubMed] [Google Scholar]
- 66. Sakamoto M, Ohkuma M. 2010. Usefulness of the hsp60 gene for the identification and classification of Gram-negative anaerobic rods. J Med Microbiol 59:1293–1302. doi: 10.1099/jmm.0.020420-0 [DOI] [PubMed] [Google Scholar]
- 67. Vancuren SJ, Hill JE. 2019. Update on cpnDB: a reference database of chaperonin sequences. Database (Oxford) 2019:baz033. doi: 10.1093/database/baz033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Chenna R, Sugawara H, Koike T, Lopez R, Gibson TJ, Higgins DG, Thompson JD. 2003. Multiple sequence alignment with the Clustal series of programs. Nucleic Acids Res 31:3497–3500. doi: 10.1093/nar/gkg500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Tamura K, Nei M. 1993. Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Mol Biol Evol 10:512–526. doi: 10.1093/oxfordjournals.molbev.a040023 [DOI] [PubMed] [Google Scholar]
- 70. Kumar S, Stecher G, Li M, Knyaz C, Tamura K. 2018. MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol 35:1547–1549. doi: 10.1093/molbev/msy096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Zhou S, Liu B, Zheng D, Chen L, Yang J. 2025. VFDB 2025: an integrated resource for exploring anti-virulence compounds. Nucleic Acids Res 53:D871–D877. doi: 10.1093/nar/gkae968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Feldgarden M, Brover V, Gonzalez-Escalona N, Frye JG, Haendiges J, Haft DH, Hoffmann M, Pettengill JB, Prasad AB, Tillman GE, Tyson GH, Klimke W. 2021. AMRFinderPlus and the Reference Gene Catalog facilitate examination of the genomic links among antimicrobial resistance, stress response, and virulence. Sci Rep 11:12728. doi: 10.1038/s41598-021-91456-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Fu L, Niu B, Zhu Z, Wu S, Li W. 2012. CD-HIT: accelerated for clustering the next-generation sequencing data. Bioinformatics 28:3150–3152. doi: 10.1093/bioinformatics/bts565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Langmead B, Salzberg SL. 2012. Fast gapped-read alignment with Bowtie 2. Nat Methods 9:357–359. doi: 10.1038/nmeth.1923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Wood DE, Lu J, Langmead B. 2019. Improved metagenomic analysis with Kraken 2. Genome Biol 20:257. doi: 10.1186/s13059-019-1891-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Lu J, Breitwieser FP, Thielen P, Salzberg SL. 2017. Bracken: estimating species abundance in metagenomics data. PeerJ Comput Sci 3:e104. doi: 10.7717/peerj-cs.104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Ondov BD, Bergman NH, Phillippy AM. 2011. Interactive metagenomic visualization in a Web browser. BMC Bioinformatics 12:385. doi: 10.1186/1471-2105-12-385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Andrews SC. 2019. FastQC. Available from: https://github.com/s-andrews/FastQC
- 79. Ewels P, Magnusson M, Lundin S, Käller M. 2016. MultiQC: summarize analysis results for multiple tools and samples in a single report. Bioinformatics 32:3047–3048. doi: 10.1093/bioinformatics/btw354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Chen S, Zhou Y, Chen Y, Gu J. 2018. Fastp: an ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 34:i884–i890. doi: 10.1093/bioinformatics/bty560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Kryukov K, Imanishi T. 2016. Human contamination in public genome assemblies. PLoS ONE 11:e0162424. doi: 10.1371/journal.pone.0162424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Stinson LF, Keelan JA, Payne MS. 2019. Identification and removal of contaminating microbial DNA from PCR reagents: impact on low-biomass microbiome analyses. Lett Appl Microbiol 68:2–8. doi: 10.1111/lam.13091 [DOI] [PubMed] [Google Scholar]
- 83. Lee M. 2022. Bit: a multipurpose collection of bioinformatics tools. F1000Res 11:122. doi: 10.12688/f1000research.79530.1 [DOI] [Google Scholar]
- 84. Wick RR, Judd LM, Gorrie CL, Holt KE. 2017. Unicycler: Resolving bacterial genome assemblies from short and long sequencing reads. PLoS Comput Biol 13:e1005595. doi: 10.1371/journal.pcbi.1005595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Gurevich A, Saveliev V, Vyahhi N, Tesler G. 2013. QUAST: quality assessment tool for genome assemblies. Bioinformatics 29:1072–1075. doi: 10.1093/bioinformatics/btt086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Seppey M, Manni M, Zdobnov EM. 2019. BUSCO: assessing genome assembly and annotation completeness. Methods Mol Biol 1962:227–245. doi: 10.1007/978-1-4939-9173-0_14 [DOI] [PubMed] [Google Scholar]
- 87. Seemann T. 2014. Prokka: rapid prokaryotic genome annotation. Bioinformatics 30:2068–2069. doi: 10.1093/bioinformatics/btu153 [DOI] [PubMed] [Google Scholar]
- 88. Kaminski J, Gibson MK, Franzosa EA, Segata N, Dantas G, Huttenhower C. 2015. High-specificity targeted functional profiling in microbial communities with ShortBRED. PLoS Comput Biol 11:e1004557. doi: 10.1371/journal.pcbi.1004557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Camacho C, Coulouris G, Avagyan V, Ma N, Papadopoulos J, Bealer K, Madden TL. 2009. BLAST+: architecture and applications. BMC Bioinformatics 10:421. doi: 10.1186/1471-2105-10-421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Seemann T. 2022. MLST. Available from: https://github.com/tseemann/mlst
- 91. Jolley KA, Bray JE, Maiden MCJ. 2018. Open-access bacterial population genomics: BIGSdb software, the PubMLST.org website and their applications. Wellcome Open Res 3:124. doi: 10.12688/wellcomeopenres.14826.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Bian S, Jia Y, Zhan Q, Wong NK, Hu Q, Zhang W, Zhang Y, Li L. 2021. VPsero: rapid serotyping of Vibrio parahaemolyticus using serogroup-specific genes based on whole-genome sequencing data. Front Microbiol 12:620224. doi: 10.3389/fmicb.2021.620224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Lee MD. 2019. GToTree: a user-friendly workflow for phylogenomics. Bioinformatics 35:4162–4164. doi: 10.1093/bioinformatics/btz188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Parks DH, Chuvochina M, Rinke C, Mussig AJ, Chaumeil P-A, Hugenholtz P. 2022. GTDB: an ongoing census of bacterial and archaeal diversity through a phylogenetically consistent, rank normalized and complete genome-based taxonomy. Nucleic Acids Res 50:D785–D794. doi: 10.1093/nar/gkab776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Mistry J, Chuguransky S, Williams L, Qureshi M, Salazar GA, Sonnhammer EL, Tosato SC, Paladin L, Raj S, Richardson LJ. 2021. Pfam: The protein families database in 2021. Nucleic Acids Res 49:D412–D419. doi: 10.1093/nar/gkaa913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Eddy SR. 2023. HMMER: Biosequence Analysis Using Profile Hidden Markov Models. Available from: http://hmmer.org
- 97. Hyatt D, Chen GL, Locascio PF, Land ML, Larimer FW, Hauser LJ. 2010. Prodigal: prokaryotic gene recognition and translation initiation site identification. BMC Bioinformatics 11:119. doi: 10.1186/1471-2105-11-119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Edgar RC. 2004. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res 32:1792–1797. doi: 10.1093/nar/gkh340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Capella-Gutiérrez S, Silla-Martínez JM, Gabaldón T. 2009. trimAl: a tool for automated alignment trimming in large-scale phylogenetic analyses. Bioinformatics 25:1972–1973. doi: 10.1093/bioinformatics/btp348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Price MN, Dehal PS, Arkin AP. 2010. FastTree 2--approximately maximum-likelihood trees for large alignments. PLoS ONE 5:e9490. doi: 10.1371/journal.pone.0009490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Letunic I, Bork P. 2024. Interactive Tree of Life (iTOL) v6: recent updates to the phylogenetic tree display and annotation tool. Nucleic Acids Res 52:W78–W82. doi: 10.1093/nar/gkae268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Shen W, Xiang H, Huang T, Tang H, Peng M, Cai D, Hu P, Ren H. 2023. KMCP: accurate metagenomic profiling of both prokaryotic and viral populations by pseudo-mapping. Bioinformatics 39:btac845. doi: 10.1093/bioinformatics/btac845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Smith SD. 2019. Phylosmith: an R-package for reproducible and efficient microbiome analysis with phyloseq-objects. JOSS 4:1442. doi: 10.21105/joss.01442 [DOI] [Google Scholar]
- 104. McMurdie PJ, Holmes S. 2013. Phyloseq: an R package for reproducible interactive analysis and graphics of microbiome census data. PLoS ONE 8:e61217. doi: 10.1371/journal.pone.0061217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Sievers F, Wilm A, Dineen D, Gibson TJ, Karplus K, Li W, Lopez R, McWilliam H, Remmert M, Söding J, Thompson JD, Higgins DG. 2011. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol Syst Biol 7:539. doi: 10.1038/msb.2011.75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Saitou N, Nei M. 1987. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454 [DOI] [PubMed] [Google Scholar]
- 107. Alock BP, Huynh W, Chalil R, Smith KW, Raphenya AR, Wlodarski MA, Edalatmand A, Syed SA, Tsang KK. 2023. CARD 2023: expanded curation, support for machine learning, and resistome prediction at the Comprehensive Antibiotic Resistance Database. Nucleic Acids Res 51:D690–D699. doi: 10.1093/nar/gkac920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Suzek BE, Huang H, McGarvey P, Mazumder R, Wu CH. 2007. UniRef: comprehensive and non-redundant UniProt reference clusters. Bioinformatics 23:1282–1288. doi: 10.1093/bioinformatics/btm098 [DOI] [PubMed] [Google Scholar]
- 109. Baker-Austin C, Trinanes J, Gonzalez-Escalona N, Martinez-Urtaza J. 2017. Non-cholera vibrios: the microbial barometer of climate change. Trends Microbiol 25:76–84. doi: 10.1016/j.tim.2016.09.008 [DOI] [PubMed] [Google Scholar]
- 110. Froelich BA, Daines DA. 2020. In hot water: effects of climate change on Vibrio-human interactions. Environ Microbiol 22:4101–4111. doi: 10.1111/1462-2920.14967 [DOI] [PubMed] [Google Scholar]
- 111. Vezzulli L. 2023. Global expansion of Vibrio spp. in hot water. Environ Microbiol Rep 15:77–79. doi: 10.1111/1758-2229.13135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Archer EJ, Baker-Austin C, Osborn TJ, Jones NR, Martínez-Urtaza J, Trinanes J, Oliver JD, González FJC, Lake IR. 2023. Climate warming and increasing Vibrio vulnificus infections in North America. Sci Rep 13:3893. doi: 10.1038/s41598-023-28247-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Vezzulli L, Baker-Austin C, Kirschner A, Pruzzo C, Martinez-Urtaza J. 2020. Global emergence of environmental non-O1/O139 Vibrio cholerae infections linked with climate change: a neglected research field? Environ Microbiol 22:4342–4355. doi: 10.1111/1462-2920.15040 [DOI] [PubMed] [Google Scholar]
- 114. Jamal Y, Usmani M, Brumfield KD, Singh K, Huq A, Nguyen TH, Colwell R, Jutla A. 2024. Quantification of climate footprints of Vibrio vulnificus in coastal human communities of the United States Gulf Coast. Geohealth 8:e2023GH001005. doi: 10.1029/2023GH001005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Smalls J, Grim C, Parveen S. 2023. AssesSments of Vibrio parahaemolyticus and Vibrio vulnificus levels and microbial community compositions in blue crabs (Callinectes sapidus) and seawater harvested from the Maryland Coastal Bays. Front Microbiol 14:1235070. doi: 10.3389/fmicb.2023.1235070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Pimentel ZT, Dufault-Thompson K, Russo KT, Scro AK, Smolowitz RM, Gomez-Chiarri M, Zhang Y. 2021. Microbiome analysis reveals diversity and function of Mollicutes associated with the Eastern Oyster, Crassostrea virginica mSphere 6:10–1128. doi: 10.1128/mSphere.00227-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Almuhaideb E, Hasan NA, Grim C, Rashed SM, Parveen S. 2024. Comparative evaluation of specimen type and processing conditions for studying oyster microbiomes. Front Microbiol 15:1504487. doi: 10.3389/fmicb.2024.1504487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Hasan NA, Grim CJ, Lipp EK, Rivera ING, Chun J, Haley BJ, Taviani E, Choi SY, Hoq M, Munk AC, Brettin TS, Bruce D, Challacombe JF, Detter JC, Han CS, Eisen JA, Huq A, Colwell RR. 2015. Deep-sea hydrothermal vent bacteria related to human pathogenic Vibrio species. Proc Natl Acad Sci USA 112:E2813–E2819. doi: 10.1073/pnas.1503928112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Gasc C, Peyret P. 2018. Hybridization capture reveals microbial diversity missed using current profiling methods. Microbiome 6:61. doi: 10.1186/s40168-018-0442-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Jiang C, Tanaka M, Nishikawa S, Mino S, Romalde JL, Thompson FL, Gomez-Gil B, Sawabe T. 2022. Vibrio clade 3.0: new Vibrionaceae evolutionary units using genome-based approach. Curr Microbiol 79:1–15. doi: 10.1007/s00284-021-02725-0 [DOI] [PubMed] [Google Scholar]
- 121. Miller JJ, Weimer BC, Timme R, Lüdeke CHM, Pettengill JB, Bandoy DD, Weis AM, Kaufman J, Huang BC, Payne J, Strain E, Jones JL. 2021. Erratum for miller et al., “phylogenetic and biogeographic patterns of Vibrio parahaemolyticus strains from North America inferred from whole-genome sequence data”. Appl Environ Microbiol 87:e0069321. doi: 10.1128/AEM.00693-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Haendiges J, Jones J, Myers RA, Mitchell CS, Butler E, Toro M, Gonzalez-Escalona N. 2016. A nonautochthonous U.S. strain of Vibrio parahaemolyticus isolated from chesapeake bay oysters caused the outbreak in Maryland in 2010. Appl Environ Microbiol 82:3208–3216. doi: 10.1128/AEM.00096-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Haendiges J, Timme R, Allard MW, Myers RA, Brown EW, Gonzalez-Escalona N. 2015. Characterization of Vibrio parahaemolyticus clinical strains from Maryland (2012-2013) and comparisons to a locally and globally diverse V. parahaemolyticus strains by whole-genome sequence analysis. Front Microbiol 6:125. doi: 10.3389/fmicb.2015.00125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. López-Pérez M, Jayakumar JM, Haro-Moreno JM, Zaragoza-Solas A, Reddi G, Rodriguez-Valera F, Shapiro OH, Alam M, Almagro-Moreno S. 2019. Evolutionary model of cluster divergence of the emergent marine pathogen Vibrio vulnificus: from genotype to ecotype. MBio 10:e02852-18. doi: 10.1128/mBio.02852-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. López-Pérez M, Jayakumar JM, Grant TA, Zaragoza-Solas A, Cabello-Yeves PJ, Almagro-Moreno S. 2021. Ecological diversification reveals routes of pathogen emergence in endemic Vibrio vulnificus populations. Proc Natl Acad Sci USA 118:e2103470118. doi: 10.1073/pnas.2103470118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Theethakaew C, Feil EJ, Castillo-Ramírez S, Aanensen DM, Suthienkul O, Neil DM, Davies RL. 2013. Genetic relationships of Vibrio parahaemolyticus isolates from clinical, human carrier, and environmental sources in Thailand, determined by multilocus sequence analysis. Appl Environ Microbiol 79:2358–2370. doi: 10.1128/AEM.03067-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Lydon KA, Kinsey T, Le C, Gulig PA, Jones JL. 2021. Biochemical and virulence characterization of Vibrio vulnificus isolates from clinical and environmental sources. Front Cell Infect Microbiol 11:637019. doi: 10.3389/fcimb.2021.637019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Thiaville PC, Bourdage KL, Wright AC, Farrell-Evans M, Garvan CW, Gulig PA. 2011. Genotype is correlated with but does not predict virulence of Vibrio vulnificus biotype 1 in subcutaneously inoculated, iron dextran-treated mice. Infect Immun 79:1194–1207. doi: 10.1128/IAI.01031-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Chen AJ, Hasan NA, Haley BJ, Taviani E, Tarnowski M, Brohawn K, Johnson CN, Colwell RR, Huq A. 2017. Characterization of Pathogenic Vibrio parahaemolyticus from the Chesapeake Bay, Maryland. Front Microbiol 8:2460. doi: 10.3389/fmicb.2017.02460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Beaudry MS, Bhuiyan MIU, Glenn TC. 2024. Enriching the future of public health microbiology with hybridization bait capture. Clin Microbiol Rev 37:e0006822. doi: 10.1128/cmr.00068-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Noyes NR, Weinroth ME, Parker JK, Dean CJ, Lakin SM, Raymond RA, Rovira P, Doster E, Abdo Z, Martin JN, Jones KL, Ruiz J, Boucher CA, Belk KE, Morley PS. 2017. Enrichment allows identification of diverse, rare elements in metagenomic resistome-virulome sequencing. Microbiome 5:1–13. doi: 10.1186/s40168-017-0361-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figures S1 to S5 and Tables S1 to S12.
Data Availability Statement
Sequencing data generated for all samples included in this study have been deposited in the NCBI Sequence Read Archive database under BioProject ID PRJNA1188529. Probe sequences for the custom Vibrio spp. hybridization panel (Tables S1 through S3), cpn60 sequences recovered from metagenomic assembled contigs (Table S7), and accession numbers for metagenomic sequencing libraries (Table S9) and draft genome assemblies (Table S10) are provided in the supplemental material.