Abstract
Aging is an intrinsic biological decline marked by multidimensional alterations spanning molecular, cellular, tissue, and organ levels. One hallmark of aging is the progressive deterioration of immune function, a condition referred to as immunosenescence. This process often involves a persistent, mild, and non-infectious inflammatory state across the body, commonly described as inflammaging. The regulation of age-related immune and inflammatory processes is critically influenced by epigenetic mechanisms, such as alterations in DNA methylation patterns, histone modifications, chromatin structure reorganization, and the regulatory actions of non-coding RNAs. Recent research has increasingly focused on the regulatory roles of post-translational modifications (PTMs), including histone methylation, acetylation, ubiquitination, and O-GlcNAcylation, have been widely recognized as fundamental modulators of immunoinflammatory processes in aging. In this review, we provide a comprehensive overview of histone modification-mediated mechanisms involved in the regulation of immunosenescence. We further highlight their functional roles from the perspective of immune inflammation and explore potential therapeutic strategies targeting histone modifications to mitigate immunosenescence.
Keywords: Aging, Immunosenescence, Inflammaging, Histone modification, Post-translational modification
Introduction
Aging is an intrinsic biological decline marked by multidimensional alterations spanning molecular, cellular, tissue, and organ levels. Recent studies have identified aging as a multifaceted biological process influenced by key hallmarks such as cellular senescence, genomic instability, telomere shortening, epigenetic changes, proteostasis collapse, dysregulated nutrient sensing, mitochondrial impairment, stem cell depletion, and disrupted intercellular signaling [1, 2]. Notably, the aging process emerges as the predominant determinant for the development of chronic disorders, declines in both physical and mental faculties, and increased susceptibility to mortality [3]. The gradual deterioration of homeostatic capacity with advancing age renders organisms increasingly vulnerable to age-associated pathologies (e.g., cardiovascular diseases, cancer, and neurodegenerative disorders) and substantially elevates mortality risk [4].
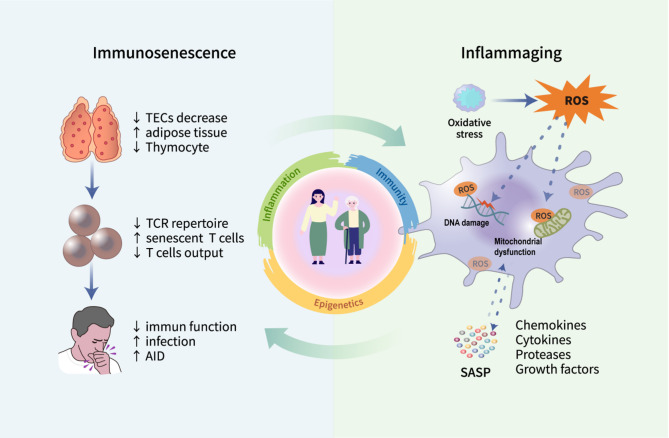
The decline in immunological competence represents a key biological consequence of aging, a phenomenon scientifically termed immunosenescence. A defining feature of this process involves the structural degeneration and reorganization of immune organs, coupled with declining efficacy in both nonspecific and antigen-specific immune mechanisms. This decline results in suboptimal vaccine efficacy and heightened vulnerability to infections and age-associated pathologies [5]. The hallmark features of immunosenescence include diminished immune responsiveness coupled with an exacerbated proinflammatory status [6]. This paradoxical phenomenon, designated as inflammaging (inflammatory aging), is considered a consequential manifestation of immunosenescence [7] and acts as a pivotal driver underlying the progression of age-associated pathologies.
In eukaryotes, epigenetic modifications play pivotal roles in transcriptional regulation [8]. As a fundamental mechanism of gene expression control, epigenetics is characterized by reversible chromatin-based regulatory processes that modulate gene activity while maintaining the DNA sequence [9]. The epigenome serves as a critical interface between genotype and phenotype, dynamically responding to environmental stimuli and orchestrating phenotypic plasticity during aging [4]. Major epigenetic regulatory mechanisms include histone modifications, DNA methylation, chromatin remodeling, and non-coding RNA-mediated regulation.Importantly, aging does not affect all components of the immune system equally, and epigenetic regulation serves as essential modulators of shaping immune cell identity and function [10]. Emerging research on post-translational modifications (PTMs), notably histone methylation, acetylation, ubiquitylation, and O-GlcNAcylation (Fig. 1), has elucidated their specialized roles in modulating immune-inflammatory responses during senescence.
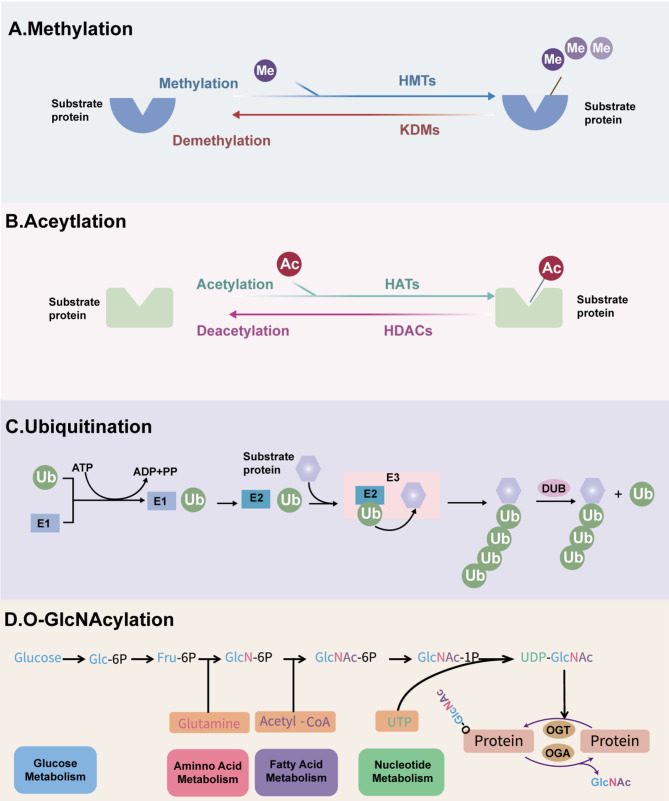
Fig. 1.
Common histone PTMs processes. A Histone methylation is dynamically regulated by HMTs and KDMs. B Histone acetylation is modulated by HATs and HDACs. C In ubiquitination, ubiquitin-activating enzyme (E1) activates Ub in an ATP-dependent manner, which is then transferred to a ubiquitin-conjugating enzyme (E2), forming a ubiquitin–E2 complex. The ubiquitin ligase (E3) facilitates the transfer of Ub from the E2 enzyme to the substrate protein. This modification can be reversed by DUBs. D O-GlcNAcylation is primarily regulated via the hexosamine biosynthetic pathway (HBP), which integrates four major metabolic pathways: glucose metabolism (glucose), amino acid metabolism (glutamine), fatty acid metabolism (acetyl-CoA), and nucleotide metabolism (UTP). OGT and OGA control the addition and removal of O-GlcNAc, respectively
During immunosenescence, PTMs reshape the immune microenvironment through epigenetic regulation and signal transduction, profoundly influencing the senescence-associated secretory phenotype (SASP), autophagy, and the perpetuation of chronic inflammation. Histone methylation, predominantly occurring at H3K4, H3K9, H3K27, and H3K36 residues, is catalyzed by methyltransferases such as KMT2A, SUV39H1, and EZH2. These enzymes modulate gene activation, autophagic processes, and transcriptional silencing, thereby regulating cellular senescence and inflammatory responses [11]. Acetylation modifications, including H3K9ac, H3K27ac, and H4K16ac, are dynamically regulated by histone acetyltransferases (HATs) and histone deacetylases (HDACs). These modifications facilitate gene expression and telomeric silencing, ultimately influencing transcriptional activity and immune function [12]. Ubiquitination, mediated by E3 ubiquitin ligases, targets histones and associated proteins, modulating proteasomal degradation, mitophagy, and inflammatory signaling pathways. This regulatory mechanism is critical for maintaining proteostasis during aging [13–15]. The non-canonical O-GlcNAcylation, catalyzed by O-GlcNAc transferase (OGT), modifies serine and threonine residues on histones. This modification enhances the activity of antioxidant transcription factors and suppresses aberrant tau phosphorylation in neurodegenerative disorders, thereby supporting cognitive function and immune defense mechanisms [16, 17]. Collectively, these PTMs orchestrate cellular responses to senescence-inducing stimuli by fine-tuning chromatin architecture and gene expression, positioning them as pivotal therapeutic targets in immunosenescence and related pathologies.
In this review, we begin by outlining the concept and defining features of immunosenescence, along with the aging-associated alterations observed across various immune cell populations. Subsequently, we elucidate the mechanistic links between immunosenescence and the pathogenesis of age-dependent diseases. Furthermore, we synthesize recent discoveries elucidating how specific histone modifications in immune aging. Finally, we critically evaluate emerging therapeutic strategies targeting histone-modifying pathways to counteract immunosenescence.
Immunosenescence: concept and characteristics
Immunosenescence describes the progressive decline of immunological competence associated with aging [18]. This process involves multifaceted changes in immune cell populations—such as T cells, B cells, and macrophages—leading to compromised immunity in older adults. Consequently, they face higher risks of infections, autoimmune disorders, and malignancies [19, 20]. Immunosenescence is characteristically manifested by thymic involution, T-cell depletion, and inflammaging(Fig. 2). The thymus is a crucial immunological structure, facilitating the generation and maturation of immune cells, particularly for T lymphocytes [21]. Thymic atrophy represents the most prominent hallmark of immunosenescence [22]. Histologically comprising epithelial components and perivascular non-epithelial elements, the thymus undergoes profound age-associated architectural changes. The pathological features include: (1) progressive loss of thymic epithelial cells (TECs), (2) ectopic adipose tissue accumulation, and (3) developmental arrest of thymocytes [23]. These alterations collectively impair naïve T-cell output and reduce peripheral T-cell receptor (TCR) diversity, ultimately compromising immune competence and predisposing to chronic low-grade inflammation [24]. Notably, patients with congenital heart disease (CHD) who underwent thymectomy exhibited accelerated immunosenescence, characterized by persistent T-cell lymphopenia and diminished naïve T-cell pools [25]. These findings highlight thymic rejuvenation as an effective intervention to mitigate immunological deterioration associated with aging.
Fig. 2.
Characteristics of immunosenescence. Immunosenescence is driven by multiple factors, including thymic involution, diminished T cell output, and the accumulation of SASP induced by inflammaging. Senescent immune cells exhibit impaired ability to clear both senescent cells and inflammatory mediators, thereby reinforcing a detrimental cycle between inflammaging and immunosenescence
Moreover, immunosenescence is often accompanied by a persistent, mild, and non-infectious inflammatory state across the body, a process termed “inflammaging,” which is now identified as a defining feature of aging [2]. Inflammaging arises from the intricate interaction between increased pro-inflammatory mediators and impaired immune cell function, leading to widespread dysregulation of both the innate and adaptive immune systems, and ultimately resulting in a diminished immune response to antigenic stimuli [26]. One proposed mechanism underlying inflammaging is the preferential preservation of innate immune responses, which tend to remain relatively intact with age, in contrast to the progressive decline in adaptive immunity. This imbalance may foster excessive pro-inflammatory activity, thereby accelerating the inflammaging process [27]. Additionally, oxidative stress, which increases during aging, exacerbates this pathological state, amplifying chronic inflammatory processes [28]. Evidence indicates that this persistent inflammatory state stems from chronic oxidative stress induced by sustained immune activation [29]. The bidirectional interplay between oxidative stress and inflammation forms the foundation of the so-called “oxi-inflamm-aging theory” of aging [30, 31]. Persistent oxidative stress induces excessive reactive oxygen species (ROS) production by immune cells, bringing about cellular damage, enhanced recruitment of inflammatory cells, and a self-perpetuating loop of inflammatory amplification and oxidative injury [32]. High ROS exposure causes DNA damage, mitochondrial dysfunction, inducing senescence in cells accompanied by the emergence of the SASP [33]. SASP is composed of various chemokines, cytokines, proteases, and growth factors that mediate detrimental paracrine effects on proximate cells, disrupt extracellular matrix composition, and impair tissue ultrastructure [34]. The sustained secretion of SASP factors is thus a major driver of inflammaging.Although immune cells serve as the primary surveillance and clearance mechanism for senescent cells, SASP factors may impair immune surveillance, leading to the accumulation of senescent cells and chronic inflammatory signaling molecules, thereby creating a vicious cycle between inflammation and aging [35]. Moreover, cellular senescence, mitochondrial dysfunction, DNA damage, and alterations in the gut microbiota have all been closely implicated in the progression of inflammaging and may act synergistically to exacerbate it [28, 36]. In conclusion, regardless of the specific nature or contribution of each component involved, inflammaging ultimately leads to a persistent subclinical inflammatory status. Whether early intervention in inflammaging can effectively prevent or delay the onset of cellular senescence, immunosenescence, and related age-associated diseases remains an important question that warrants further validation in clinical studies.
Alterations in immune cell populations during Immunosenescence
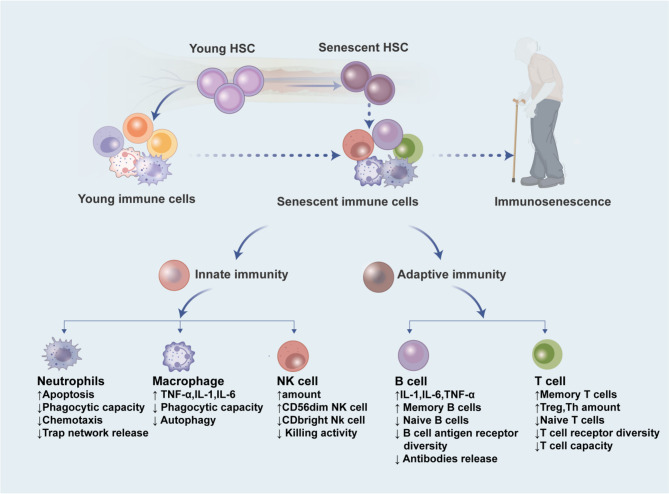
Immunosenescence manifests through progressive deterioration of immune components, encompassing both innate and adaptive immunity systems [37, 38]. The innate arm primarily involves neutrophils, natural killer (NK) cells, monocytes, macrophages and dendritic cells, whereas the adaptive system mainly comprises T and B lymphocytes [39]. This age-related immunological decline constitutes a multifactorial cascade process, with heterogeneous susceptibility observed across distinct immune cell populations [40]. Consequently, immunosenescence serves as a critical role in cellular subset homeostasis(Fig. 3).
Fig. 3.
Alterations of immune cells during immunosenescence. Immunosenescence leads to both quantitative and functional changes in various immune cell types, including neutrophils, macrophages, NK cells, B cells, and T cells
Neutrophils and Immunosenescence
Representing approximately 70% of circulating leukocytes [41], neutrophils serve as the primary cellular mediators of innate immune defense in humans. Their key immune functions include phagocytosis of pathogens, degranulation (release of antimicrobial factors), reactive ROS production, and neutrophil extracellular trap (NET) formation [42]. However, these functions decline significantly with aging [43]. In murine sepsis models, neutrophils from aged individuals exhibited reduced recruitment efficiency and impaired phagocytic capacity relative to young controls [44, 45]. A similar age-dependent decline in chemotaxis and pathogen clearance has been revealed in older individuals with community-acquired pneumonia (CAP) [46]. This functional deterioration of neutrophils might have compromised early immune defense, exacerbating infection progression. Furthermore, polymorphonuclear leukocytes (PMNs) from older individuals exhibited reduced phagocytic activity, accompanied by an increase in the proportion of immunosuppressive PMN subsets (CD16bright/CD62Ldim), leading to decreased ROS levels and phagocytic index. These alterations significantly increased susceptibility to bacterial and fungal infections [47]. NETs, a unique extracellular meshwork composed of DNA, histones, and granular proteins, play a crucial role in pathogen trapping and killing [48]. However, in HIV-infected individuals, aging impaired calcium signaling and TLR8 activation in blood and genital tract neutrophils, suppressing NET release. This dysfunction weakened anti-HIV immune responses, potentially increasing HIV infection risk in women [49].
Age-related defects in neutrophil chemotaxis were closely associated with increased PI3K activity. Studies demonstrated that pharmacological inhibition of PI3K effectively restored chemotactic function in neutrophils from aged individuals [50]. Immunosenescence also profoundly alters neutrophil migratory behavior [51]. Barkaway et al. [52] revealed that aging elevated mast cell-derived CXCL1, promoting reverse transendothelial migration (rTEM) of neutrophils in inflamed tissues. This aberrant process led to neutrophils re-entering circulation and subsequent migration to distal organs, exacerbating vascular leakage and remote organ damage.Similarly, Rolas et al. [53] demonstrated that progerin-expressing endothelial cells (ECs) enhance CXCL1 production, driving pathological neutrophil recruitment to inflamed tissues and amplifying tissue injury and inflammatory responses.
NK cells and immunosenescence
Serving as primary cytolytic executors in innate immune responses, NK cells are crucial for recognizing and eliminating various compromised cells, particularly virus-infected cells and cancerous cells [54, 55]. Intriguingly, although overall immune cell levels decline with aging, NK cell populations exhibit a paradoxical expansion [56, 57]. However, in certain malignancies, NK cell abundance is markedly reduced. For instance, studies in non-small cell lung cancer (NSCLC) laboratory mouse paradigms revealed significantly diminished NK cell infiltration in geriatric relative to juvenile murine subjects [58]. Similarly, hepatocellular carcinoma (HCC) patients demonstrated a notable reduction in NK cell infiltration within the tumor microenvironment (TME), with this decline strongly correlating with advancing age and age-associated phenotypic changes [59]. In endometrial cancer, dysregulation of key chemokines (CXCL12, IP-10, and CCL27) and cytokines (IL-1β and IL-6) within the TME impaired NK cell functionality and recruitment, suggesting that the TME actively remodeled NK cell characteristics to support cancer progression [60]. Furthermore, aging influenced NK cell subtypes, with aged individuals exhibiting decreased proportions of CD56bright NK cells and increased CD56dim populations [56, 61]. Phenotypic shifts also occured during aging; Deng et al. identified an elevated percentage of PD-1⁺ NK cells in older individuals, which co-expressed CD57 and CD69, revealing that PD-1⁺ NK cells represented a senescence-associated immunophenotype [62]. Mechanistically, Chen et al. reported that Vps34 (the sole class III PI3K family member) deficiency impaired autophagy in terminally differentiated NK cells, leading to ROS accumulation and a premature senescence phenotype—a process reversible upon antioxidant intervention [63]. Functionally, age-related NK cell dysfunction manifested as reduced cytotoxicity, diminished secretion of key cytokines (e.g., IL-2 and IL-12), and consequently, compromised immune surveillance, increasing susceptibility to infections, oncogenesis, and mortality. Notably, visceral adipose tissue (VAT)-derived IL-15 and its receptor IL-15Rα may partially mitigated these detrimental effects [57, 64].
Fortunately, emerging therapeutic strategies have shown promise in counteracting aging-induced NK cell dysfunction. For instance, melatonin enhanced NK cell maturation and activation by stimulating the JAK3/STAT5 pathway, upregulated T-bet expression, and consequently augmented NK cell proliferation, degranulation, and IFN-γ secretion—offering a potential immunomodulatory approach for the elderly [65]. Another intervention, arabinoxylan rice bran (Biobran/MGN-3), boosted NK cell cytotoxicity by upregulating CD107a expression, potentially improving elderly individuals’ immune defense against oncological and viral challenges [66]. Similarly, Lactobacillus plantarum HY7712, a probiotic strain derived from kimchi, mitigated radiation- or senescence-associated NK cell impairment via the TLR2/NF-κB pathway [67]. More definitively, NK cell adoptive transfer has demonstrated efficacy in rejuvenating immunosenescent systems, clearing senescent cells (SNCs) from tissues, and reducing SASP factors—highlighting its therapeutic potential for age-related pathologies [68–70].
Macrophages and immunosenescence
Macrophages are found in nearly every organ system and are essential for immune homeostasis, thanks to their exceptional phagocytic capacity, complement component recognition, and antigen-presenting functions [71]. However, aging induces significant alterations in macrophage phenotype and functionality, characterized by diminished phagocytosis and chemotaxis, impaired autophagy, and heightened production of pro-inflammatory cytokines alongside excessive reactive ROS release [72]. For instance, upon LPS stimulation, macrophages derived from elderly individuals exhibited markedly increased secretion of IL-1, TNF-α, and IL-6 relative to those from younger subjects [73]. Vida et al. [74] further demonstrated that macrophages from older mice accumulated greater quantities of ROS and lipofuscin, concomitant with functional impairment. These results highlighted macrophages’ pivotal involvement in age-associated chronic oxidative stress, positioning them as key contributors to immunosenescence and the “oxidative-inflammatory aging” axis [75]. Additionally, the decline in both abundance and rhythmicity of Krüppel-like factor 4 (Klf4) in aged macrophages disrupted circadian regulation, subsequently attenuating immune responsiveness [76]. Such functional decline exacerbated chronic inflammation and tissue degeneration, predisposing to multiple age-related pathologies. Pappert et al. [77] identified that senescent macrophages released cytotoxic mediators, accelerating osteoporosis. Similarly, age-altered dermal macrophages drove adaptive immune dysfunction, fostering pro-inflammatory microenvironments conducive to conditions like skin cancer [78]. Immunosenescence may also impair hyalocytes (vitreous-resident macrophages), thereby promoting vitreoretinal disorders [79]. Notably, Liu et al. [80] observed an elevated proportion of senescent macrophages in murine muscular dystrophy models. Treatment with the senolytic agent fisetin reduced senescent macrophage burden, restored myocyte populations, and improved muscle phenotype, suggesting that senescent macrophages inhibited muscle stem cell function and contributed to dystrophic progression—highlighting fisetin as a potential therapeutic strategy.Mechanistically, deficient type 2 cytokine signaling accelerated macrophage aging, whereas the IL-4–STAT6 axis conferred protection against senescence [81]. Furthermore, T-2 toxin triggered immunosenescence in RAW264.7 cells via cGAS-STING pathway activation, implicating this pathway as a potential therapeutic target [82]. Autophagy, an intracellular degradation process linked to longevity, critically regulated macrophage homeostasis. Atg7-deficient macrophages exhibited metabolic shifts toward glycolysis, accompanied by hallmark senescent phenotypes: proliferative arrest, loss of phagocytic function, and hyperinflammatory cytokine responses [83]. Thus, autophagy serves as a guardian against immunosenescence by sustaining metabolic flexibility and suppressing inflammatory cascades.
T-cell and immunosenescence
The aging process triggers profound structural and functional remodeling across immune cell subsets, encompassing comprehensive adaptations in cellular phenotypes and physiological activities. Of particular interest are T lymphocytes, the central effectors of cellular immunity, whose dynamic evolution during aging has attracted significant research attention [84]. Immunosenescence is marked by the quiescent persistence of naïve and memory T cells, which retain the potential for rapid activation, proliferation, and differentiation upon antigenic challenge [85, 86]. Notably, age-related decline in thymic output capacity emerges as a hallmark of immunosenescence, concomitant with elevated SASP, enhanced glycolytic flux, and reactive oxygen species accumulation [18]. Concurrently, elderly individuals exhibit reduced pools of proliferative naïve B/T cells, constricted receptor repertoire diversity, and amplified pro-inflammatory senescent secretory profiles. Furthermore, aging impairs germinal center reactions and compromises the architecture/function of secondary lymphoid organs, resulting in dysregulated T-B cell dynamics and increased autoreactivity [87].
Senescent T cells may propagate inflammatory cascades through direct cell-cell contact, paracrine cytokine signaling, or tissue-targeting effects, ultimately driving tissue damage and contributing to aging pathogenesis [88]. Intriguingly, B cells serve as a pivotal regulator of T cell fate determination, with studies demonstrating B cells’ involvement in age-associated naïve T cell loss through modulation of TCR clonal expansion and pathogenic T subset differentiation [89]. Aging also fosters the accumulation of specialized T cell subsets, including: (i) highly cytotoxic NK-like populations, (ii) cytokine-dysregulated exhausted subsets, and (iii) pro-inflammatory regulatory T cells. These populations share impaired lymphoid homing capacity, preferentially infiltrating non-lymphoid tissues to exacerbate local inflammation and tissue degeneration [90]. Importantly, T cell senescence can be pathologically accelerated—coinfection with SARS-CoV-2 and CMV potently drives premature immunosenescence via inflammatory microenvironment induction and exhaustion mechanisms, thereby elevating risks for cardiovascular and other chronic conditions [91].
B-cell and immunosenescence
Immunosenescence exerts multifaceted effects on immune function, with particularly complex consequences for B cells [92]. The hallmarks of B cell immunosenescence include dysregulated cytokine secretion, aberrant immunoglobulin production, and impaired immunomodulatory capacity [93]. With advancing age, senescence is accompanied by adipose tissue expansion and elevated palmitic acid (FA) levels. Obesity exacerbates B cell immunosenescence through dual mechanisms involving leptin (an inflammatory driver) and FA (metabolic reprogramming). Increased adipocyte-derived leptin acts directly on B cells, upregulating mRNA expression of proinflammatory cytokines (TNF-α, IL-6, IL-8), chemokines (e.g., CXCL13), pattern recognition receptor TLR4, and the cell cycle inhibitor p16, thereby promoting a proinflammatory B cell phenotype. Additionally, leptin-treated B cells exhibit reduced class-switching capacity (IgM→IgG) and compromised regulatory function. Concurrently, FA enters B cells via metabolic pathways, inducing T-bet (a Th1-associated transcription factor) expression and stimulating autoreactive IgG antibody secretion. These changes accelerate the shift toward a proinflammatory/autoreactive B cell phenotype, leading to age-associated functional defects such as diminished proliferative capacity and impaired immune response efficiency [92, 94, 95]. Immunosenescence further modulates B cell trafficking under inflammatory conditions. Age-dependent elevation in inflammatory mediators alters homeostatic B cell migration into the peritoneal cavity, exacerbating inflammation-related pathologies [96]. This process heightens systemic inflammation, increasing vulnerability to infections and impairing vaccine responsiveness [92]. For instance, elderly individuals exhibited weaker antibody responses to influenza vaccine strains, though elevated BTLA levels on mature B cells enhances IgG reactivity against H1N1. Heightened BTLA levels on class-switched memory B lymphocytes also correlate with maintained neutralizing antibody levels against viral pathogens and enhanced anamnestic immune reactivity following booster immunization [97]. While age-related immune decline predominantly affects adaptive immunity [98], IL-15—a pleiotropic cytokine—offers therapeutic potential. IL-15 monoclonal antibody (mAb) treatment reduces the frequency of age-related B cells (ABCs; B220 + CD11c + T-bet+) and germinal center (GC) B cells, ameliorating manifestations of age-related autoimmunity [99].
Impact of immunosenescence on age-related diseases
With advancing age, immunosenescence emerges as a critical physiological phenomenon that not only compromises immune defense mechanisms but also significantly promotes the pathogenesis of multiple aging-associated disorders. Investigating the intrinsic connections and underlying mechanisms between immunosenescence and these disorders is essential for designing targeted interventions and promoting healthy longevity in the elderly(Fig. 4).
Fig. 4.
Schematic representation of immunosenescence-related diseases. Immunosenescence contributes to age-related immune dysfunction and is implicated in the onset and progression of various age-associated diseases through multiple mechanisms, including neurodegenerative disorders, cardiovascular diseases, malignancies, and autoimmune conditions.AD, Alzheimer’s disease; PD, Parkinson’s disease; MI, myocardial infarction; HF, heart failure; HBP, hypertension; SLE, systemic lupus erythematosus
Neurodegenerative diseases
Neurodegenerative diseases (NDs) are age-associated progressive neurological diseases featured by the gradual deterioration of cognitive, emotional, and motor functions in patients [86]. The pathological basis involves cellular senescence within the nervous system, particularly the accumulation and increased senescence of glial cells [100]. Representative disorders include Alzheimer’s disease (AD) and Parkinson’s disease (PD) [101, 102].
In AD, immunosenescence exacerbates neuroinflammation and neuronal damage through multiple mechanisms. First, age-related immune dysregulation disrupts microbial homeostasis, such as the excessive proliferation of oral anaerobic bacteria, which activates innate immune responses and elevates inflammatory mediators (e.g., TNF-α). This weakens the blood-brain barrier (BBB), establishing a detrimental inflammation-microbiome cycle that accelerates AD progression [103]. Second, immunosenescence impairs immune surveillance against latent pathogens like herpesviruses [104, 105], whose reactivation induces chronic inflammation and promotes AD pathogenesis [106]. Zoonotic pathogens such as Mycobacterium avium subspecies Paratuberculosis (MAP) further disrupt cellular metabolism and autophagy under immunosenescent conditions, leading to Aβ and tau accumulation and exacerbating neurodegeneration [107]. Additionally, senescent immune cells contribute to oxidative stress imbalance and enhanced lipid peroxidation, activating ferroptosis pathways [108, 109]. Dysregulation of the Nrf2-GPX4 axis impairs lipid peroxide clearance, triggering ferroptotic cascades that amplify neuronal damage in AD [110, 111]. Meanwhile, microglia in a pro-inflammatory state exhibit upregulated cytokine secretion and impaired Aβ clearance, forming a self-perpetuating cycle of neurodegeneration [112, 113]. Complement components (e.g., C3a) further increase BBB permeability and microglial activation, worsening neuroinflammatory responses [114].
PD pathogenesis is also closely linked to immunosenescence [115], which manifests through progressive deterioration of dopamine-producing neurons in the substantia nigra (SN) and aberrant α-synuclein (α-syn) aggregation, often accompanied by systemic chronic inflammation and immune dysregulation [116]. Immunosenescence disrupts T/B lymphocyte homeostasis, driving gut microbiota dysbiosis with pathogenic bacterial overgrowth and depletion of commensal species. This elevates circulating pro-inflammatory metabolites (e.g., lipopolysaccharides), inducing systemic inflammation. Immunosenescence modulates PD via the gut-microbiome-brain axis through two primary mechanisms: (1) Gut microbiota (GM) influences brain-gut axis dynamics by regulating immune, neuroendocrine, and enteric nervous systems, directly impacting motor function and PD onset [117]; (2) Microbial toxins induce α-syn aggregation in the enteric nervous system (ENS), which propagates to the central nervous system (CNS) through the vagus nerve, initiating neurodegeneration [118, 119]. α-syn aggregates activate microglia, promoting NLRP3 inflammasome-dependent IL-1β release and amplifying neuroinflammation in PD [120]. Furthermore, IL-17 A disrupts the BBB, activates microglia, and enhances TNF-α secretion and T-cell infiltration, exacerbating neurodegeneration [121]. Enteric glial cells also contribute to PD pathogenesis, as Toll-like receptor-mediated immune activation compromises intestinal barrier integrity, representing another critical mechanism in PD development [122, 123].
Cardiovascular diseases
Studies have confirmed that immunosenescence plays a critical role in the development of cardiovascular disorders (CVD) [124]. DNA viruses (e.g., cytomegalovirus, CMV) accelerate immunosenescence by promoting endothelial injury and fostering a pro-inflammatory vascular microenvironment, thereby exacerbating atherogenesis [125]. Conversely, RNA viruses (e.g., HCV, HIV, and SARS-CoV-2) induce oxidative stress, DNA damage, and SASP, further aggravating vascular inflammation and cellular senescence [126]. The decline in dehydroepiandrosterone (DHEA) concentrations that accompanies aging has been shown to negatively impact the functional capacity of macrophages, leading to uncontrolled overproduction of IL-6 and TNF-α. Notably, foam macrophages (CD14 + CD16+) and intermediate-phenotype macrophages (LB-foam macrophages) aberrantly secrete human endogenous retrovirus K102 (HERV-K102) particles, amplifying inflammatory responses via “trained innate immunity” mechanisms, which play a critical role in advanced atherosclerotic plaque formation [127]. Telomere attrition exerts a dual effect: it not only diminishes immune cell replicative capacity and impairs lymphocyte and macrophage function but also promotes the secretion of pro-inflammatory mediators, resulting in “dysfunctional yet hyperactive” senescent immune cells that perpetuate vascular inflammation [128]. Additionally, immunosenescence modulates atherosclerosis through SASP, where senescent immune cells continuously release inflammatory mediators (IL-1β, IL-18) and chemokines, recruiting more immune cells into the vascular wall and establishing a chronic inflammatory loop that disrupts vascular integrity, accelerates lipid deposition, and promotes plaque instability [126]. Furthermore, immunosenescence drives the expansion of pro-inflammatory immune subsets, including granzyme K (GzmK) + CD8 + T cells and integrin α11b (CD11b) + integrin α11c (CD11c) + T-bet + B cells (ABC cells). These ABC cells exhibit enhanced antigen presentation and co-stimulatory molecule expression, intensifying intraplaque immune responses and accelerating disease progression [129]. Age-related decline in endoplasmic reticulum (ER) chaperones and foldases compromises protein folding and the unfolded protein response (UPR), increasing susceptibility to CVD in the elderly [130]. These intertwined mechanisms collectively establish a “immunosenescence-inflammation-atherosclerosis” vicious cycle, ultimately leading to vascular dysfunction and plaque formation.Beyond vascular effects, aging reduces ventricular nerve density and disrupts vasculature-derived neuromodulatory genes, impairing cardiac function. Studies revealed that aging downregulated microRNA-145 (miR-145), reducing semaphorin-3 A (Sema3a) expression and diminishing cardiac axon density, thereby destabilizing electrical signaling [131]. Moreover, age-associated adipose tissue expansion exacerbated systemic inflammation via adipokine dysregulation, disrupting metabolic homeostasis and increasing myocardial infarction risk [130]. Additionally, the CD4/CD8 ratio rose with age, and this T-cell subset imbalance was linked to elevated hypertension risk [132].
Malignant tumors
Malignant tumors are frequently associated with marked immunosuppression [133]. The immunoregulatory network within the TME critically modulates cancer progression through intercellular communication mechanisms [110]. During immunosenescence, senescent T cells exhibit diminished responsiveness to cognate antigens and remain in a terminally differentiated, proliferatively exhausted state, compromising their capacity to identify and eliminate malignant cells, thereby fostering an immunosuppressive TME. Early hypotheses posited immunosenescence as an adaptive regulatory mechanism that maintains homeostasis by preventing excessive immune activation. However, recent studies demonstrate that SASP secretion exacerbates oncogenesis. For instance, senescent macrophages enhanced the proliferative, invasive, and metastatic potential of lung cancer cells via inflammatory mediators (e.g., IL-6, MMPs, ROS) within the TME [134]. Furthermore, metabolic dysregulation in immune cells (T cells and macrophages) may drove TME remodeling, creating a permissive niche for cancerous cells growth in elderly patients [135]. Notably, age-dependent tumorigenesis correlates strongly with impaired immune surveillance and dysregulated cancer immunoediting [136]. Compared to younger patients, geriatric oncology patients (≥ 75 years) often faced poorer prognoses due to immunocompromised status, higher comorbidity burdens, and reduced tolerance to treatment-related toxicity [137].
Autoimmune diseases
The deterioration of immune function with aging exhibits a significant correlation with autoimmune diseases (AIDs), as it often coincides with elevated inflammatory levels, these mechanisms collectively represent major pathogenic factors in aging-associated disorders [138]. The progression of autoimmune diseases may be influenced by immunosenescence through multiple mechanisms. For instance, with increasing age, the generative ability of T cells markedly diminished, leading to an insufficient immune response to foreign pathogens.Immunosenescence disrupts immune tolerance and activates autoreactive T cells by reducing TCR diversity, impairing regulatory T-cell (Treg) function, and promoting the release of senescence-associated inflammatory factors. This perpetuates a vicious cycle of chronic inflammation and tissue antigen exposure, ultimately driving AIDs pathogenesis. Notably, TCR repertoire analysis has been employed to explore the influence of aging on multiple sclerosis (MS) [139]. Furthermore, immunosenescence affects autoimmune disease progression through age-related B cells (ABCs). These cells acquire a proinflammatory phenotype due to senescence-related dysfunction (e.g., mitochondrial abnormalities, epigenetic dysregulation), secreting IL-6 and TNF-α while producing autoreactive antibodies, thereby disrupting immune tolerance. Additionally, ABCs exacerbate chronic inflammatory microenvironments by activating senescence-related signaling pathways (e.g., mTOR/NF-κB), promoting abnormal activation and tissue infiltration of autoimmune cells (e.g., Th17), ultimately accelerating the pathological progression of rheumatoid arthritis (RA), systemic lupus erythematosus (SLE), and related disorders [140].
Immunosenescence-based therapies are emerging as novel treatment strategies for AIDs. In SLE, accelerated senescence of immune cells is accompanied by elevated IL-15 levels, leading to systemic immune hyperactivation. Studies demonstrated that targeting IL-15 or selectively eliminating senescent immune cells effectively reduced anti-dsDNA antibody titers and ameliorated renal damage, offering a promising therapeutic avenue [99]. Similarly, immunosenescence is implicated in disease progression, treatment response, and prognosis in MS. Recent research has explored innovative approaches, such as senolytic agents, neuroprotective interventions, and remyelination therapies, which may benefit elderly MS patients [141]. Concurrently, soluble costimulatory molecules (e.g., sCD163, sCD28, sCD80, sCTLA-4) have been proposed as promising indicators for assessing immunosenescence, facilitating the timely identification of AIDs and therapeutic response evaluation [142].
Immunosenescence and histone post-translational modifications
PTMs serve as critical epigenetic regulators of cellular function during aging. In eukaryotic cells, numerous essential proteins - particularly those governing gene expression, cell cycle progression, DNA repair mechanisms, and metabolic regulation - undergo diverse PTMs. The eukaryotic genome is compacted into chromatin, with nucleosomes constituting its fundamental repeating units. Each nucleosomal core particle contains 146 bp of DNA wrapped around an octameric histone core comprising H3, H4, H2A, and H2B [143]. Specialized histone-modifying enzymes catalyze various PTMs at specific amino acid residues (lysine, serine, and arginine) within the flexible N-terminal tails of histones. These modifications encompass a broad spectrum of chemical alterations, including acetylation, methylation, phosphorylation, citrullination, ubiquitination, ADP-ribosylation, deamidation, formylation, O-linked β-N-acetylglucosamine modification (O-GlcNAcylation), as well as various acylations (propionylation, butyrylation, crotonylation) and proline isomerization [144, 145]. This section focuses on four major histone modifications (methylation, acetylation, ubiquitination, O-GlcNAcylation and PARylation(Fig. 5)) and reviews recent advances in understanding their implications in immunosenescence(Tables 1 and 2).
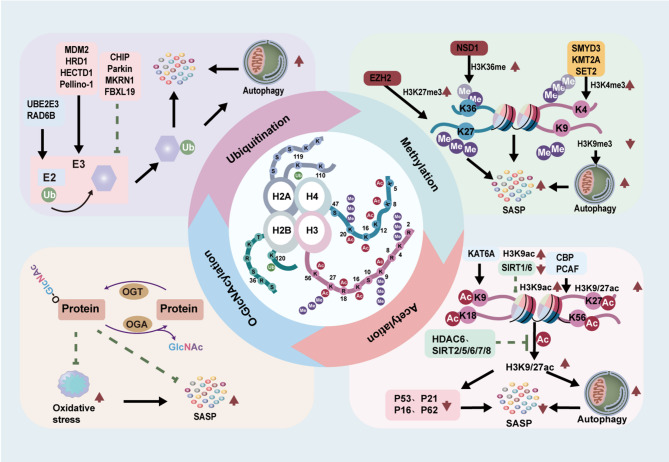
Fig. 5.
Histone modifications in immunosenescence. The four core histones—H2A, H2B, H3, and H4—undergo various post-translational modifications, including methylation, acetylation, ubiquitination, and O-GlcNAcylation at specific amino acid residues. These modifications play critical roles in the regulation of immunosenescence and the development of related diseases
Table 1.
Histone methylation and acetylation during Immunosenescence
| Potential Target | Modification Sites | Enzyme | Model | Mechanism | Effect | References |
|---|---|---|---|---|---|---|
| Methylation | H3K4me3 | KMT2A | IVDD | KMT2A upregulates H3K4me3, promotes METTL3 expression, mediates m⁶A methylation, inhibits autophagy, and induces SASP | Promotes aging | [152, 153] |
| H3K4me3 | SET-2 | Dietary restriction (DR) C. elegans model | Inhibits SET-2, reduces H3K4me3, promotes HLH-30/TFEB and PHA-4/FOXA, and promotes autophagy | Delays aging | [154] | |
| H3K4me2/3 | Smyd3 | Angiotensin II (Ang II)-induced rat endothelial cell aging model | Binds to the P21 promoter, promotes H3K4me3, and induces SASP | Promotes aging | [155] | |
| H3K9me3 | – | Mouse myocardial infarction (MI) model | Lack of H3K9me3 promotes autophagy | Promotes aging | [156] | |
| H3K27me3 | PRC2 | Diploid fibroblast (HDF) aging model | After EZH2 downregulation, reduced H3K27me3 demethylation induces DNA damage, activates P16 and SASP | Promotes aging | [161] | |
| H3K27me3 | EZH2 | Naturally aging mice | Upregulation of H3K27me3 | Promotes aging | [164] | |
| H3K36me | NSD1 | Osteoarthritis mouse model | miR-350-3p inhibits NSD1, reducing H3K36me levels | Promotes aging | [165] | |
| – | PRMT1 | PRMT1 knockout model | PRMT1 deficiency promotes cell death | Promotes aging | [166] | |
| H3K4me1/3 | KDM1A | Kdm1a knockout model | KDM1A deficiency leads to downregulation of H3K4me1 and upregulation of H3K4me3, protecting the gene silencing boundary mediated by PRC and preventing age-related euchromatinization in neurons | Delays aging | [171] | |
| H3K36me2 | KDM2A | SIRT6 knockout model | Deletion increases H3K36me2 levels, recruits DNA repair factors, and enhances genome stability | Delays aging | [172] | |
| H3K9me3 | KDM3A/4 C | Mesenchymal stromal cell (MSCs) aging model | Downregulation of H3K9me3 activates condensins NCAPD2 and NCAPG2, regulates heterochromatin reorganization, and induces DNA damage | Promotes aging | [173] | |
| H3K27me3 | KDM6A | KDM6A knockout model | KDM6A deficiency causes H3K27me3 accumulation and regulatory disorders, combined with X-chromosome escape, amplifying the pro-inflammatory response | Promotes aging | [174] | |
| H3K27me3 | KDM6A | Spinal cord microvascular endothelial cell (SCMECs) aging model | Downregulation of H3K27me3 interacts with CNN1, affecting neuroinflammation and functional recovery | Promotes aging | [175] | |
| Acetylation | H3K9/27ac | CBP, PCAF, and other HATs | – | Increases H3K9/K27ac in the promoter region of neuronal genes, binds to TFEB to promote H3K9ac in the promoter of target genes, and drives lysosomal biogenesis and autophagy | Delays aging | [177, 178] |
| H3K9ac | KAT6A | Lymphoma mouse model | Upregulation of H3K9ac regulates the repressor at the CDKN2A locus | Delays aging | [181] | |
| H3ac | Elp3 | – | Upregulation of H3ac affects neuronal protein and organelle transport, exacerbating neurodegeneration in aging-related diseases | Promotes aging | [182] | |
| HDAC6 and SIRT2/5/6/7/8 | – | Downregulation of H3ac negatively regulates neuronal protein and organelle transport | Delays aging | [182] | ||
| H4K16ac | Sir2/Sas2 | Saccharomyces cerevisiae cell model | Imbalance of Sir2/Sas2, upregulation of H4K16ac, induces subtelomeric gene silencing | Promotes aging | [182] | |
| H4ac | Tip60 | C. elegans model | Catalyzes H4 hyperacetylation, remodels chromatin structure, and establishes a pro-inflammatory microenvironment | Promotes aging | [184] | |
| H3K9/H4K16 | SIRT1 | Human fibroblast model | SIRT1 deficiency leads to upregulation of H3K9/H4K16ac, transcriptional activation and increased expression of SASP factors | Promotes aging | [188] | |
| H3K9 | SIRT6 | Hypertensive mouse model | SIRT6 deacetylates H3K9, regulates the Nkx3.2 - GATA5 pathway, protects endothelial function and resists hypertension | Delays aging | [189] | |
| K81 | SIRT2 | Sirt2 knockout mouse model | Sirt2 deficiency leads to downregulation of deacetylation of the aging regulatory protein p66Shc at K81, accumulation of superoxide and ROS, exacerbating age-related arterial stiffness and impaired contraction and relaxation | Delays aging | [190] | |
| – | HDAC1 /2 | Nphs2-Cre Hdac1/2 | HDAC1/2 deficiency leads to increased SA-β-gal activity and lipofuscin accumulation | Delays aging | [191] | |
| – | HDAC3 | HDAC3 knockout mouse model | HDAC3 deficiency causes chromatin changes related to lipid droplet (LD) formation | Delays aging | [192] |
Table 2.
Histone ubiquitination and O-GlcNAcylation during Immunosenescence
| Potential Target | Modification Sites | Enzyme | Model | Mechanism | Effect | References |
|---|---|---|---|---|---|---|
| Ubiquitination | K1642 | CHIP | Mouse model of osteoarthritis (OA) | DPP4 competitively binds to the K1642 site of MYH9, blocking CHIP-mediated ubiquitin-proteasomal degradation, and the accumulation of MYH9 triggers excessive mitochondrial fission and exacerbates oxidative stress | Promotes aging | [13] |
| - | Parkin | IVDD rat model | SPP1 inhibits mitophagy by blocking the PINK1/PARKIN pathway, accelerating nucleus pulposus cell degeneration and inducing calcification | Promotes aging | [198] | |
| – | Diabetic nephropathy (DN) mouse model | Parkin ubiquitinates GATA4, downregulating the GATA4/GAS1 signaling pathway and alleviating inflammation and fibrosis in renal tubular epithelial cells (RTEC) | Delays aging | [14] | ||
| – | FBXL19 | Cardiovascular injury model related to influenza A virus (IAV) infection | Enhances the innate immune response, inhibits inflammation and cell aging | Delays aging and protects the heart | [15] | |
| – | MKRN1 | Endothelial cell (ECs) aging and activation model | High expression of MKRN1 induced by d-flow ubiquitinates and degrades related regulatory molecules, activates the NF - κB pathway and the cell aging program, and exacerbates endothelial cell dysfunction and promotes atherosclerosis | Promotes aging | [199] | |
| K142 | HRD1 | Neuron aging model exposed to cadmium chloride (CdCl₂) | Cadmium chloride exposure activates the SEL1L/ hrd1-mediated ERAD system, leading to ubiquitination of SigmaR1 | Promotes aging | [200] | |
| – | HECTD1 | RAW264.7 cell aging model | KL targets HECTD1, inhibits IRS1 ubiquitin-proteasomal degradation, regulates macrophage aging and function, and alleviates diabetic retinopathy | Promotes aging | [201] | |
| K63 | Pellino-1 | D-gal-induced mouse aging model | Pellino - 1 ubiquitinates p21 via the K63 site and participates in the aging process of lung cells and the development of COPD | Promotes aging | [202] | |
| – | MDM2 | 2-year naturally aging mouse model | MDM2 ubiquitination promotes HDAC1 degradation and promotes kidney aging | Promotes aging | [191] | |
| – | UBE2E3 | Aging Sertoli cell (SCs) model | miR - 143 - 3p targets UBE2E3, restricting SCs proliferation and disrupting the blood-testis barrier | Promotes aging | [202] | |
| – | RAD6B | RAD6B knockout (KO) mouse model | RAD6B deficiency disrupts the PEDF signal, activates DNA damage, aging, and apoptosis pathways, and accelerates retinal degeneration | Delays aging | [204] | |
| K13/K15 | USP14 | D - gal-induced POI mouse model | Abnormally high expression of USP14 disrupts the DDR function of granulosa cells | Promotes ovarian aging | [205] | |
| – | USP11 | Unilateral ureteral obstruction (UUO) mouse model | USP11 deubiquitinates Tgfbr2 to reduce its degradation, activating the downstream aging signaling pathway | Promotes kidney aging and fibrosis | [206] | |
| – | USP13 | USP13 knockout mouse model | USP13 participates in the lung aging signaling pathway by regulating the K63-linked ubiquitination and protein stability of MDM2 | Promotes lung aging | [207] | |
| – | USP18 | Human coronary artery endothelial cells (HCAECs) | USP18 participates in the regulation of inflammation via the ubiquitination pathway | Delays aging | [208] | |
| – | USP30 | D - gal-treated cell aging model | USP30 antagonizes Parkin and negatively regulates mitophagy, accelerating cardiomyocyte aging | Promotes aging | [209] | |
| – | USP3 | Primary articular chondrocyte model | USP3 upregulates SIRT3 and inhibits IL - 1β-induced chondrocyte aging | Delays aging | [210] | |
| O-GlcNAcylation | Ser470/Thr493 | OGT | C. elegans model | OGT-1 can catalyze the O-GlcNAc glycosylation modification of the Ser470 and Thr493 sites of the SKN-1 protein, thereby affecting the accumulation, activity of SKN-1 in the intestinal nucleus, and the lifespan and oxidative stress resistance of nematodes | Delays aging | [16] |
| – | OGT | OGT knockout mouse model | Decreased levels of OGT and O - GlcNAcylation in the brains of aging mice lead to cognitive impairment. Regulating OGT promotes cognitive rejuvenation | Delays aging | [214] | |
| – | OGA | O - GlcNAcylation overexpressing C57BL/6J mouse model | Inhibition of OGA affects AD treatment | Delays aging | [17, 215] | |
| – | OGT, OGA | Transient cerebral ischemia stroke model | The XBP1s/HBP/O - GlcNAc axis regulates neuroprotection in ischemic stroke | Delays aging | [216] | |
| Thr699 | OGT, OGA | – | Modifies β - hydroxybutyrylation (Kbhb), enhances the interaction between STAT1 and IFNAR2, and enhances the antiviral response mediated by ifn - i | Delays aging | [217] |
Methylation
Methylation represents a pivotal form of PTMs, extensively occurring in various intracellular proteins, particularly in chromatin-associated histones and non-histone molecules. Among these, histone methylation stands out as one of the most stable and long-lasting PTMs, serving as an extensively studied mechanism for transcriptional inhibition and genetic regulation [146]. However, depending on the specific amino acid modified, histone methylation can also facilitate transcriptional activation [147]. This modification involves the addition of one or more methyl (–CH₃) groups to amino acid residues, primarily lysine (K) or arginine (R) [148]. Lysine residues can undergo mono- (me1), di- (me2), or trimethylation (me3) at their ε-amino groups, whereas arginine methylation may occur as monomethylation (me), symmetric dimethylation (me2s), or asymmetric dimethylation (me2a) [149]. The methylation process is predominantly catalyzed by histone methyltransferases (HMTs), which are divided into two major classes depending on their target amino acids: histone lysine methyltransferases (KMTs) and protein arginine methyltransferases (PRMTs). The KMT family includes enzymes such as EZH2, G9a, DOT1L, and SETD2, while the PRMT family in mammals comprises PRMT1 through PRMT9 [150]. Histone H3 serves as the primary site for methylation, though other core histones also undergo this modification [151]. Specifically, five lysine residues on histone H3 (K4, K9, K27, K36, and K79) are susceptible to methylation, whereas histone H4 is predominantly methylated at K20 by specific KMTs. Notably, methylation at H3K4 and H3K36 is associated with transcriptional activation, whereas methylation at H3K9, H3K27, H3K79, and H4K20 correlates with transcriptional repression [11].
Emerging research has increasingly linked histone methylation to immunosenescence and age-associated diseases. Emerging research indicated that site-specific histone methylation profoundly influences aging by modulating gene expression, autophagy, and inflammatory cytokine release. For instance, H3K4 methylation generally exhibited pro-aging effects. In intervertebral disc degeneration (IVDD) models, KMT2A-mediated H3K4me3 upregulated methyltransferase-like 3 (METTL3), leading to reduced ATG4a mRNA stability via m6A modification, thereby suppressing autophagy in nucleus pulposus cells (NPCs) and accelerating SASP expression [152, 153]. Conversely, in dietary-restricted (DR) C. elegans models, limited S-adenosylmethionine (SAM) synthesis inhibited SET-2 (a homolog of human KMT2), thereby reducing H3K4me3 levels and subsequently activating TFEB/HLH-30 and PHA-4/FOXA pathways to enhance autophagy and extend lifespan [154]. Similarly, in angiotensin II (Ang II)-induced endothelial senescence, the histone methyltransferase Smyd3 promoted H3K4me3 deposition at the p21 promoter, enhancing its transcription and accelerating cellular aging [155]. In contrast, H3K9me3 modification is predominantly localized to heterochromatin, and its abundance declines with advancing age [156]. Heterochromatin plays a pivotal role in transcriptional silencing and the maintenance of genomic stability [157]. The widespread loss of heterochromatin, especially constitutive heterochromatin, has been consistently recognized as a defining feature of aging across various cell types and species. This epigenetic alteration is primarily characterized by the depletion of H3K9me3, leading to loss of gene silencing and driving senescence-associated pathways [158]. Studies in progeria-derived fibroblasts (PSFs) demonstrated that heterochromatin reduction, marked by diminished H3K9me3 levels, suppressed autophagic flux, exacerbated SASP secretion, and subsequently induced cardiac fibrosis [159]. In a murine myocardial infarction (MI) model, loss of H3K9me3 sensitized mesenchymal stem cells (MSCs) to stress-induced apoptosis and premature senescence [160]. H3K27 methylation also represents a pivotal epigenetic regulator of cellular senescence and organismal aging. The Polycomb repressive complex 2 (PRC2), composed of EZH2, EED, and SUZ12, exclusively catalyzes H3K27me3, with PRC2 binding further activating EZH2 [161]. In human diploid fibroblasts (HDFs), EZH2 downregulation triggered DNA damage responses, subsequently activating p16 and SASP to induce senescence, establishing H3K27me3 as a senescence marker [162, 163]. Recent studies have demonstrated that both EZH2 and its associated histone modification marker, H3K27me3, exhibit age-dependent upregulation in the gastric tissues of aged mice, including both naturally aged and Klotho-deficient models [164]. Additionally, in mechanically induced osteoarthritis, miR-350-3p suppressed the H3K36 methyltransferase NSD1, reducing H3K36me levels and promoting chondrocyte senescence [165]. Arginine methylation also contributes to aging. PRMT1, the most prominent PRMT, regulated STAT1, lymphocyte signaling, and TNFα/NF-κB pathways, implicating it in immune responses [166]. PRMT1 deficiency induced mitochondrial dysfunction, exacerbating age-related sarcopenia [167]. In COPD models, PRMT1 depletion increased cellular senescence and death, worsening disease progression [168].
Lysine demethylases (KDMs), which utilize iron and 2-oxoglutarate (2OG) to oxidatively remove methyl groups, play pivotal roles in gene regulation and chromatin structure [169, 170]. Among these, KDM1A and KDM2A are key regulators of aging. KDM1A safeguarded PRC2-mediated gene silencing boundaries, preventing age-associated euchromatinization in neurons [171]. KDM2A depletion elevated H3K36me2, which recruited DNA repair factors and enhanced genomic stability, thereby delaying senescence [172]. Conversely, KDM3A and KDM4C regulated heterochromatin reorganization in mesenchymal stromal cells (MSCs) by activating condensins NCAPD2 and NCAPG2. Their inhibition induced DNA damage responses, exacerbating cellular aging [173]. KDM6A, however, exerted detrimental effects by demethylating H3K27me3/H3K4me3, promoting IRF5 transcription and X-chromosome escape, thereby amplifying pro-inflammatory microglial responses in aging [174]. Additionally, KDM6A interacted with calponin 1 (CNN1) to form an epigenetic axis that regulated trauma-induced senescence in spinal cord microvascular endothelial cells (SCMECs), influencing neuroinflammation and functional recovery [175].
Acetylation
The balance of histone acetylation is maintained by two counteracting enzymes: HATs and HDACs, which maintain a delicate equilibrium to ensure precise gene expression control [12]. HATs catalyze the transfer of acetyl groups to lysine residues on histone tails and are phylogenetically classified into two major families: the Gcn5-related N-acetyltransferases (GNAT) and the MYST family [176, 177]. The GNAT family, comprising Gcn5, p300/CBP-associated factor (PCAF), Elp3, and Hpa2, primarily mediates acetylation at lysine sites of histone H3 through an ordered sequential mechanism involving direct nucleophilic attack of the ε-amino group on enzyme-bound acetyl-CoA. In contrast, the MYST family—including catalytic subunits such as Moz, Ybf2/Sas3, Sas2, Tip60, MORF, and HBO1—preferentially acetylates histone H4 lysine residues via a direct attack mechanism within the Esa1×acetyl-CoA×histone ternary complex. Notably, certain HATs also post-translationally modify non-histone proteins, including high-mobility group chromatin proteins, transcriptional activators (e.g., p53), coactivators, and general transcription factors [177, 178]. The reverse reaction, involving acetyl group removal from histone tails, is catalyzed by HDACs. Mammalian HDACs are currently categorized into four classes: Class I (HDAC1-3, HDAC8), Class II (HDAC4-7, HDAC9-10), Class III (SIRT1-7), and Class IV (HDAC11). Mechanistically, Class I, II, and IV HDACs are zinc-dependent metalloenzymes, whereas Class III HDACs (sirtuins) require NAD + as an essential cofactor for their deacetylase activity [179].
HATs are primarily regulated by acetyl-CoA levels. In tissues, fasting or caloric restriction reduces cytoplasmic acetyl-CoA, leading to diminished p300 HAT activity, which in turn promotes longevity-associated autophagy. Conversely, elevated nuclear acetyl-CoA enhances HAT activity and increases histone acetylation, paradoxically extending lifespan. Additionally, The nuclear translocation of cytoplasmic acetyl-CoA synthetase 2 (ACSS2) enhances the activity of HATs, including CREB-binding protein (CBP), p300/CBP-associated factor (PCAF), and others. This activation increases H3K9 and H3K27 acetylation at neuronal gene promoter regions. Moreover, ACSS2 interacts with transcription factor EB (TFEB) to facilitate H3K9 acetylation at TFEB target gene promoters, thereby promoting lysosomal biogenesis and autophagy. These processes collectively counteract age-related cellular decline [180]. Among the lysine acetyltransferases (KATs) of the MYST family (KAT5-KAT8), KAT6A suppresses cellular senescence by modulating repressors at the CDKN2A locus via H3K9 acetylation [181]. Elongator complex subunit 3 (Elp3), the catalytic component of the elongator complex, facilitates H3 acetylation of α-tubulin, which functions as a molecular beacon for motor protein attachment to microtubules. This process influences neuronal protein and organelle transport, exacerbating neurodegeneration in aging-related disorders. Furthermore, α-tubulin H3 acetylation is negatively regulated by HDAC6 and SIRT2/5/6/7/8 [182]. During aging, depletion of the mediator complex subunit Med5 or mutations in Med7 lead to its reduced occupancy near subtelomeric X-elements, disrupting the balance between silent information regulator 2 (Sir2) and silence-associated sequence 2 (Sas2). The relative increase in Sas2 activity elevates H4K16 acetylation near telomeres, inducing subtelomeric gene silencing. This epigenetic alteration aligns with the observed increase in telomeric H4K16 acetylation in senescent cells, revealing Sas2’s role in aging via modulation of telomeric histone acetylation [183]. Under persistent DNA damage, nuclear DNA damage signaling activates mitochondrial β-oxidation, triggering adipose tissue breakdown and generating excess acetyl-CoA to fuel histone acetylation. Tip60, a central metabolic-epigenetic regulator, not only facilitates polyunsaturated fatty acid accumulation and lipid mediator production but also directly catalyzes H4 hyperacetylation, collectively reshaping chromatin architecture. This process alters the expression profiles of immune effectors and cytochrome genes, establishing a pro-inflammatory microenvironment resembling immune activation, ultimately driving age-related functional decline [184]. In aged hematopoietic stem cells (HSCs), a marked reduction in H4K77ac acetylation had been observed, which was associated with enhanced HDAC3 activity. Notably, inhibition or knockdown of HDAC3 in HSCs significantly promoted lymphoid lineage differentiation [185]. Moreover, histone acetylation profiling of the choroid in aged mice revealed a distinct molecular signature characterized by decreased levels of H3K14ac, H3K56ac, and H4K16ac [186].
Sirtuins, classified as class III HDACs, serve as critical sentinels for maintaining genomic stability by mediating lysine residue deacetylation in an NAD⁺-dependent manner. SIRT1 was downregulated by lysosomal activity in hematopoietic and immune organs following natural aging of T cells in mice and elderly humans [187]. In SIRT1-deficient cells, SASP components, including IL-6 and IL-8, rapidly accumulated. Mechanistically, SIRT1 bound to the promoter regions of IL-8 and IL-6 but dissociated during cellular senescence, coinciding with progressive hyperacetylation of histone H3K9 and H4K16 at these loci [188]. SIRT6 exerted pleiotropic protective effects in endothelial cells by deacetylating histone H3K9, thereby enhancing endothelium-dependent vasodilation, promoting vascular NO bioavailability, decreasing membrane permeability, attenuating endothelial senescence and apoptosis, and stimulating autophagy [189]. In aged mice, SIRT2 deficiency led to superoxide and ROS accumulation, exacerbating age-related arterial stiffness and impaired contractile relaxation, suggesting that SIRT2-mediated deacetylation served as an adaptive response to delay vascular aging [190]. Furthermore, HDAC1/2-deficient podocytes in mice exhibited hallmark senescence features, including elevated age-associated β-galactosidase (SA-β-gal) activity and lipofuscin accumulation [191]. Intriguingly, HDAC1 can be ubiquitinated and degraded by murine double minute 2 (MDM2), thereby promoting senescence [192]. In COPD, lymphocyte senescence was linked to HDAC2 loss in CD28-null CD8⁺ T cells and NKT-like cells [193]. Additionally, HDAC3-deficient bone marrow stromal cells (BMSCs) displayed chromatin alterations associated with premature senescence and lipid droplet (LD) formation, indicating that HDAC3 is crucial in suppressing skeletal aging by regulating LD biogenesis and senescence in osteochondroprogenitor cells [194].
Ubiquitination
Ubiquitin (Ub), an evolutionarily conserved 76-amino acid regulatory polypeptide, was initially characterized by Goldstein and colleagues in 1975 [195]. Ubiquitination involves the covalent conjugation of Ub to the ε-amino group of lysine residues on substrate proteins, a process mediated by the sequential action of E1 activating enzymes, E2 conjugating enzymes, and E3 ubiquitin ligases [196]. Ub itself contains seven lysine residues (K6, K11, K27, K29, K33, K48, and K63) and an N-terminal amine, enabling the formation of polyubiquitin chains through linkage at these sites—a phenomenon termed polyubiquitination. Conversely, deubiquitinating enzymes (DUBs) catalyze the removal of ubiquitination from substrates [197].
During aging, ubiquitination modifications extensively participate in cell fate determination and tissue functional decline through diverse mechanisms, including proteostasis regulation, mitochondrial function, oxidative stress, and inflammatory responses. E3 ubiquitin ligases serve as pivotal regulators of senescence by facilitating the ubiquitin-dependent degradation of specific substrates. For instance, the activity of the CHIP ligase was modulated by the DPP4-MYH9 axis, wherein DPP4 competitively bound MYH9 to suppress CHIP function, thereby exacerbating oxidative stress and senescence in chondrocytes [13]. In nucleus pulposus cells, secreted phosphoprotein 1 (SPP1) impeded PINK1/Parkin-mediated mitophagy via the integrin α5β1 pathway, accelerating cellular senescence and intervertebral disc degeneration [198]. Furthermore, studies in diabetic nephropathy (DN) mouse models demonstrated that the E3 ligase Parkin mitigated renal tubular epithelial cell (RTEC) senescence by alleviating inflammation and fibrosis [14]. Similarly, FBXL19 exerted cardioprotective effects during cardiac aging by enhancing innate immune responses and suppressing inflammation and cellular senescence [15]. Atherosclerosis research further revealed a close association between E3 ligases and the SASP. MKRN1 deficiency activated the NF-κB pathway in endothelial cells, aggravating endothelial dysfunction and inflammation [199].In the nervous system, cadmium chloride exposure activated the SEL1L/HRD1-mediated ERAD system, leading to SigmaR1 ubiquitination and accelerated neuronal senescence [200]. Analogously, HECTD1 mediated 4-hydroxynonenal (4HNE)-induced senescence in RAW264.7 cells by targeting IRS1 for degradation, a process counteracted by the soluble anti-aging factor α-Klotho [201]. In COPD models, Pellino-1 modulated p21-dependent SASP production, and its silencing attenuated chronic inflammation and tissue aging [202]. Additionally, MDM2 exacerbated renal aging by ubiquitinating HDAC1 and promoting its degradation [192]. E2 ubiquitin-conjugating enzymes also play critical roles in senescence. For example, miR-143-3p restricted Sertoli cell proliferation and disrupted the blood-testis barrier by targeting the ubiquitin-conjugating enzyme UBE2E3 [203]. RAD6B deficiency accelerated retinal aging, resulting in increased apoptosis and DNA damage [204].
DUBs influence aging by modulating the ubiquitination status of specific proteins. In premature ovarian insufficiency (POI), USP14 promoted DNA damage accumulation, hastening ovarian aging [205]. USP11 enhanced renal senescence and fibrosis by deubiquitinating and stabilizing TGF-β type II receptor (Tgfbr2), thereby amplifying senescence-associated signaling [206]. USP13 contributed to pulmonary aging by regulating K63-linked ubiquitination of MDM2, inducing cellular senescence [207]. Conversely, USP18 alleviated inflammation and senescence in coronary endothelial cells [208], while USP30 silencing ameliorated mitochondrial dysfunction and cardiomyocyte senescence [209]. Notably, USP3 exerted protective effects in age-related disorders by inhibiting chondrocyte senescence via SIRT3 and FOXO3 deacetylation [210].
O-GlcNAcylation
O-GlcNAcylation represents a non-canonical glycosylation modification marked by the covalent conjugation of a single O-linked N-acetylglucosamine (O-GlcNAc) moiety to serine and threonine residues of cytosolic, nuclear, and mitochondrial proteins, constituting a dynamic and reversible PTMs [211]. This modification is catalyzed by OGT, while its removal is mediated by O-GlcNAcase (OGA). Together, these enzymes maintain the dynamic equilibrium of O-GlcNAc levels in response to cellular metabolic states and environmental fluctuations.
Recent studies underscored the critical involvement of O-GlcNAcylation in aging and associated pathologies, particularly in neurodegenerative disorders and metabolic homeostasis [212, 213]. In mammals and the model organism Caenorhabditis elegans, O-GlcNAcylation directly modulated key longevity-associated transcription factors, such as Nrf-2 (SKN-1 in C. elegans), promoting their nuclear accumulation and enhancing antioxidant defense mechanisms, thereby extending lifespan [16]. O-GlcNAcylation had also been demonstrated to be essential for synaptic plasticity and cognitive function. Studies revealed that aged mice exhibited reduced OGT expression and altered O-GlcNAcylation patterns in the brain, which correlated with cognitive decline. Notably, OGT overexpression in the hippocampus of aged mice partially restored spatial learning and memory, underscoring the critical role of O-GlcNAc regulation in maintaining neuroplasticity and cognitive function [214].The engagement of O-GlcNAcylation in NDs is particularly significant. Enhanced O-GlcNAcylation mitigates the phosphorylation and aggregation of tau and β-amyloid in AD, alleviating neurotoxicity [17, 215]. Intriguingly, ischemia-induced O-GlcNAcylation was compromised in the aged brain, whereas augmenting O-GlcNAcylation improved functional recovery after ischemic injury [216]. Furthermore, O-GlcNAcylation modulated antiviral defense pathways. Recent findings indicated that O-GlcNAcylation suppressed senescence-induced β-hydroxybutyrylation (Kbhb) at Thr699 of STAT1, thereby strengthening the interaction between STAT1 and interferon-α/β receptor 2 (IFNAR2) and enhancing IFN-I-mediated antiviral responses. This mechanism offered a potential therapeutic strategy to bolster antiviral defenses in the elderly [217].
PARylation
Poly ADP-ribosylation (PARylation) is a post-translational modification catalyzed by poly(ADP-ribose) polymerases (PARPs), in which ADP-ribose units derived from NAD⁺ are covalently attached to target proteins as linear or branched polymers, thereby modulating their structure and function. PARP1, the most extensively studied member of the PARP family, is rapidly activated in response to DNA damage and facilitates the recruitment of repair proteins through auto-PARylation [218]. Since its discovery, accumulating evidence has demonstrated that PARylation plays a critical role in cellular stress responses and DNA repair, closely associated with repair efficiency and fidelity.Beyond its canonical role in genome maintenance, PARylation has emerged as a pleiotropic regulator implicated in inflammation, metabolism, and cell death, highlighting its dual impact on cellular homeostasis. Under physiological conditions, PARylation contributes to immune defense by promoting the release of pro-inflammatory cytokines such as TNF-α and IL-6 in macrophages. However, during aging, its excessive activation can trigger inflammaging, thereby accelerating immunosenescence. Chronic inflammation, in turn, has been linked to telomere shortening and functional decline in T cells, as well as an increased risk of cardiovascular disease [219, 220].Recent studies have further revealed the multifaceted involvement of PARylation in the progression of immunosenescence. For example, PARylation has been shown to regulate the activation of both T and B lymphocytes [219]. In senescent T cells, aberrant PARP1 activity disrupted the modification balance of LAT, a critical adaptor in TCR signaling, thereby impairing T cell proliferation and differentiation. Similarly, in aged B cells, dysregulated expression of PARylation-related factors reduced their ability to produce high-affinity antibodies.Moreover, PARylation also modulated macrophage metabolic polarization. In aging, abnormal PARP activity enhanced glycolysis in pro-inflammatory M1 macrophages and suppressed the anti-inflammatory functions of M2 macrophages [220]. Notably, PARylation was implicated in the regulation of DNA methylation patterns and metabolic homeostasis, with its overactivation associated with disrupted glucose metabolism and the development of type 2 diabetes [221].Taken together, PARylation contributed to immunosenescence through multiple dimensions, including immune cell activation, metabolic reprogramming, and epigenetic regulation, offering new mechanistic insights and potential therapeutic avenues for delaying immune aging and preventing age-related diseases.
Therapeutic strategies targeting post-translational modifications in Immunosenescence
During immunosenescence, PTMs—including histone methylation, acetylation, ubiquitination, and O-GlcNAcylation—serve as pivotal regulators that influence both modulating gene expression and proteostasis, while also promoting pathological processes such as inflammatory activation and cellular functional decline. Targeting these modifications presents a promising avenue for anti-aging interventions(Table 3).
Table 3.
Potential treatments targeting histone modifcations in Immunosenescence
| Potential Target | Mechanism | Therapeutic Strategy | Efficacy | References |
|---|---|---|---|---|
| Methylation | Inhibits KMT2D and reduces H3K4me1 | High - intensity interval training (HIIT) | Blocks the cycle of lipid metabolic disorder and inflammation | [222] |
| Enhances H3K9me3/H3K27me3 modification | Dasatinib + Quercetin | Reduces inflammation and improves cognitive function | [223] | |
| Enhances H4R3me2as modification | Metformin, Rapamycin, Resveratrol | Regulates SASP and anti - apoptotic genes | [225] | |
| Acetylation | Inhibits HDAC1/2 and enhances H3K9 and H4 acetylation | Trichostatin A (TSA), Suberoylanilide hydroxamic acid (SAHA) | Restores EC - SOD expression and inhibits ROS | [226] |
| Inhibits H3K9/14 acetylation | TSA | Anti - fibrotic and inhibits SASP | [227] | |
| Regulates H3 acetylation | Honokiol (HNK) | Inhibits NF - κB and promotes IL - 10 | [228] | |
| Inhibits HDAC3 | Chitinase 1 (CHIT1) | Inhibits neuroinflammation and improves cognition | [229] | |
| Inhibits HDAC | Valproic acid (VPA) | Alleviates diabetes - related aging and SASP phenotypes | [230] | |
| Activates SIRT1/SIRT6 | SIRT activators | Inhibits COPD immune aging and provides vascular protection | [189, 231] | |
| Ubiquitination | Upregulates CHIP and promotes MYH9 ubiquitination | 4,5 - Dicaffeoylquinic acid | Inhibits mitochondria and SASP | [13] |
| Upregulates MARCHF3 and promotes NLRP3 ubiquitination | Bushen Huoxue acupuncture | Inhibits pyroptosis and inflammatory factors and improves AD | [234] | |
| Promotes K48 ubiquitination of p65 | Friedelin(FR) | Inhibits NF - κB and alleviates tendon degeneration | [235] | |
| O-GlcNAcylation | Inhibits OGA and enhances O - GlcNAcylation | Thiamet-G | Reduces tau pathology and slows down neuroinflammation | [17] |
| Enhances O - GlcNAcylation | Thiamet-G | Enhances the utilization of UDP - GlcNAc | [216] |
Histone methylation serves as a central epigenetic regulator with substantial therapeutic potential. High-intensity interval training (HIIT) has been demonstrated to downregulate KMT2D and its target gene IDI1, thereby suppressing H3K4me1-mediated activation of lipid metabolism-related promoters and disrupting the vicious cycle between metabolic dysregulation and chronic inflammation [222]. Notably, senolytic drug combinations (e.g., dasatinib + quercetin) modulate the H3K9me3/H3K27me3 landscape, attenuating systemic inflammation and improving cognitive function in aging models [223]. The EZH2 inhibitor EPZ6438 (Tazemetostat) has been shown to alleviate age-related decline of interstitial cells of Cajal (ICCs) in the mouse stomach [224]. Additionally, PRMT1-catalyzed H4R3me2a modification inhibits PA200-mediated H4 degradation. Intriguingly, anti-aging agents such as metformin, rapamycin, and resveratrol restore H4R3me2a levels, stabilize nucleosome architecture, and regulate SASP-related and anti-apoptotic gene expression, offering novel strategies for epigenetic-based senescence intervention [225].
As a fundamental epigenetic mechanism, histone acetylation exhibits broad therapeutic effects in aging modulation. HDAC1/2 inhibitors (e.g., trichostatin(TSA) and suberoylanilide hydroxamic acid(SAHA)) restored acetylation at H3K9 and multiple H4 residues, counteracting ROS accumulation and inflammation caused by EC-SOD downregulation in aged pulmonary fibroblasts [226]. Moreover, TSA suppressed SASP gene activation by reducing H3K9/14 hyperacetylation at promoter regions, demonstrating anti-fibrotic efficacy in Duchenne muscular dystrophy(DMD) [227]. Honokiol modulated H3 acetylation and γ-H2AX, inhibiting NF-κB while upregulating IL-10, thereby conferring dual anti-inflammatory and neuroprotective benefits in neurodegenerative disorders [228]. Chitinase 1 (CHIT1) ameliorated cognitive deficits and neuroinflammation via HDAC3 inhibition [229]. Valproic acid (VPA) mitigated diabetes-associated cellular senescence and SASP by suppressing C5a receptor expression, expanding the clinical applicability of HDAC inhibitors [230]. Furthermore, class III HDACs (e.g., SIRT1/SIRT6) regulated histone acetylation to counteract lymphocyte senescence in COPD and conferred vascular protection [189, 231].A clinical study demonstrated that time-restricted feeding (TRF) enhanced the expression of the deacetylase SIRT1 and promoted autophagy, thereby exerting anti-aging effects in humans [232]. Although nicotinamide, a class III HDAC inhibitor, was shown to improve cognitive function in Alzheimer’s disease (AD) mouse models, a phase 2a proof-of-concept clinical trial indicated that further development of nicotinamide as a potential intervention for AD must address challenges related to its limited bioavailability and rapid methylation metabolism [233].
As a critical component of the protein degradation system, ubiquitination modulated inflammatory signaling and aberrant protein clearance. In osteoarthritis (OA), DPP4 bound MYH9, impeding its CHIP-mediated ubiquitination and degradation, thereby exacerbating mitochondrial fission and SASP activation. The small-molecule 4,5-dicaffeoylquinic acid disrupted this interaction, restoring ubiquitin-proteasome function and alleviating cartilage degeneration [13]. In AD, the herbal compound “Bushen Huoxue Zhen” upregulated the E3 ligase MARCHF3, promoting NLRP3 ubiquitination and degradation, which suppressed pyroptosis and reduced IL-1β/IL-18 release, ultimately ameliorating neuroinflammation [234]. The natural triterpenoid Friedelin recruited RNF182 to facilitate K48-linked ubiquitination and autophagic clearance of p65, blocking NF-κB activation and attenuating tendinopathy-associated senescence [235].
O-GlcNAcylation, a nutrient and stress-responsive PTM, exerted dual roles in age-related neuropathology. For instance, the OGA inhibitor Thiamet-G enhanced O-GlcNAcylation in AD, reducing tau pathology and neuroinflammation [17]. Ischemia-induced O-GlcNAcylation impairment in the aging brain was linked to insufficient UDP-GlcNAc availability; however, Thiamet-G treatment improved neurofunctional outcomes following transient cerebral ischemia in both juvenile and senescent animal models [216].
Conclusion and future perspectives
Epigenetic modifications play a multifaceted role in immune-inflammatory responses. Among these, histone modifications constitute a critical regulatory mechanism governing immune cell function during immunosenescence and age-related pathologies. With advancing research in this field, novel histone modifications closely associated with aging continue to be identified. Diverse experimental approaches have been employed to elucidate the functional implications of these modifications. Consequently, therapeutic strategies targeting histone-modifying enzymes have been developed and are currently under evaluation in both preclinical and clinical settings. Nevertheless, the current understanding of histone modifications remains incomplete, leaving numerous unresolved questions that warrant further investigation. Future therapeutic interventions aimed at modulating histone modifications to maintain immune homeostasis may hold significant clinical promise. In summary, deciphering the role of histone modifications in aging-associated immune inflammation is essential for unraveling the mechanisms of immunosenescence and developing novel treatment modalities.
Acknowledgements
Not applicable.
Author contributions
The completion of this manuscript is due to the close cooperation and in-depth discussion of all the authors. L.Q. and S.S. were responsible for the overall conception and framework design of the article. J.X., X.Q. and W.C. wrote the first draft of the manuscript and were responsible for drawing the charts. X.Q. and Y.M. revised the content of the first draft. W.H., H.O and Y.B. revised the language of the first draft. All the authors reviewed every aspect of the manuscript and approved the version as submitted.
Funding
This article was supported by the National Natural Science Foundation of China (No. 82302365), Natural Science Foundation of Hubei Province, China (No. 2024AFB517), Hubei University of Science and Technology Development Fund Project (No. BK202442) and Foundation of Hubei University of Science and Technology Science “Special Project on Diabetes and Angiopathy” (No.2024TNB05).
Data availability
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that there is no conflict of interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jiaqi Xiao, Xuan Qin and WenTao Chen contributed equally to this work.
Contributor Information
Lihua Qu, Email: lihuaqu@whu.edu.cn.
Shigang Shan, Email: ssgang121@163.com.
References
- 1.Zhang L, Pitcher LE, Yousefzadeh MJ, Niedernhofer LJ, Robbins PD, Zhu Y. Cellular senescence: a key therapeutic target in aging and diseases. J Clin Invest. 2022; 132(15). [DOI] [PMC free article] [PubMed]
- 2.López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. Hallmarks of aging: an expanding universe. Cell. 2023;186(2):243–78. [DOI] [PubMed] [Google Scholar]
- 3.Moqri M, Herzog C, Poganik JR, Ying K, Justice JN, Belsky DW, Higgins-Chen AT, Chen BH, Cohen AA, Fuellen G, et al. Validation of biomarkers of aging. Nat Med. 2024;30(2):360–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang K, Liu H, Hu Q, Wang L, Liu J, Zheng Z, Zhang W, Ren J, Zhu F, Liu GH. Epigenetic regulation of aging: implications for interventions of aging and diseases. Signal Transduct Target Ther. 2022;7(1):374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu Z, Liang Q, Ren Y, Guo C, Ge X, Wang L, Cheng Q, Luo P, Zhang Y, Han X. Immunosenescence: molecular mechanisms and diseases. Signal Transduct Target Ther. 2023;8(1):200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fane M, Weeraratna AT. How the ageing microenvironment influences tumour progression. Nat Rev Cancer. 2020;20(2):89–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Solana R, Tarazona R, Gayoso I, Lesur O, Dupuis G, Fulop T. Innate immunosenescence: effect of aging on cells and receptors of the innate immune system in humans. Semin Immunol. 2012;24(5):331–41. [DOI] [PubMed] [Google Scholar]
- 8.Soto-Palma C, Niedernhofer LJ, Faulk CD, Dong X. Epigenetics, DNA damage, and aging. J Clin Invest 2022; 132(16). [DOI] [PMC free article] [PubMed]
- 9.Saul D, Kosinsky RL. Epigenetics of aging and aging-Associated diseases. Int J Mol Sci 2021; 22(1). [DOI] [PMC free article] [PubMed]
- 10.Patin E, Hasan M, Bergstedt J, Rouilly V, Libri V, Urrutia A, Alanio C, Scepanovic P, Hammer C, Jönsson F, et al. Natural variation in the parameters of innate immune cells is preferentially driven by genetic factors. Nat Immunol. 2018;19(3):302–14. [DOI] [PubMed] [Google Scholar]
- 11.Yi X, Jiang XJ, Li XY, Jiang DS. Histone methyltransferases: novel targets for tumor and developmental defects. Am J Transl Res. 2015;7(11):2159–75. [PMC free article] [PubMed] [Google Scholar]
- 12.Jiang W, Zhang LX, Tan XY, Yu P, Dong M. Inflammation and histone modification in chronic pain. Front Immunol. 2022;13:1087648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li X, Zhang Z, Jiang W, Ju Y, Guo W, Huang Z. Dipeptidyl peptidase 4 (DPP4) exacerbates osteoarthritis progression in an Enzyme-Independent manner. Adv Sci (Weinh). 2025;12(6):e2410525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen K, Chen J, Wang L, Yang J, Xiao F, Wang X, Yuan J, Wang L, He Y. Parkin ubiquitinates GATA4 and attenuates the GATA4/GAS1 signaling and detrimental effects on diabetic nephropathy. Faseb J. 2020;34(7):8858–75. [DOI] [PubMed] [Google Scholar]
- 15.Xia B, Chen H, Taleb SJ, Xi X, Shaheen N, Baoyinna B, Soni S, Mebratu YA, Yount JS, Zhao J, Zhao Y. FBXL19 in endothelial cells protects the heart from influenza A infection by enhancing antiviral immunity and reducing cellular senescence programs. Am J Physiol Heart Circ Physiol. 2024;327(4):H937–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li H, Liu X, Wang D, Su L, Zhao T, Li Z, Lin C, Zhang Y, Huang B, Lu J, Li X. O-GlcNAcylation of SKN-1 modulates the lifespan and oxidative stress resistance in caenorhabditis elegans. Sci Rep. 2017;7:43601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bell MB, Kane MS, Ouyang X, Young ME, Jegga AG, Chatham JC, Darley-Usmar V, Zhang J. Brain transcriptome changes associated with an acute increase of protein O-GlcNAcylation and implications for neurodegenerative disease. J Neurochem. 2025;169(1):e16302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lian J, Yue Y, Yu W, Zhang Y. Immunosenescence: a key player in cancer development. J Hematol Oncol. 2020;13(1):151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barbé-Tuana F, Funchal G, Schmitz CRR, Maurmann RM, Bauer ME. The interplay between Immunosenescence and age-related diseases. Semin Immunopathol. 2020;42(5):545–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou D, Borsa M, Simon AK. Hallmarks and detection techniques of cellular senescence and cellular ageing in immune cells. Aging Cell. 2021;20(2):e13316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baran-Gale J, Morgan MD, Maio S, Dhalla F, Calvo-Asensio I, Deadman ME, Handel AE, Maynard A, Chen S, Green F et al. Ageing compromises mouse thymus function and remodels epithelial cell differentiation. Elife 2020; 9. [DOI] [PMC free article] [PubMed]
- 22.Luo M, Xu L, Qian Z, Sun X. Infection-Associated thymic atrophy. Front Immunol. 2021;12:652538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li YR, Zúñiga-Pflücker JC. Thymus aging and immune reconstitution, progresses and challenges. Semin Immunol. 2023;70:101837. [DOI] [PubMed] [Google Scholar]
- 24.Duah M, Li L, Shen J, Lan Q, Pan B, Xu K. Thymus degeneration and regeneration. Front Immunol. 2021;12:706244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bremer SJ, Boxnick A, Glau L, Biermann D, Joosse SA, Thiele F, Billeb E, May J, Kolster M, Hackbusch R, et al. Thymic atrophy and immune dysregulation in infants with complex congenital heart disease. J Clin Immunol. 2024;44(3):69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Frasca D, Blomberg BB. Inflammaging decreases adaptive and innate immune responses in mice and humans. Biogerontology. 2016;17(1):7–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fulop T, Larbi A, Dupuis G, Le Page A, Frost EH, Cohen AA, Witkowski JM, Franceschi C. Immunosenescence and Inflamm-Aging as two sides of the same coin: friends or foes?? Front Immunol. 2017;8:1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bauer ME, Fuente Mde L. The role of oxidative and inflammatory stress and persistent viral infections in Immunosenescence. Mech Ageing Dev. 2016;158:27–37. [DOI] [PubMed] [Google Scholar]
- 29.Martínez de Toda I, Ceprián N, Díaz-Del Cerro E. De La Fuente M. The role of immune cells in Oxi-Inflamm-Aging. Cells 2021; 10(11). [DOI] [PMC free article] [PubMed]
- 30.Vida C, González EM, De la Fuente M. Increase of oxidation and inflammation in nervous and immune systems with aging and anxiety. Curr Pharm Des. 2014;20(29):4656–78. [DOI] [PubMed] [Google Scholar]
- 31.De la Fuente M, Miquel J. An update of the oxidation-inflammation theory of aging: the involvement of the immune system in oxi-inflamm-aging. Curr Pharm Des. 2009;15(26):3003–26. [DOI] [PubMed] [Google Scholar]
- 32.Federico A, Morgillo F, Tuccillo C, Ciardiello F, Loguercio C. Chronic inflammation and oxidative stress in human carcinogenesis. Int J Cancer. 2007;121(11):2381–6. [DOI] [PubMed] [Google Scholar]
- 33.Chandrasekaran A, Idelchik M, Melendez JA. Redox control of senescence and age-related disease. Redox Biol. 2017;11:91–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guo J, Huang X, Dou L, Yan M, Shen T, Tang W, Li J. Aging and aging-related diseases: from molecular mechanisms to interventions and treatments. Signal Transduct Target Ther. 2022;7(1):391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li X, Li C, Zhang W, Wang Y, Qian P, Huang H. Inflammation and aging: signaling pathways and intervention therapies. Signal Transduct Target Ther. 2023;8(1):239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Byun HO, Lee YK, Kim JM, Yoon G. From cell senescence to age-related diseases: differential mechanisms of action of senescence-associated secretory phenotypes. BMB Rep. 2015;48(10):549–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schultze JL, Aschenbrenner AC. COVID-19 and the human innate immune system. Cell. 2021;184(7):1671–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fang ZE, Wang Y, Bian S, Qin S, Zhao H, Wen J, Liu T, Ren L, Li Q, Shi W, et al. Helenine blocks NLRP3 activation by disrupting the NEK7-NLRP3 interaction and ameliorates inflammatory diseases. Phytomedicine. 2024;122:155159. [DOI] [PubMed] [Google Scholar]
- 39.Sette A, Crotty S. Adaptive immunity to SARS-CoV-2 and COVID-19. Cell. 2021;184(4):861–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pawelec G. Age and immunity: what is immunosenescence? Exp Gerontol. 2018;105:4–9. [DOI] [PubMed] [Google Scholar]
- 41.Tu H, Ren H, Jiang J, Shao C, Shi Y, Li P. Dying to defend: neutrophil death pathways and their implications in immunity. Adv Sci (Weinh). 2024;11(8):e2306457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Geh D, Leslie J, Rumney R, Reeves HL, Bird TG, Mann DA. Neutrophils as potential therapeutic targets in hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2022;19(4):257–73. [DOI] [PubMed] [Google Scholar]
- 43.Tseng CW, Liu GY. Expanding roles of neutrophils in aging hosts. Curr Opin Immunol. 2014;29:43–8. [DOI] [PubMed] [Google Scholar]
- 44.He W, Xiao K, Fang M, Xie L. Immune cell number, phenotype, and function in the elderly with sepsis. Aging Dis. 2021;12(1):277–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Demaret J, Venet F, Friggeri A, Cazalis MA, Plassais J, Jallades L, Malcus C, Poitevin-Later F, Textoris J, Lepape A, Monneret G. Marked alterations of neutrophil functions during sepsis-induced immunosuppression. J Leukoc Biol. 2015;98(6):1081–90. [DOI] [PubMed] [Google Scholar]
- 46.Grudzinska FS, Brodlie M, Scholefield BR, Jackson T, Scott A, Thickett DR, Sapey E. Neutrophils in community-acquired pneumonia: parallels in dysfunction at the extremes of age. Thorax. 2020;75(2):164–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sauce D, Dong Y, Campillo-Gimenez L, Casulli S, Bayard C, Autran B, Boddaert J, Appay V, Elbim C. Reduced oxidative burst by primed neutrophils in the elderly individuals is associated with increased levels of the CD16bright/CD62Ldim immunosuppressive subset. J Gerontol Biol Sci Med Sci. 2017;72(2):163–72. [DOI] [PubMed] [Google Scholar]
- 48.Yang C, Wang Z, Li L, Zhang Z, Jin X, Wu P, Sun S, Pan J, Su K, Jia F et al. Aged neutrophils form mitochondria-dependent vital NETs to promote breast cancer lung metastasis. J Immunother Cancer 2021; 9(10). [DOI] [PMC free article] [PubMed]
- 49.Moreno de Lara L, Werner A, Borchers A, Carrillo-Salinas FJ, Marmol W, Parthasarathy S, Iyer V, Vogell A, Illanes D, Abadía-Molina AC, et al. Aging dysregulates neutrophil extracellular trap formation in response to HIV in blood and genital tissues. Front Immunol. 2023;14:1256182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sapey E, Greenwood H, Walton G, Mann E, Love A, Aaronson N, Insall RH, Stockley RA, Lord JM. Phosphoinositide 3-kinase Inhibition restores neutrophil accuracy in the elderly: toward targeted treatments for Immunosenescence. Blood. 2014;123(2):239–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fulop T, Larbi A, Douziech N, Fortin C, Guérard KP, Lesur O, Khalil A, Dupuis G. Signal transduction and functional changes in neutrophils with aging. Aging Cell. 2004;3(4):217–26. [DOI] [PubMed] [Google Scholar]
- 52.Barkaway A, Rolas L, Joulia R, Bodkin J, Lenn T, Owen-Woods C, Reglero-Real N, Stein M, Vázquez-Martínez L, Girbl T, et al. Age-related changes in the local milieu of inflamed tissues cause aberrant neutrophil trafficking and subsequent remote organ damage. Immunity. 2021;54(7):1494–e15101497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rolas L, Stein M, Barkaway A, Reglero-Real N, Sciacca E, Yaseen M, Wang H, Vazquez-Martinez L, Golding M, Blacksell IA, et al. Senescent endothelial cells promote pathogenic neutrophil trafficking in inflamed tissues. EMBO Rep. 2024;25(9):3842–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Myers JA, Miller JS. Exploring the NK cell platform for cancer immunotherapy. Nat Rev Clin Oncol. 2021;18(2):85–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vivier E, Rebuffet L, Narni-Mancinelli E, Cornen S, Igarashi RY, Fantin VR. Natural killer cell therapies. Nature. 2024;626(8000):727–36. [DOI] [PubMed] [Google Scholar]
- 56.Campos C, Pera A, Sanchez-Correa B, Alonso C, Lopez-Fernandez I, Morgado S, Tarazona R, Solana R. Effect of age and CMV on NK cell subpopulations. Exp Gerontol. 2014;54:130–7. [DOI] [PubMed] [Google Scholar]
- 57.Gounder SS, Abdullah BJJ, Radzuanb N, Zain F, Sait NBM, Chua C, Subramani B. Effect of Aging on NK Cell Population and Their Proliferation at Ex Vivo Culture Condition. Anal Cell Pathol (Amst). 2018; 2018: 7871814. [DOI] [PMC free article] [PubMed]
- 58.Hou J, Xie S, Gao J, Jiang T, Zhu E, Yang X, Jin Z, Long H, Zhang A, Yang F, et al. NK cell transfer overcomes resistance to PD-(L)1 therapy in aged mice. Exp Hematol Oncol. 2024;13(1):48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jain SS, Barefoot ME, Varghese RS, Ressom HW. Cell-type Deconvolution and Age Estimation Using DNA Methylation Reveals NK Cell Deficiency in the Hepatocellular Carcinoma Microenvironment. Proceedings (IEEE Int Conf Bioinformatics Biomed). 2022; 2022: 444–449. [DOI] [PMC free article] [PubMed]
- 60.Degos C, Heinemann M, Barrou J, Boucherit N, Lambaudie E, Savina A, Gorvel L, Olive D. Endometrial tumor microenvironment alters human NK cell recruitment, and resident NK cell phenotype and function. Front Immunol. 2019;10:877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rodrigues-Santos P, López-Sejas N, Almeida JS, Ruzičková L, Couceiro P, Alves V, Campos C, Alonso C, Tarazona R, Freitas-Tavares P, et al. Effect of age on NK cell compartment in chronic myeloid leukemia patients treated with tyrosine kinase inhibitors. Front Immunol. 2018;9:2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Deng M, Zeng Y, Liu Y, Wang X, Chen N, Zhang M, Jiang M, Zhao H, Du J. Increased PD-1(+) NK cell subset in the older population. Int J Gen Med. 2024;17:651–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chen S, Li Z, Feng J, Quan Y, He J, Hao J, Dong Z. Dual activity of type III PI3K kinase Vps34 is critical for NK cell development and senescence. Adv Sci (Weinh). 2024;11(21):e2309315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Solana R, Mariani E. NK and NK/T cells in human senescence. Vaccine. 2000;18(16):1613–20. [DOI] [PubMed] [Google Scholar]
- 65.Liang C, Song R, Zhang J, Yao J, Guan Z, Zeng X. Melatonin enhances NK cell function in aged mice by increasing T-bet expression via the JAK3-STAT5 signaling pathway. Immun Ageing. 2024;21(1):59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Elsaid AF, Shaheen M, Ghoneum M. Biobran/MGN-3, an arabinoxylan rice bran, enhances NK cell activity in geriatric subjects: A randomized, double-blind, placebo-controlled clinical trial. Exp Ther Med. 2018;15(3):2313–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lee H, Ahn YT, Park SH, Park DY, Jin YW, Kim CS, Sung SH, Huh CS, Kim DH. Lactobacillus plantarum HY7712 protects against the impairment of NK-cell activity caused by whole-body γ-irradiation in mice. J Microbiol Biotechnol. 2014;24(1):127–31. [DOI] [PubMed] [Google Scholar]
- 68.Deng X, Terunuma H. Adoptive NK cell therapy: a potential revolutionary approach in longevity therapeutics. Immun Ageing. 2024;21(1):43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tang X, Deng B, Zang A, He X, Zhou Y, Wang D, Li D, Dai X, Chen J, Zhang X, et al. Characterization of age-related immune features after autologous NK cell infusion: protocol for an open-label and randomized controlled trial. Front Immunol. 2022;13:940577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bai Z, Yang P, Yu F, Li Z, Yao Z, Martinez J, Li M, Xu H. Combining adoptive NK cell infusion with a dopamine-releasing peptide reduces senescent cells in aged mice. Cell Death Dis. 2022;13(4):305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Khan AH, Mulfaul K. Choroidal macrophages in homeostasis, aging and age-related macular degeneration. Exp Eye Res. 2025;250:110159. [DOI] [PubMed] [Google Scholar]
- 72.De Maeyer RPH, Chambers ES. The impact of ageing on monocytes and macrophages. Immunol Lett. 2021;230:1–10. [DOI] [PubMed] [Google Scholar]
- 73.Mitsuhashi M, Taub DD, Kapogiannis D, Eitan E, Zukley L, Mattson MP, Ferrucci L, Schwartz JB, Goetzl EJ. Aging enhances release of Exosomal cytokine mRNAs by Aβ1-42-stimulated macrophages. Faseb J. 2013;27(12):5141–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Vida C, de Toda IM, Cruces J, Garrido A, Gonzalez-Sanchez M, De la Fuente M. Role of macrophages in age-related oxidative stress and Lipofuscin accumulation in mice. Redox Biol. 2017;12:423–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Suchy D, Łabuzek K, Bułdak Ł, Szkudłapski D, Okopień B. Comparison of chosen activation markers of human monocytes/macrophages isolated from the peripheral blood of young and elderly volunteers. Pharmacol Rep. 2014;66(5):759–65. [DOI] [PubMed] [Google Scholar]
- 76.Blacher E, Tsai C, Litichevskiy L, Shipony Z, Iweka CA, Schneider KM, Chuluun B, Heller HC, Menon V, Thaiss CA, Andreasson KI. Aging disrupts circadian gene regulation and function in macrophages. Nat Immunol. 2022;23(2):229–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pappert M, Khosla S, Doolittle M. Influences of aged bone marrow macrophages on skeletal health and senescence. Curr Osteoporos Rep. 2023;21(6):771–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Guimarães GR, Almeida PP, de Oliveira Santos L, Rodrigues LP, de Carvalho JL, Boroni M. Hallmarks of aging in macrophages: consequences to skin inflammaging. Cells. 2021; 10(6). [DOI] [PMC free article] [PubMed]
- 79.Rosmus DD, Koch J, Hausmann A, Chiot A, Arnhold F, Masuda T, Kierdorf K, Hansen SM, Kuhrt H, Fröba J, et al. Redefining the ontogeny of hyalocytes as yolk sac-derived tissue-resident macrophages of the vitreous body. J Neuroinflammation. 2024;21(1):168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Liu L, Yue X, Sun Z, Hambright WS, Feng Q, Cui Y, Huard J, Robbins PD, Wang Z, Mu X. Senolytic elimination of senescent macrophages restores muscle stem cell function in severely dystrophic muscle. Aging. 2022;14(19):7650–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhou Z, Yao J, Wu D, Huang X, Wang Y, Li X, Lu Q, Qiu Y. Type 2 cytokine signaling in macrophages protects from cellular senescence and organismal aging. Immunity. 2024;57(3):513–e527516. [DOI] [PubMed] [Google Scholar]
- 82.Deng Y, Gao H, Wu Q. T-2 toxin induces Immunosenescence in RAW264.7 macrophages by activating the HIF-1α/cGAS-STING pathway. J Agric Food Chem. 2024;72(43):24046–57. [DOI] [PubMed] [Google Scholar]
- 83.Stranks AJ, Hansen AL, Panse I, Mortensen M, Ferguson DJ, Puleston DJ, Shenderov K, Watson AS, Veldhoen M, Phadwal K, et al. Autophagy controls acquisition of aging features in macrophages. J Innate Immun. 2015;7(4):375–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Shirakawa K, Sano M. T cell Immunosenescence in aging, obesity, and cardiovascular disease. Cells 2021; 10(9). [DOI] [PMC free article] [PubMed]
- 85.Goronzy JJ, Weyand CM. Mechanisms underlying T cell ageing. Nat Rev Immunol. 2019;19(9):573–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gao Y, Lu Y, Liang X, Zhao M, Yu X, Fu H, Yang W. CD4(+) T-Cell senescence in neurodegenerative disease: pathogenesis and potential therapeutic targets. Cells 2024; 13(9). [DOI] [PMC free article] [PubMed]
- 87.Snijckers RPM, Foks AC. Adaptive immunity and atherosclerosis: aging at its crossroads. Front Immunol. 2024;15:1350471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sharabi A, Tsokos GC. T cell metabolism: new insights in systemic lupus erythematosus pathogenesis and therapy. Nat Rev Rheumatol. 2020;16(2):100–12. [DOI] [PubMed] [Google Scholar]
- 89.Khan S, Chakraborty M, Wu F, Chen N, Wang T, Chan YT, Sayad A, Vásquez JDS, Kotlyar M, Nguyen K et al. B cells promote T cell Immunosenescence and mammalian aging parameters. bioRxiv. 2023.
- 90.Soto-Heredero G, Gómez de Las Heras MM, Escrig-Larena JI, Mittelbrunn M. Extremely differentiated T cell subsets contribute to tissue deterioration during aging. Annu Rev Immunol. 2023;41:181–205. [DOI] [PubMed] [Google Scholar]
- 91.Álvarez-Heredia P, Reina-Alfonso I, Domínguez-Del-Castillo JJ, Gutiérrez-González C, Hassouneh F, Batista-Duharte A, Pérez AB, Tarazona R, Solana R, Pera A. Accelerated T-Cell Immunosenescence in Cytomegalovirus-Seropositive individuals after severe acute respiratory syndrome coronavirus 2 infection. J Infect Dis. 2023;228(5):576–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Frasca D, Diaz A, Romero M, Garcia D, Blomberg BB. B cell Immunosenescence. Annu Rev Cell Dev Biol. 2020;36:551–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.De Biasi S, Ciobanu AL, Santacroce E, Lo Tartaro D, Degliesposti G, D’Angerio M, Leccese M, Cardi M, Trenti T, Cuccorese M et al. SARS-CoV-2 vaccination responses in Anti-CD20-Treated progressive multiple sclerosis patients show Immunosenescence in Antigen-Specific B and T cells. Vaccines (Basel). 2024; 12(8). [DOI] [PMC free article] [PubMed]
- 94.Frasca D, Blomberg BB. Obesity accelerates age defects in mouse and human B cells. Front Immunol. 2020;11:2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Frasca D, Romero M, Garcia D, Diaz A, Blomberg BB. Obesity accelerates Age-Associated defects in human B cells through a metabolic reprogramming induced by the fatty acid palmitate. Front Aging. 2021;2:828697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hopkin SJ, Pezhman L, Begum J, Kavanagh D, McGettrick HM, Iqbal AJ, Chimen M. Aging modulates homeostatic leukocyte trafficking to the peritoneal cavity in a sex-specific manner. J Leukoc Biol. 2023;114(4):301–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kannan S, Kurupati RK, Doyle SA, Freeman GJ, Schmader KE, Ertl HC. BTLA expression declines on B cells of the aged and is associated with low responsiveness to the trivalent influenza vaccine. Oncotarget. 2015;6(23):19445–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Dema M, Eixarch H, Hervera A, Castillo M, Villar LM, Montalban X, Espejo C. Disease aggravation with age in an experimental model of multiple sclerosis: role of Immunosenescence. Aging Cell. 2025;24(5):e14491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Jiang J, Yang M, Yang B, Wu H, Lu Q. Elevated IL-15 levels in systemic lupus erythematosus: potential pathogenesis insight and therapeutic target. Int Immunopharmacol. 2024;142(Pt A):112973. [DOI] [PubMed] [Google Scholar]
- 100.Lau V, Ramer L, Tremblay M. An aging, pathology burden, and glial senescence build-up hypothesis for late onset alzheimer’s disease. Nat Commun. 2023;14(1):1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Fu J, Huang Y, Bao T, Liu C, Liu X, Chen X. The role of Th17 cells/IL-17A in AD, PD, ALS and the strategic therapy targeting on IL-17A. J Neuroinflammation. 2022;19(1):98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gu Z, Zhao H, Song Y, Kou Y, Yang W, Li Y, Li X, Ding L, Sun Z, Lin J, et al. PEGylated-liposomal Astaxanthin ameliorates Aβ neurotoxicity and Alzheimer-related phenotypes by scavenging formaldehyde. J Control Release. 2024;366:783–97. [DOI] [PubMed] [Google Scholar]
- 103.Sharma R, Kumar R, Sharma A, Goel A, Padwad Y. Long-term consumption of green tea EGCG enhances murine health span by mitigating multiple aspects of cellular senescence in mitotic and post-mitotic tissues, gut dysbiosis, and Immunosenescence. J Nutr Biochem. 2022;107:109068. [DOI] [PubMed] [Google Scholar]
- 104.Theodorakis N, Feretzakis G, Hitas C, Kreouzi M, Kalantzi S, Spyridaki A, Kollia Z, Verykios VS, Nikolaou M. Immunosenescence: how aging increases susceptibility to bacterial infections and virulence factors. Microorganisms 2024; 12(10). [DOI] [PMC free article] [PubMed]
- 105.Soraci L, Beccacece A, Princiotto M, Villalta Savedra E, Gambuzza ME, Aguennouz M, Corsonello A, Luciani F, Muglia L, Filicetti E, et al. The emerging links between Immunosenescence in innate immune system and neurocryptococcosis. Front Immunol. 2024;15:1410090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Biagio P, Isabella DF, Federica C, Elena S, Ivan G. Alzheimer’s disease and herpes viruses: current events and perspectives. Rev Med Virol. 2024;34(3):e2550. [DOI] [PubMed] [Google Scholar]
- 107.Dow CT, Warm. Sweetened milk at the Twilight of Immunity - Alzheimer’s Disease - Inflammaging, insulin resistance, M. paratuberculosis and Immunosenescence. Front Immunol. 2021;12:714179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Shoemark DK, Allen SJ. The Microbiome and disease: reviewing the links between the oral Microbiome, aging, and alzheimer’s disease. J Alzheimers Dis. 2015;43(3):725–38. [DOI] [PubMed] [Google Scholar]
- 109.Lathe R, St Clair D. Programmed ageing: decline of stem cell renewal, immunosenescence, and alzheimer’s disease. Biol Rev Camb Philos Soc. 2023;98(4):1424–58. [DOI] [PubMed] [Google Scholar]
- 110.Wang Y, Cao X, Yang C, Fan J, Zhang X, Wu X, Guo W, Sun S, Liu M, Zhang L, Li T. Ferroptosis and Immunosenescence in colorectal cancer. Semin Cancer Biol. 2024;106–107:156–65. [DOI] [PubMed] [Google Scholar]
- 111.Wang C, Chen S, Guo H, Jiang H, Liu H, Fu H, Wang D, Forsythoside. A mitigates Alzheimer’s-like pathology by inhibiting Ferroptosis-mediated neuroinflammation via Nrf2/GPX4 axis activation. Int J Biol Sci. 2022;18(5):2075–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kaur D, Sharma V, Deshmukh R. Activation of microglia and astrocytes: a roadway to neuroinflammation and alzheimer’s disease. Inflammopharmacology. 2019;27(4):663–77. [DOI] [PubMed] [Google Scholar]
- 113.Wang RP, Huang J, Chan KWY, Leung WK, Goto T, Ho YS, Chang RC. IL-1β and TNF-α play an important role in modulating the risk of periodontitis and alzheimer’s disease. J Neuroinflammation. 2023;20(1):71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Propson NE, Roy ER, Litvinchuk A, Köhl J, Zheng H. Endothelial C3a receptor mediates vascular inflammation and blood-brain barrier permeability during aging. J Clin Invest 2021; 131(1). [DOI] [PMC free article] [PubMed]
- 115.Kesidou E, Theotokis P, Damianidou O, Boziki M, Konstantinidou N, Taloumtzis C, Sintila SA, Grigoriadis P, Evangelopoulos ME, Bakirtzis C, Simeonidou C. CNS ageing in health and neurodegenerative disorders. J Clin Med. 2023; 12(6). [DOI] [PMC free article] [PubMed]
- 116.Komleva Y, Chernykh A, Lopatina O, Gorina Y, Lokteva I, Salmina A, Gollasch M. Inflamm-Aging and brain insulin resistance: new insights and role of Life-style strategies on cognitive and social determinants in aging and neurodegeneration. Front Neurosci. 2020;14:618395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Mulak A, Bonaz B. Brain-gut-microbiota axis in parkinson’s disease. World J Gastroenterol. 2015;21(37):10609–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.O’Donovan SM, Crowley EK, Brown JR, O’Sullivan O, O’Leary OF, Timmons S, Nolan YM, Clarke DJ, Hyland NP, Joyce SA, et al. Nigral overexpression of α-synuclein in a rat parkinson’s disease model indicates alterations in the enteric nervous system and the gut Microbiome. Neurogastroenterol Motil. 2020;32(1):e13726. [DOI] [PubMed] [Google Scholar]
- 119.Dogra N, Mani RJ, Katare DP. The Gut-Brain axis: two ways signaling in parkinson’s disease. Cell Mol Neurobiol. 2022;42(2):315–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Pike AF, Longhena F, Faustini G, van Eik JM, Gombert I, Herrebout MAC, Fayed M, Sandre M, Varanita T, Teunissen CE, et al. Dopamine signaling modulates microglial NLRP3 inflammasome activation: implications for parkinson’s disease. J Neuroinflammation. 2022;19(1):50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Liu Z, Qiu AW, Huang Y, Yang Y, Chen JN, Gu TT, Cao BB, Qiu YH, Peng YP. IL-17A exacerbates neuroinflammation and neurodegeneration by activating microglia in rodent models of parkinson’s disease. Brain Behav Immun. 2019;81:630–45. [DOI] [PubMed] [Google Scholar]
- 122.Montalbán-Rodríguez A, Abalo R, López-Gómez L. From the Gut to the Brain: The Role of Enteric Glial Cells and Their Involvement in the Pathogenesis of Parkinson’s Disease. Int J Mol Sci 2024; 25(2). [DOI] [PMC free article] [PubMed]
- 123.Pellegrini C, Fornai M, D’Antongiovanni V, Antonioli L, Bernardini N, Derkinderen P. The intestinal barrier in disorders of the central nervous system. Lancet Gastroenterol Hepatol. 2023;8(1):66–80. [DOI] [PubMed] [Google Scholar]
- 124.Sellami M, Gasmi M, Denham J, Hayes LD, Stratton D, Padulo J, Bragazzi N. Effects of acute and chronic exercise on immunological parameters in the elderly aged: can physical activity counteract the effects of aging?? Front Immunol. 2018;9:2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Rodríguez-Goncer I, Fernández-Ruiz M, Aguado JM. A critical review of the relationship between post-transplant atherosclerotic events and cytomegalovirus exposure in kidney transplant recipients. Expert Rev Anti Infect Ther. 2020;18(2):113–25. [DOI] [PubMed] [Google Scholar]
- 126.Ghamar Talepoor A, Doroudchi M. Immunosenescence in atherosclerosis: A role for chronic viral infections. Front Immunol. 2022;13:945016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Laderoute M. The paradigm of Immunosenescence in atherosclerosis-cardiovascular disease (ASCVD). Discov Med. 2020;29(156):41–51. [PubMed] [Google Scholar]
- 128.Poch E, Carbonell P, Franco S, Díez-Juan A, Blasco MA, Andrés V. Short telomeres protect from diet-induced atherosclerosis in Apolipoprotein E-null mice. Faseb J. 2004;18(2):418–20. [DOI] [PubMed] [Google Scholar]
- 129.Smit V, de Mol J, Schaftenaar FH, Depuydt MAC, Postel RJ, Smeets D, Verheijen FWM, Bogers L, van Duijn J, Verwilligen RAF, et al. Single-cell profiling reveals age-associated immunity in atherosclerosis. Cardiovasc Res. 2023;119(15):2508–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Guarner V, Rubio-Ruiz ME. Low-grade systemic inflammation connects aging, metabolic syndrome and cardiovascular disease. Interdiscip Top Gerontol. 2015;40:99–106. [DOI] [PubMed] [Google Scholar]
- 131.Wagner JUG, Tombor LS, Malacarne PF, Kettenhausen LM, Panthel J, Kujundzic H, Manickam N, Schmitz K, Cipca M, Stilz KA, et al. Aging impairs the neurovascular interface in the heart. Science. 2023;381(6660):897–906. [DOI] [PubMed] [Google Scholar]
- 132.Tylutka A, Morawin B, Gramacki A, Zembron-Lacny A. Pre-Existing Hypertension Is Related with Disproportions in T-Lymphocytes in Older Age. J Clin Med. 2022; 11(2). [DOI] [PMC free article] [PubMed]
- 133.Yang Y, Fan L, Li M, Wang Z. Immune senescence: A key player in cancer biology. Semin Cancer Biol. 2025;108:71–82. [DOI] [PubMed] [Google Scholar]
- 134.Gu M, Liu Y, Zheng W, Jing Z, Li X, Guo W, Zhao Z, Yang X, Liu Z, Zhu X, Gao W. Combined targeting of senescent cells and senescent macrophages: A new Idea for integrated treatment of lung cancer. Semin Cancer Biol. 2024;106–107:43–57. [DOI] [PubMed] [Google Scholar]
- 135.Chen X, Wang Z, Zhu B, Deng M, Qiu J, Feng Y, Ding N, Huang C. Metabolic Reprogramming Induced by Aging Modifies the Tumor Microenvironment. Cells. 2024; 13(20). [DOI] [PMC free article] [PubMed]
- 136.Rodriguez JE, Naigeon M, Goldschmidt V, Roulleaux Dugage M, Seknazi L, Danlos FX, Champiat S, Marabelle A, Michot JM, Massard C, et al. Immunosenescence, inflammaging, and cancer immunotherapy efficacy. Expert Rev Anticancer Ther. 2022;22(9):915–26. [DOI] [PubMed] [Google Scholar]
- 137.Al-Danakh A, Safi M, Jian Y, Yang L, Zhu X, Chen Q, Yang K, Wang S, Zhang J, Yang D. Aging-related biomarker discovery in the era of immune checkpoint inhibitors for cancer patients. Front Immunol. 2024;15:1348189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Wang Y, Dong C, Han Y, Gu Z, Sun C. Immunosenescence, aging and successful aging. Front Immunol. 2022;13:942796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Amoriello R, Mariottini A, Ballerini C. Immunosenescence and autoimmunity: exploiting the T-Cell receptor repertoire to investigate the impact of aging on multiple sclerosis. Front Immunol. 2021;12:799380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ruan P, Wang S, Yang M, Wu H. The ABC-associated Immunosenescence and lifestyle interventions in autoimmune disease. Rheumatol Immunol Res. 2022;3(3):128–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Neațu M, Hera-Drăguț A, Ioniță I, Jugurt A, Davidescu EI, Popescu BO. Understanding the complex dynamics of Immunosenescence in multiple sclerosis: from pathogenesis to treatment. Biomedicines 2024; 12(8). [DOI] [PMC free article] [PubMed]
- 142.Aprilia A, Handono K, Sujuti H, Sabarudin A, Winaris N. sCD163, sCD28, sCD80, and sCTLA-4 as soluble marker candidates for detecting Immunosenescence. Immun Ageing. 2024;21(1):9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Talati M, Seeley E, Ihida-Stansbury K, Delisser H, McDonald H, Ye F, Zhang X, Shyr Y, Caprioli R, Meyrick B. Altered expression of nuclear and cytoplasmic histone H1 in pulmonary artery and pulmonary artery smooth muscle cells in patients with IPAH. Pulm Circ. 2012;2(3):340–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Ma Y, Lv W, Guo Y, Yin T, Bai Y, Liu Z, Chen C, WenjuanYang, Feng J, Qian W, et al. Histone demethylases in autophagy and inflammation. Cell Commun Signal. 2025;23(1):24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Maity S, Farrell K, Navabpour S, Narayanan SN, Jarome TJ. Epigenetic Mechanisms in Memory and Cognitive Decline Associated with Aging and Alzheimer’s Disease. Int J Mol Sci 2021; 22(22). [DOI] [PMC free article] [PubMed]
- 146.Kouzarides T. Chromatin modifications and their function. Cell. 2007;128(4):693–705. [DOI] [PubMed] [Google Scholar]
- 147.Bannister AJ, Kouzarides T. Reversing histone methylation. Nature. 2005;436(7054):1103–6. [DOI] [PubMed] [Google Scholar]
- 148.Michalak EM, Burr ML, Bannister AJ, Dawson MA. The roles of DNA, RNA and histone methylation in ageing and cancer. Nat Rev Mol Cell Biol. 2019;20(10):573–89. [DOI] [PubMed] [Google Scholar]
- 149.Gong F, Miller KM. Histone methylation and the DNA damage response. Mutat Res Rev Mutat Res. 2019;780:37–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Yang C, Zhang J, Ma Y, Wu C, Cui W, Wang L. Histone methyltransferase and drug resistance in cancers. J Exp Clin Cancer Res. 2020;39(1):173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Jambhekar A, Dhall A, Shi Y. Roles and regulation of histone methylation in animal development. Nat Rev Mol Cell Biol. 2019;20(10):625–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Wu O, Jin Y, Zhang Z, Zhou H, Xu W, Chen L, Jones M, Kwan KYH, Gao J, Zhang K, et al. KMT2A regulates the autophagy-GATA4 axis through METTL3-mediated m(6)A modification of ATG4a to promote NPCs senescence and IVDD progression. Bone Res. 2024;12(1):67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Xu J, Chen Q, Tian K, Liang R, Chen T, Gong A, Mathy NW, Yu T, Chen X. m6A methyltransferase METTL3 maintains colon cancer tumorigenicity by suppressing SOCS2 to promote cell proliferation. Oncol Rep. 2020;44(3):973–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Lim CY, Lin HT, Kumsta C, Lu TC, Wang FY, Kang YH, Hansen M, Ching TT, Hsu AL. SAMS-1 coordinates HLH-30/TFEB and PHA-4/FOXA activities through histone methylation to mediate dietary restriction-induced autophagy and longevity. Autophagy. 2023;19(1):224–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Yang D, Wei G, Long F, Nie H, Tian X, Qu L, Wang S, Li P, Qiu Y, Wang Y, et al. Histone methyltransferase Smyd3 is a new regulator for vascular senescence. Aging Cell. 2020;19(9):e13212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Sarkar TJ, Quarta M, Mukherjee S, Colville A, Paine P, Doan L, Tran CM, Chu CR, Horvath S, Qi LS, et al. Transient non-integrative expression of nuclear reprogramming factors promotes multifaceted amelioration of aging in human cells. Nat Commun. 2020;11(1):1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Grewal SIS. The molecular basis of heterochromatin assembly and epigenetic inheritance. Mol Cell. 2023;83(11):1767–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Wu Z, Qu J, Liu GH. Roles of chromatin and genome instability in cellular senescence and their relevance to ageing and related diseases. Nat Rev Mol Cell Biol. 2024;25(12):979–1000. [DOI] [PubMed] [Google Scholar]
- 159.Jin M, Li C, Wu Z, Tang Z, Xie J, Wei G, Yang Z, Huang S, Chen Y, Li X, et al. Inhibiting the histone demethylase Kdm4a restrains cardiac fibrosis after myocardial infarction by promoting autophagy in premature senescent fibroblasts. Adv Sci (Weinh). 2025;12(21):e2414830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Zhang F, Hu G, Chen X, Zhang L, Guo L, Li C, Zhao H, Cui Z, Guo X, Sun F, et al. Excessive branched-chain amino acid accumulation restricts mesenchymal stem cell-based therapy efficacy in myocardial infarction. Signal Transduct Target Ther. 2022;7(1):171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Chu L, Qu Y, An Y, Hou L, Li J, Li W, Fan G, Song BL, Li E, Zhang L, Qi W. Induction of senescence-associated secretory phenotype underlies the therapeutic efficacy of PRC2 Inhibition in cancer. Cell Death Dis. 2022;13(2):155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Ito T, Teo YV, Evans SA, Neretti N, Sedivy JM. Regulation of cellular senescence by polycomb chromatin modifiers through distinct DNA Damage- and histone Methylation-Dependent pathways. Cell Rep. 2018;22(13):3480–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Schwab N, Grenier K, Hazrati LN. DNA repair deficiency and senescence in concussed professional athletes involved in contact sports. Acta Neuropathol Commun. 2019;7(1):182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Ro S. Improving gastric motility in aging through EZH2 Inhibition and preservation of interstitial cells of Cajal. Cell Mol Gastroenterol Hepatol. 2024;18(5):101382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Lin R, Yin J, Huang J, Zou L, Liu L, Tang W, Zhang H, Yang L, Zhang Y, Li G, et al. Macrophage-derived ectosomal miR-350-3p promotes osteoarthritis progression through downregulating chondrocyte H3K36 methyltransferase NSD1. Cell Death Discov. 2024;10(1):223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Reintjes A, Fuchs JE, Kremser L, Lindner HH, Liedl KR, Huber LA, Valovka T. Asymmetric arginine dimethylation of rela provides a repressive mark to modulate TNFα/NF-κB response. Proc Natl Acad Sci U S A. 2016;113(16):4326–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.HK S, Kim H, Lee J, You CL, Yun CE, Jeong HJ, Jin EJ, Jo Y, Ryu D, Bae GU, Kang JS. Protein arginine methyltransferase 1 ablation in motor neurons causes mitochondrial dysfunction leading to Age-related motor neuron degeneration with muscle loss. Res (Wash D C). 2023;6:0158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Tran TTV, Jeong Y, Kim S, Yeom JE, Lee J, Lee W, Bae GU, Kang JS. PRMT1 ablation in endothelial cells causes endothelial dysfunction and aggravates COPD attributable to dysregulated NF-κB signaling. Adv Sci (Weinh). 2025;12(19):e2411514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Kuwik J, Scott V, Chedid S, Stransky S, Hinkelman K, Kavoosi S, Calderon M, Watkins S, Sidoli S, Islam K. Analogue-Sensitive Inhibition of histone demethylases uncovers Member-Specific function in ribosomal protein synthesis. J Am Chem Soc. 2025;147(4):3341–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Vicioso-Mantis M, Aguirre S, Martínez-Balbás MA. JmjC family of histone demethylases form nuclear condensates. Int J Mol Sci 2022; 23(14). [DOI] [PMC free article] [PubMed]
- 171.Del Blanco B, Niñerola S, Martín-González AM, Paraíso-Luna J, Kim M, Muñoz-Viana R, Racovac C, Sanchez-Mut JV, Ruan Y, Barco Á. Kdm1a safeguards the topological boundaries of PRC2-repressed genes and prevents aging-related euchromatinization in neurons. Nat Commun. 2024;15(1):1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Rezazadeh S, Yang D, Biashad SA, Firsanov D, Takasugi M, Gilbert M, Tombline G, Bhanu NV, Garcia BA, Seluanov A, Gorbunova V. SIRT6 mono-ADP ribosylates KDM2A to locally increase H3K36me2 at DNA damage sites to inhibit transcription and promote repair. Aging. 2020;12(12):11165–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Huang B, Wang B, Yuk-Wai Lee W, Pong UK, Leung KT, Li X, Liu Z, Chen R, Lin JC, Tsang LL et al. KDM3A and KDM4C Regulate Mesenchymal Stromal Cell Senescence and Bone Aging via Condensin-mediated Heterochromatin Reorganization. iScience. 2019; 21: 375–390. [DOI] [PMC free article] [PubMed]
- 174.Ngwa C, Misrani A, Manyam KV, Xu Y, Qi S, Sharmeen R, Lee J, Wu LJ, McCullough L, Liu F. Escape of Kdm6a from X chromosome is detrimental to ischemic brains via IRF5 signaling. Transl Stroke Res. 2025. [DOI] [PMC free article] [PubMed]
- 175.Li C, Qin T, Zhao J, Jin Y, Qin Y, He R, Wu T, Duan C, Jiang L, Yuan F, et al. Kdm6a-CNN1 axis orchestrates epigenetic control of trauma-induced spinal cord microvascular endothelial cell senescence to balance neuroinflammation for improved neurological repair. Bone Res. 2024;12(1):19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Lee KK, Workman JL. Histone acetyltransferase complexes: one size doesn’t fit all. Nat Rev Mol Cell Biol. 2007;8(4):284–95. [DOI] [PubMed] [Google Scholar]
- 177.Sterner DE, Berger SL. Acetylation of histones and transcription-related factors. Microbiol Mol Biol Rev. 2000;64(2):435–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Berndsen CE, Denu JM. Catalysis and substrate selection by histone/protein lysine acetyltransferases. Curr Opin Struct Biol. 2008;18(6):682–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Gregoretti IV, Lee YM, Goodson HV. Molecular evolution of the histone deacetylase family: functional implications of phylogenetic analysis. J Mol Biol. 2004;338(1):17–31. [DOI] [PubMed] [Google Scholar]
- 180.Bradshaw PC. Acetyl-CoA metabolism and histone acetylation in the regulation of aging and lifespan. Antioxid (Basel). 2021; 10(4). [DOI] [PMC free article] [PubMed]
- 181.Baell JB, Leaver DJ, Hermans SJ, Kelly GL, Brennan MS, Downer NL, Nguyen N, Wichmann J, McRae HM, Yang Y, et al. Inhibitors of histone acetyltransferases KAT6A/B induce senescence and arrest tumour growth. Nature. 2018;560(7717):253–7. [DOI] [PubMed] [Google Scholar]
- 182.Nguyen L, Humbert S, Saudou F, Chariot A. Elongator - an emerging role in neurological disorders. Trends Mol Med. 2010;16(1):1–6. [DOI] [PubMed] [Google Scholar]
- 183.Zhu X, Liu B, Carlsten JO, Beve J, Nyström T, Myers LC, Gustafsson CM. Mediator influences telomeric Silencing and cellular life span. Mol Cell Biol. 2011;31(12):2413–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Hamsanathan S, Anthonymuthu T, Han S, Shinglot H, Siefken E, Sims A, Sen P, Pepper HL, Snyder NW, Bayir H, et al. Integrated -omics approach reveals persistent DNA damage rewires lipid metabolism and histone hyperacetylation via MYS-1/Tip60. Sci Adv. 2022;8(7):eabl6083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Gong Y, Zhan H, Wei N, Liu M, Liu Y, Guan P, Xie Y, Deng Y, Pu Q, Lou X et al. Acetylation profiling by Iseq-Kac reveals insights into HSC aging and lineage decision. Nat Chem Biol. 2025. [DOI] [PubMed]
- 186.Dubey SK, Dubey R, Prajapati SC, Jung K, Mohan K, Liu X, Roney J, Tian W, Abney J, Giarmarco MM, et al. Histone deficiency and hypoacetylation in the aging retinal pigment epithelium. Aging Cell. 2024;23(5):e14108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Wang L, Xu C, Johansen T, Berger SL, Dou Z. SIRT1 - a new mammalian substrate of nuclear autophagy. Autophagy. 2021;17(2):593–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Hayakawa T, Iwai M, Aoki S, Takimoto K, Maruyama M, Maruyama W, Motoyama N. SIRT1 suppresses the senescence-associated secretory phenotype through epigenetic gene regulation. PLoS ONE. 2015;10(1):e0116480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Guo J, Wang Z, Wu J, Liu M, Li M, Sun Y, Huang W, Li Y, Zhang Y, Tang W, et al. Endothelial SIRT6 is vital to prevent hypertension and associated cardiorenal injury through targeting Nkx3.2-GATA5 signaling. Circ Res. 2019;124(10):1448–61. [DOI] [PubMed] [Google Scholar]
- 190.Zhang Y, Wang X, Li XK, Lv SJ, Wang HP, Liu Y, Zhou J, Gong H, Chen XF, Ren SC, et al. Sirtuin 2 deficiency aggravates ageing-induced vascular remodelling in humans and mice. Eur Heart J. 2023;44(29):2746–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Medina Rangel PX, Cross E, Liu C, Pedigo CE, Tian X, Gutiérrez-Calabrés E, Nagata S, Priyadarshini A, Lerner G, Bunda P, et al. Cell cycle and senescence regulation by podocyte histone deacetylase 1 and 2. J Am Soc Nephrol. 2023;34(3):433–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Xiang HL, Yuan Q, Zeng JY, Xu ZY, Zhang HZ, Huang J, Song AN, Xiong J, Zhang C. MDM2 accelerated renal senescence via ubiquitination and degradation of HDAC1. Acta Pharmacol Sin. 2024;45(11):2328–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Hodge G, Jersmann H, Tran HB, Roscioli E, Holmes M, Reynolds PN, Hodge S. Lymphocyte senescence in COPD is associated with decreased histone deacetylase 2 expression by pro-inflammatory lymphocytes. Respir Res. 2015;16:130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Yeo D, Zars Fisher EL, Khosla S, Farr JN, Westendorf JJ. Hdac3-deficiency increases senescence-associated distention of satellite DNA and telomere-associated foci in osteoprogenitor cells. J Bone Min Res. 2024;39(7):994–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Goldstein G, Scheid M, Hammerling U, Schlesinger DH, Niall HD, Boyse EA. Isolation of a polypeptide that has lymphocyte-differentiating properties and is probably represented universally in living cells. Proc Natl Acad Sci U S A. 1975;72(1):11–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Celebi G, Kesim H, Ozer E, Kutlu O. The Effect of Dysfunctional Ubiquitin Enzymes in the Pathogenesis of Most Common Diseases. Int J Mol Sci. 2020; 21(17). [DOI] [PMC free article] [PubMed]
- 197.Clague MJ, Urbé S, Komander D. Breaking the chains: deubiquitylating enzyme specificity begets function. Nat Rev Mol Cell Biol. 2019;20(6):338–52. [DOI] [PubMed] [Google Scholar]
- 198.Gu H, Li Q, Liu Z, Li Y, Liu K, Kong X, Zhang Y, Meng Q, Song K, Xie Q, et al. SPP1-ITGα5/β1 accelerates calcification of nucleus pulposus cells by inhibiting mitophagy via Ubiquitin-Dependent PINK1/PARKIN pathway Blockade. Adv Sci (Weinh). 2025;12(7):e2411162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Kotla S, Le NT, Vu HT, Ko KA, Gi YJ, Thomas TN, Giancursio C, Lusis AJ, Cooke JP, Fujiwara K, Abe JI. Endothelial senescence-associated secretory phenotype (SASP) is regulated by Makorin-1 ubiquitin E3 ligase. Metabolism. 2019;100:153962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Qian B, Li TY, Zheng ZX, Zhang HY, Xu WQ, Mo SM, Cui JJ, Chen WJ, Lin YC, Lin ZN. The involvement of SigmaR1(K142) degradation mediated by ERAD in neural senescence linked with CdCl(2) exposure. J Hazard Mater. 2024;472:134466. [DOI] [PubMed] [Google Scholar]
- 201.Li Q, Wang P, Gong Y, Xu M, Wang M, Luan R, Liu J, Li X, Shao Y. α-Klotho prevents diabetic retinopathy by reversing the senescence of macrophages. Cell Commun Signal. 2024;22(1):449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Ma JH, Zhang YT, Wang LP, Sun QY, Zhang H, Li JJ, Han NN, Zhu YY, Xie XY, Li X. K63 Ubiquitination of P21 Can Facilitate Pellino-1 in the Context of Chronic Obstructive Pulmonary Disease and Lung Cellular Senescence. Cells. 2022; 11(19). [DOI] [PMC free article] [PubMed]
- 203.Xiao Z, Liang J, Huang R, Chen D, Mei J, Deng J, Wang Z, Li L, Li Z, Xia H et al. Inhibition of miR-143-3p restores Blood-Testis barrier function and ameliorates Sertoli cell senescence. Cells 2024; 13(4). [DOI] [PMC free article] [PubMed]
- 204.Ye Q, Wang J, Liu X, Liu Z, BaZong L, Ma J, Shen R, Ye W, Zhang W, Wang D. The role of RAD6B and PEDF in retinal degeneration. Neuroscience. 2022;480:19–31. [DOI] [PubMed] [Google Scholar]
- 205.Ma LZ, Wang A, Lai YH, Zhang J, Zhang XF, Chen SL, Zhou XY. USP14 Inhibition promotes DNA damage repair and represses ovarian granulosa cell senescence in premature ovarian insufficiency. J Transl Med. 2024;22(1):834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Ni JY, Wang X, Xie HY, Yang NH, Li JY, Sun XA, Guo HJ, Zhou L, Zhang W, Liu J, Lu LM. Deubiquitinating enzyme USP11 promotes renal tubular cell senescence and fibrosis via inhibiting the ubiquitin degradation of TGF-β receptor II. Acta Pharmacol Sin. 2023;44(3):584–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.He J, Baoyinna B, Taleb SJ, Zhao J, Zhao Y. USP13 regulates cell senescence through mediating MDM2 stability. Life Sci. 2023;331:122044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Auclair M, Guénantin AC, Fellahi S, Garcia M, Capeau J. HIV antiretroviral drugs, dolutegravir, Maraviroc and ritonavir-boosted Atazanavir use different pathways to affect inflammation, senescence and insulin sensitivity in human coronary endothelial cells. PLoS ONE. 2020;15(1):e0226924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Pan W, Wang Y, Bai X, Yin Y, Dai L, Zhou H, Wu Q, Wang Y. Deubiquitinating enzyme USP30 negatively regulates mitophagy and accelerates myocardial cell senescence through antagonism of parkin. Cell Death Discov. 2021;7(1):187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Zhou Q, Wang W, Wu J, Qiu S, Yuan S, Fu PL, Qian QR, Xu YZ. Ubiquitin-specific protease 3 attenuates interleukin-1β-mediated chondrocyte senescence by deacetylating forkhead box O-3 via sirtuin-3. Bioengineered. 2022;13(2):2017–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Yang X, Qian K. Protein O-GlcNAcylation: emerging mechanisms and functions. Nat Rev Mol Cell Biol. 2017;18(7):452–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Banerjee PS, Lagerlöf O, Hart GW. Roles of O-GlcNAc in chronic diseases of aging. Mol Aspects Med. 2016;51:1–15. [DOI] [PubMed] [Google Scholar]
- 213.Mueller T, Ouyang X, Johnson MS, Qian WJ, Chatham JC, Darley-Usmar V, Zhang J. New insights into the biology of protein O-GlcNAcylation: approaches and observations. Front Aging. 2020;1:620382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Wheatley EG, Albarran E, White CW 3rd, Bieri G, Sanchez-Diaz C, Pratt K, Snethlage CE, Ding JB, Villeda SA. Neuronal O-GlcNAcylation improves cognitive function in the aged mouse brain. Curr Biol. 2019;29(20):3359–e33693354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Bell M, Kane MS, Ouyang X, Young ME, Jegga AG, Chatham JC, Darley-Usmar V, Zhang J. Acute increase of protein O-GlcNAcylation in mice leads to transcriptome changes in the brain opposite to what is observed in Alzheimer’s Disease. bioRxiv. 2024.
- 216.Wang Z, Li X, Spasojevic I, Lu L, Shen Y, Qu X, Hoffmann U, Warner DS, Paschen W, Sheng H, Yang W. Increasing O-GlcNAcylation is neuroprotective in young and aged brains after ischemic stroke. Exp Neurol. 2021;339:113646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Zuo Y, Wang Q, Tian W, Zheng Z, He W, Zhang R, Zhao Q, Miao Y, Yuan Y, Wang J, Zheng H. β-hydroxybutyrylation and O-GlcNAc modifications of STAT1 modulate antiviral defense in aging. Cell Mol Immunol. 2025;22(4):403–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.D’Amours D, Desnoyers S, D’Silva I, Poirier GG. Poly(ADP-ribosyl)ation reactions in the regulation of nuclear functions. Biochem J. 1999;342(Pt 2):249–68. [PMC free article] [PubMed] [Google Scholar]
- 219.Kang M, Park S, Park SH, Lee HG, Park JH. A Double-Edged sword: the two faces of parylation. Int J Mol Sci 2022; 23(17). [DOI] [PMC free article] [PubMed]
- 220.Santinelli-Pestana DV, Aikawa E, Singh SA, Aikawa M. PARPs and ADP-Ribosylation in Chronic Inflammation: A Focus on Macrophages. Pathogens. 2023; 12(7). [DOI] [PMC free article] [PubMed]
- 221.Zampieri M, Bacalini MG, Barchetta I, Scalea S, Cimini FA, Bertoccini L, Tagliatesta S, De Matteis G, Zardo G, Cavallo MG, Reale A. Increased parylation impacts the DNA methylation process in type 2 diabetes mellitus. Clin Epigenetics. 2021;13(1):114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Fan X, Wang H, Wang W, Shen J, Wang Z. Exercise training alleviates cholesterol and lipid accumulation in mice with non-alcoholic steatohepatitis: reduction of KMT2D-mediated histone methylation of IDI1. Exp Cell Res. 2024;442(2):114265. [DOI] [PubMed] [Google Scholar]
- 223.Krzystyniak A, Wesierska M, Petrazzo G, Gadecka A, Dudkowska M, Bielak-Zmijewska A, Mosieniak G, Figiel I, Wlodarczyk J, Sikora E. Combination of dasatinib and Quercetin improves cognitive abilities in aged male Wistar rats, alleviates inflammation and changes hippocampal synaptic plasticity and histone H3 methylation profile. Aging. 2022;14(2):572–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Taheri N, Choi EL, Nguyen VTT, Zhang Y, Huynh NM, Kellogg TA, van Wijnen AJ, Ordog T, Hayashi Y. Inhibition of EZH2 reduces Aging-Related decline in interstitial cells of Cajal of the mouse stomach. Cell Mol Gastroenterol Hepatol. 2024;18(4):101376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Lin C, Li H, Liu J, Hu Q, Zhang S, Zhang N, Liu L, Dai Y, Cao D, Li X, et al. Arginine hypomethylation-mediated proteasomal degradation of histone H4-an early biomarker of cellular senescence. Cell Death Differ. 2020;27(9):2697–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Roman J, Zhu J, Ritzenthaler JD, Zelko IN. Epigenetic regulation of EC-SOD expression in aging lung fibroblasts: role of histone acetylation. Free Radic Biol Med. 2017;112:212–23. [DOI] [PubMed] [Google Scholar]
- 227.Consalvi S, Tucciarone L, Macrì E, De Bardi M, Picozza M, Salvatori I, Renzini A, Valente S, Mai A, Moresi V, Puri PL. Determinants of epigenetic resistance to HDAC inhibitors in dystrophic fibro-adipogenic progenitors. EMBO Rep. 2022;23(6):e54721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Sasia C, Borgonetti V, Mancini C, Lori G, Arbiser JL, Taddei ML, Galeotti N. The Neolignan Honokiol and Its Synthetic Derivative Honokiol Hexafluoro Reduce Neuroinflammation and Cellular Senescence in Microglia Cells. Cells. 2024; 13(19). [DOI] [PMC free article] [PubMed]
- 229.Yu X, Yu W, Wu L, Yang W, Lü Y. Chitotriosidase attenuates brain inflammation via HDAC3/NF-κB pathway in D-galactose and aluminum-induced rat model with cognitive impairments. Neurosci Res. 2021;172:73–9. [DOI] [PubMed] [Google Scholar]
- 230.Coughlan MT, Ziemann M, Laskowski A, Woodruff TM, Tan SM. Valproic acid attenuates cellular senescence in diabetic kidney disease through the Inhibition of complement C5a receptors. Sci Rep. 2022;12(1):20278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Hodge G, Tran HB, Reynolds PN, Jersmann H, Hodge S. Lymphocyte senescence in COPD is associated with decreased Sirtuin 1 expression in steroid resistant pro-inflammatory lymphocytes. Ther Adv Respir Dis. 2020;14:1753466620905280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Jamshed H, Beyl RA, Della Manna DL, Yang ES, Ravussin E, Peterson CM. Early Time-Restricted feeding improves 24-Hour glucose levels and affects markers of the circadian clock, aging, and autophagy in humans. Nutrients. 2019; 11(6). [DOI] [PMC free article] [PubMed]
- 233.Ketron GL, Grun F, Grill JD, Feldman HH, Rissman RA, Brewer GJ. Pharmacokinetic and pharmacodynamic assessment of oral nicotinamide in the NEAT clinical trial for early alzheimer’s disease. Alzheimers Res Ther. 2025;17(1):59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Zhu H, Zhang T, Li R, Ren D, Xu J, Xiao L. Bushen Huoxue acupuncture ameliorates alzheimer’s disease by upregulating MARCHF3 to induce NLRP3 ubiquitination and inhibit caspase-1-dependent pyroptosis. Metab Brain Dis. 2024;40(1):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Jiang H, Lin X, Liang W, Li Y, Yu X. Friedelin alleviates the pathogenesis of Collagenase-Induced tendinopathy in mice by promoting the selective autophagic degradation of p65. Nutrients 2022; 14(8). [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.