Abstract
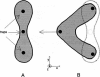
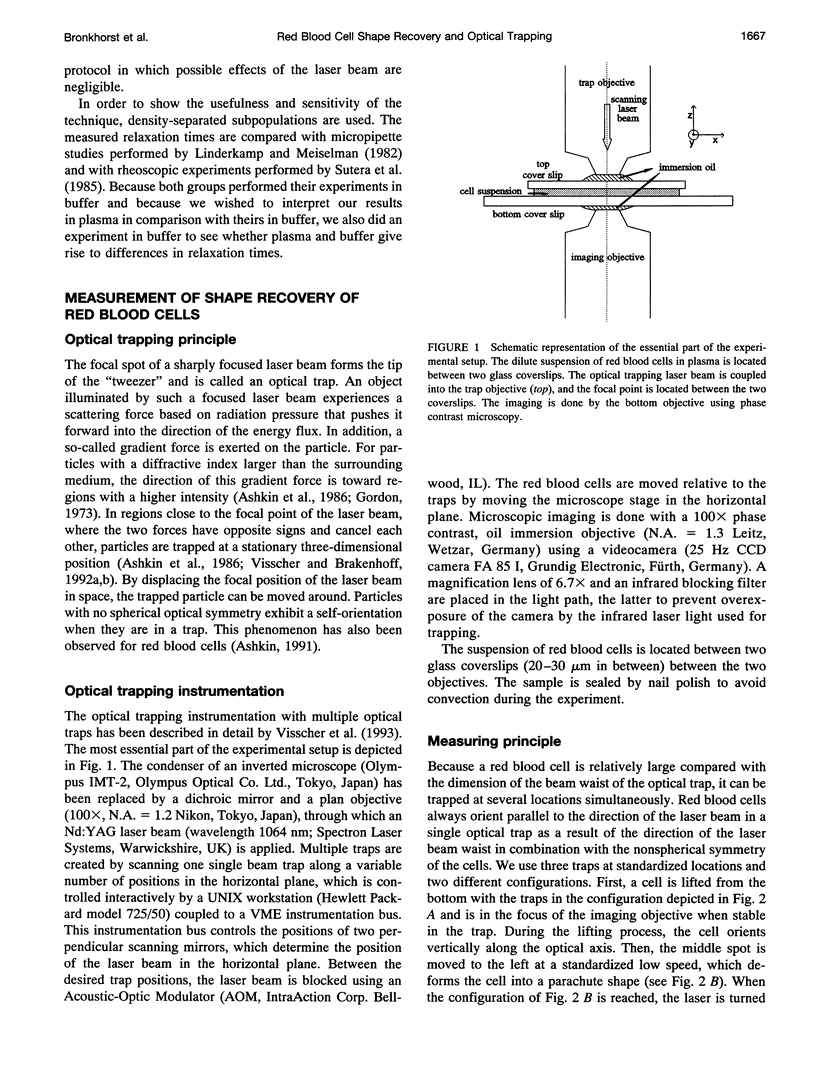
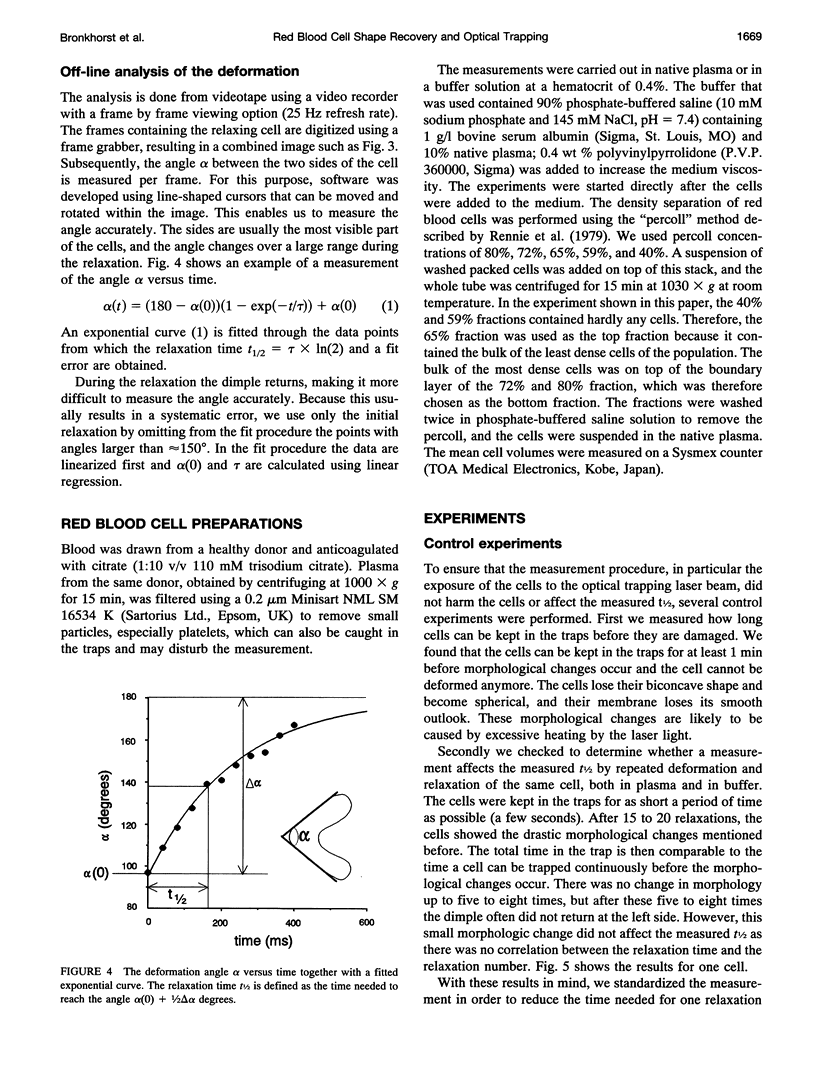
In this new method for studying the shape recovery of deformed red blood cells, three optical traps ("optical tweezers") induce a parachute-shaped red cell deformation, which is comparable to the deformation in small capillaries. The shape recovery is recorded, and a relaxation time is obtained for each individual red blood cell. The sensitivity of this technique for the detection of differences in relaxation times is demonstrated on subpopulations of density-separated red blood cells: "young" cells have shorter (162 ms) and "old" cells have longer (353 ms) relaxation times compared with the total population (271 ms). The relaxation time is remarkably shorter (114 ms) when the plasma surrounding the cells is replaced by a phosphate-buffered saline solution. The main advantages of this technique are the relatively short measuring and preparation time and the physiological type of deformation and shape recovery in which all relevant cell properties play a role. Therefore, especially when automated further, the technique may be a powerful tool for the study of (sub)populations of pathological red blood cells.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashkin A., Dziedzic J. M. Optical trapping and manipulation of viruses and bacteria. Science. 1987 Mar 20;235(4795):1517–1520. doi: 10.1126/science.3547653. [DOI] [PubMed] [Google Scholar]
- Ashkin A. The study of cells by optical trapping and manipulation of living cells using infrared laser beams. ASGSB Bull. 1991 Jul;4(2):133–146. [PubMed] [Google Scholar]
- Block S. M. Making light work with optical tweezers. Nature. 1992 Dec 3;360(6403):493–495. doi: 10.1038/360493a0. [DOI] [PubMed] [Google Scholar]
- Chien S. Red cell deformability and its relevance to blood flow. Annu Rev Physiol. 1987;49:177–192. doi: 10.1146/annurev.ph.49.030187.001141. [DOI] [PubMed] [Google Scholar]
- Clark M. R., Mohandas N., Feo C., Jacobs M. S., Shohet S. B. Separate mechanisms of deformability loss in ATP-depleted and Ca-loaded erythrocytes. J Clin Invest. 1981 Feb;67(2):531–539. doi: 10.1172/JCI110063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans E. A. Structure and deformation properties of red blood cells: concepts and quantitative methods. Methods Enzymol. 1989;173:3–35. doi: 10.1016/s0076-6879(89)73003-2. [DOI] [PubMed] [Google Scholar]
- Evans E., Mohandas N., Leung A. Static and dynamic rigidities of normal and sickle erythrocytes. Major influence of cell hemoglobin concentration. J Clin Invest. 1984 Feb;73(2):477–488. doi: 10.1172/JCI111234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer T. M. On the energy dissipation in a tank-treading human red blood cell. Biophys J. 1980 Nov;32(2):863–868. doi: 10.1016/S0006-3495(80)85022-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaehtgens P., Dührssen C., Albrecht K. H. Motion, deformation, and interaction of blood cells and plasma during flow through narrow capillary tubes. Blood Cells. 1980;6(4):799–817. [PubMed] [Google Scholar]
- Hochmuth R. M., Worthy P. R., Evans E. A. Red cell extensional recovery and the determination of membrane viscosity. Biophys J. 1979 Apr;26(1):101–114. doi: 10.1016/S0006-3495(79)85238-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koutsouris D., Guillet R., Lelievre J. C., Guillemin M. T., Bertholom P., Beuzard Y., Boynard M. Determination of erythrocyte transit times through micropores. I--Basic operational principles. Biorheology. 1988;25(5):763–772. doi: 10.3233/bir-1988-25504. [DOI] [PubMed] [Google Scholar]
- Leblond P. F., Coulombe L. The measurement of erythrocyte deformability using micropore membranes. A sensitive technique with clinical applications. J Lab Clin Med. 1979 Jul;94(1):133–143. [PubMed] [Google Scholar]
- Linderkamp O., Meiselman H. J. Geometric, osmotic, and membrane mechanical properties of density-separated human red cells. Blood. 1982 Jun;59(6):1121–1127. [PubMed] [Google Scholar]
- Nash G. B., Meiselman H. J. Red cell and ghost viscoelasticity. Effects of hemoglobin concentration and in vivo aging. Biophys J. 1983 Jul;43(1):63–73. doi: 10.1016/S0006-3495(83)84324-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palek J., Liu S. C. Dependence of spectrin organization in red blood cell membranes on cell metabolism: implications for control of red cell shape, deformability, and surface area. Semin Hematol. 1979 Jan;16(1):75–93. [PubMed] [Google Scholar]
- Rennie C. M., Thompson S., Parker A. C., Maddy A. Human erythrocyte fraction in "Percoll" density gradients. Clin Chim Acta. 1979 Oct 15;98(1-2):119–125. doi: 10.1016/0009-8981(79)90172-4. [DOI] [PubMed] [Google Scholar]
- Sutera S. P., Gardner R. A., Boylan C. W., Carroll G. L., Chang K. C., Marvel J. S., Kilo C., Gonen B., Williamson J. R. Age-related changes in deformability of human erythrocytes. Blood. 1985 Feb;65(2):275–282. [PubMed] [Google Scholar]
- Svoboda K., Schmidt C. F., Branton D., Block S. M. Conformation and elasticity of the isolated red blood cell membrane skeleton. Biophys J. 1992 Sep;63(3):784–793. doi: 10.1016/S0006-3495(92)81644-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visscher K., Brakenhoff G. J., Krol J. J. Micromanipulation by "multiple" optical traps created by a single fast scanning trap integrated with the bilateral confocal scanning laser microscope. Cytometry. 1993;14(2):105–114. doi: 10.1002/cyto.990140202. [DOI] [PubMed] [Google Scholar]
- Weed R. I. The importance of erythrocyte deformability. Am J Med. 1970 Aug;49(2):147–150. doi: 10.1016/s0002-9343(70)80069-9. [DOI] [PubMed] [Google Scholar]
- Williams A. R., Morris D. R. The internal viscosity of the human erythrocyte may determine its lifespan in vivo. Scand J Haematol. 1980 Jan;24(1):57–62. doi: 10.1111/j.1600-0609.1980.tb01318.x. [DOI] [PubMed] [Google Scholar]
- Williamson J. R., Gardner R. A., Boylan C. W., Carroll G. L., Chang K., Marvel J. S., Gonen B., Kilo C., Tran-Son-Tay R., Sutera S. P. Microrheologic investigation of erythrocyte deformability in diabetes mellitus. Blood. 1985 Feb;65(2):283–288. [PubMed] [Google Scholar]