Abstract
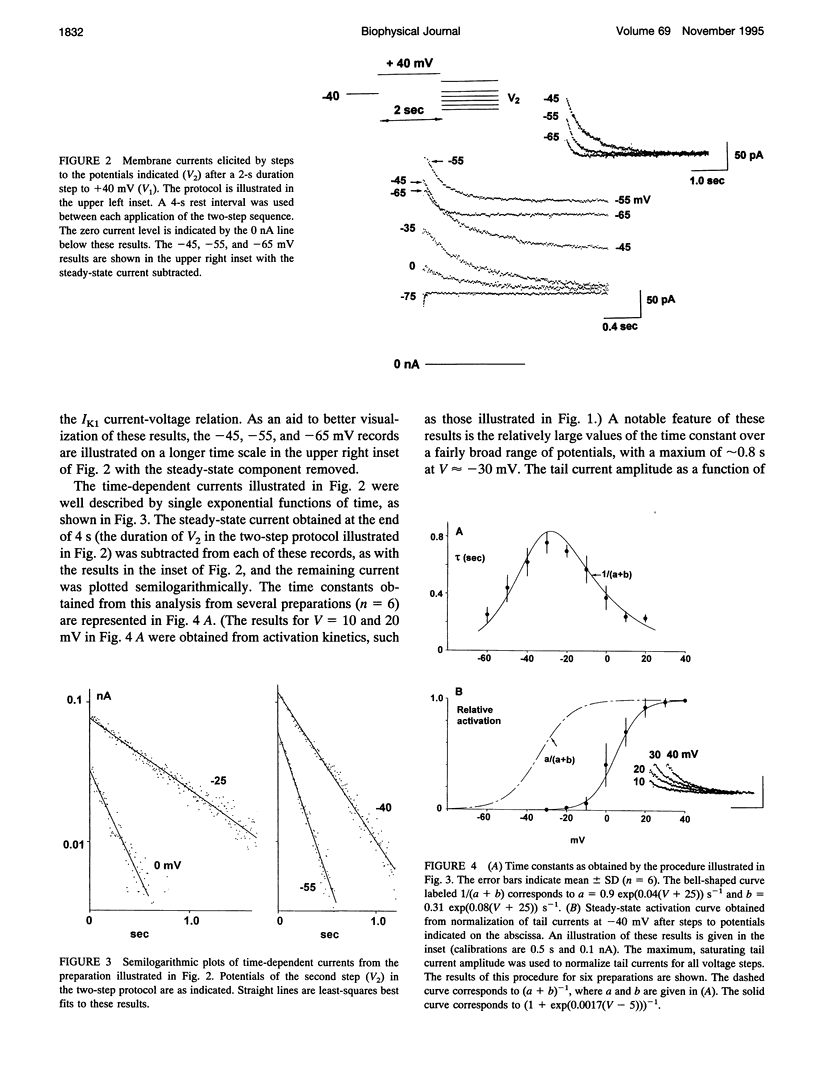
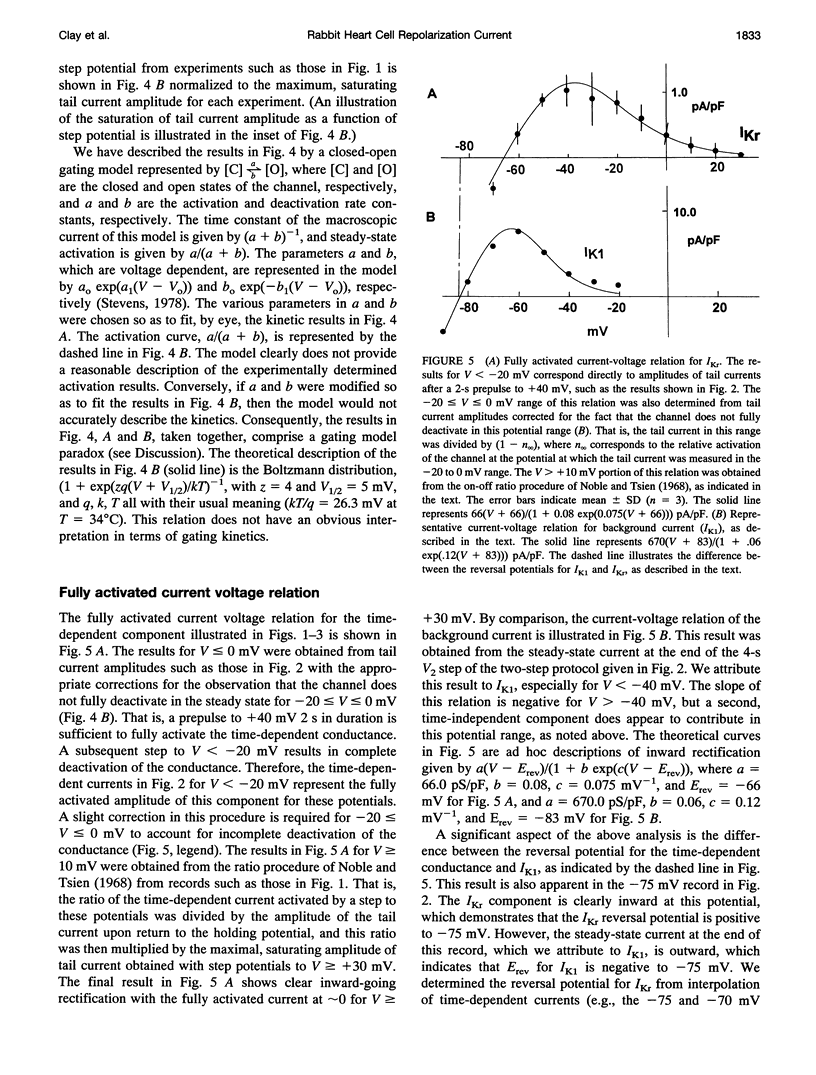
We have measured the E-4031-sensitive repolarization current (IKr) in single ventricular myocytes isolated from rabbit hearts. The primary goal of this analysis was a description of the IKr kinetic and ion transfer properties. Surprisingly, the maximum time constant of this component was 0.8 s at 33-34 degrees C, which is significantly greater than the value of 0.18 s previously reported under similar conditions in the original measurements of IKr from guinea pig ventricular myocytes. The primary, novel feature of our analysis concerns the relationship of the bell-shaped curve that describes the voltage dependence of the kinetics and the sigmoidal curve that describes the activation of IKr. The midpoint of the latter occurred at approximately +10 mV on the voltage axis, as compared to -30 mV for the point on the voltage axis at which the maximum time constant occurred. Moreover, the voltage dependence of the kinetics was much broader than the steepness of the activation curve would predict. Taken together, these results comprise a gating current paradox that is not resolved by the incorporation of a fast inactivated state in the analysis. The fully activated current-voltage relation for IKr exhibited strong inward-going rectification, so much so that the current was essentially nil at +30 mV, even though the channel opens rapidly in this voltage range. This result is consistent with the lack of effect of E-4031 on the early part of the plateau phase of the action potential. Surprisingly, the reversal potential Of /Kr was ~15 mV positive to the potassium ion equilibrium potential,which indicates that this channel carries inward current during the latter part of the repolarization phase of the action potential.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennett P. B., McKinney L. C., Kass R. S., Begenisich T. Delayed rectification in the calf cardiac Purkinje fiber. Evidence for multiple state kinetics. Biophys J. 1985 Oct;48(4):553–567. doi: 10.1016/S0006-3495(85)83813-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmeliet E. Use-dependent block and use-dependent unblock of the delayed rectifier K+ current by almokalant in rabbit ventricular myocytes. Circ Res. 1993 Nov;73(5):857–868. doi: 10.1161/01.res.73.5.857. [DOI] [PubMed] [Google Scholar]
- Carmeliet E. Voltage- and time-dependent block of the delayed K+ current in cardiac myocytes by dofetilide. J Pharmacol Exp Ther. 1992 Aug;262(2):809–817. [PubMed] [Google Scholar]
- Clay J. R. Comparison of the effects of internal TEA+ and Cs+ on potassium current in squid giant axons. Biophys J. 1985 Dec;48(6):885–892. doi: 10.1016/S0006-3495(85)83850-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clay J. R., Hill C. E., Roitman D., Shrier A. Repolarization current in embryonic chick atrial heart cells. J Physiol. 1988 Sep;403:525–537. doi: 10.1113/jphysiol.1988.sp017262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coraboeuf E., Carmeliet E. Existence of two transient outward currents in sheep cardiac Purkinje fibers. Pflugers Arch. 1982 Feb;392(4):352–359. doi: 10.1007/BF00581631. [DOI] [PubMed] [Google Scholar]
- Duan D. Y., Fermini B., Nattel S. Sustained outward current observed after I(to1) inactivation in rabbit atrial myocytes is a novel Cl- current. Am J Physiol. 1992 Dec;263(6 Pt 2):H1967–H1971. doi: 10.1152/ajpheart.1992.263.6.H1967. [DOI] [PubMed] [Google Scholar]
- Follmer C. H., Colatsky T. J. Block of delayed rectifier potassium current, IK, by flecainide and E-4031 in cat ventricular myocytes. Circulation. 1990 Jul;82(1):289–293. doi: 10.1161/01.cir.82.1.289. [DOI] [PubMed] [Google Scholar]
- Follmer C. H., Lodge N. J., Cullinan C. A., Colatsky T. J. Modulation of the delayed rectifier, IK, by cadmium in cat ventricular myocytes. Am J Physiol. 1992 Jan;262(1 Pt 1):C75–C83. doi: 10.1152/ajpcell.1992.262.1.C75. [DOI] [PubMed] [Google Scholar]
- Giles W. R., van Ginneken A. C. A transient outward current in isolated cells from the crista terminalis of rabbit heart. J Physiol. 1985 Nov;368:243–264. doi: 10.1113/jphysiol.1985.sp015856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952 Aug;117(4):500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Harvey R. D., Hume J. R. Isoproterenol activates a chloride current, not the transient outward current, in rabbit ventricular myocytes. Am J Physiol. 1989 Dec;257(6 Pt 1):C1177–C1181. doi: 10.1152/ajpcell.1989.257.6.C1177. [DOI] [PubMed] [Google Scholar]
- Harvey R. D., Ten Eick R. E. Characterization of the inward-rectifying potassium current in cat ventricular myocytes. J Gen Physiol. 1988 Apr;91(4):593–615. doi: 10.1085/jgp.91.4.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isenberg G., Klockner U. Calcium tolerant ventricular myocytes prepared by preincubation in a "KB medium". Pflugers Arch. 1982 Oct;395(1):6–18. doi: 10.1007/BF00584963. [DOI] [PubMed] [Google Scholar]
- Josephson I. R., Sanchez-Chapula J., Brown A. M. Early outward current in rat single ventricular cells. Circ Res. 1984 Feb;54(2):157–162. doi: 10.1161/01.res.54.2.157. [DOI] [PubMed] [Google Scholar]
- Jurkiewicz N. K., Sanguinetti M. C. Rate-dependent prolongation of cardiac action potentials by a methanesulfonanilide class III antiarrhythmic agent. Specific block of rapidly activating delayed rectifier K+ current by dofetilide. Circ Res. 1993 Jan;72(1):75–83. doi: 10.1161/01.res.72.1.75. [DOI] [PubMed] [Google Scholar]
- Matsuura H., Ehara T., Imoto Y. An analysis of the delayed outward current in single ventricular cells of the guinea-pig. Pflugers Arch. 1987 Dec;410(6):596–603. doi: 10.1007/BF00581319. [DOI] [PubMed] [Google Scholar]
- McDonald T. F., Trautwein W. The potassium current underlying delayed rectification in cat ventricular muscle. J Physiol. 1978 Jan;274:217–246. doi: 10.1113/jphysiol.1978.sp012144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra R., Morad M. A uniform enzymatic method for dissociation of myocytes from hearts and stomachs of vertebrates. Am J Physiol. 1985 Nov;249(5 Pt 2):H1056–H1060. doi: 10.1152/ajpheart.1985.249.5.H1056. [DOI] [PubMed] [Google Scholar]
- Noble D., Tsien R. W. Outward membrane currents activated in the plateau range of potentials in cardiac Purkinje fibres. J Physiol. 1969 Jan;200(1):205–231. doi: 10.1113/jphysiol.1969.sp008689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble D., Tsien R. W. The kinetics and rectifier properties of the slow potassium current in cardiac Purkinje fibres. J Physiol. 1968 Mar;195(1):185–214. doi: 10.1113/jphysiol.1968.sp008454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogbaghebriel A., Shrier A. Inhibition of metabolism abolishes transient outward current in rabbit atrial myocytes. Am J Physiol. 1994 Jan;266(1 Pt 2):H182–H190. doi: 10.1152/ajpheart.1994.266.1.H182. [DOI] [PubMed] [Google Scholar]
- Sakmann B., Trube G. Conductance properties of single inwardly rectifying potassium channels in ventricular cells from guinea-pig heart. J Physiol. 1984 Feb;347:641–657. doi: 10.1113/jphysiol.1984.sp015088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanguinetti M. C., Jiang C., Curran M. E., Keating M. T. A mechanistic link between an inherited and an acquired cardiac arrhythmia: HERG encodes the IKr potassium channel. Cell. 1995 Apr 21;81(2):299–307. doi: 10.1016/0092-8674(95)90340-2. [DOI] [PubMed] [Google Scholar]
- Sanguinetti M. C., Jurkiewicz N. K. Two components of cardiac delayed rectifier K+ current. Differential sensitivity to block by class III antiarrhythmic agents. J Gen Physiol. 1990 Jul;96(1):195–215. doi: 10.1085/jgp.96.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibasaki T. Conductance and kinetics of delayed rectifier potassium channels in nodal cells of the rabbit heart. J Physiol. 1987 Jun;387:227–250. doi: 10.1113/jphysiol.1987.sp016571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrier A., Clay J. R. Repolarization currents in embryonic chick atrial heart cell aggregates. Biophys J. 1986 Nov;50(5):861–874. doi: 10.1016/S0006-3495(86)83527-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens C. F. Interactions between intrinsic membrane protein and electric field. An approach to studying nerve excitability. Biophys J. 1978 May;22(2):295–306. doi: 10.1016/S0006-3495(78)85490-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldkamp M. W., van Ginneken A. C., Bouman L. N. Single delayed rectifier channels in the membrane of rabbit ventricular myocytes. Circ Res. 1993 Apr;72(4):865–878. doi: 10.1161/01.res.72.4.865. [DOI] [PubMed] [Google Scholar]
- Wang Z., Fermini B., Nattel S. Rapid and slow components of delayed rectifier current in human atrial myocytes. Cardiovasc Res. 1994 Oct;28(10):1540–1546. doi: 10.1093/cvr/28.10.1540. [DOI] [PubMed] [Google Scholar]
- Yang T., Wathen M. S., Felipe A., Tamkun M. M., Snyders D. J., Roden D. M. K+ currents and K+ channel mRNA in cultured atrial cardiac myocytes (AT-1 cells). Circ Res. 1994 Nov;75(5):870–878. doi: 10.1161/01.res.75.5.870. [DOI] [PubMed] [Google Scholar]


