Abstract
During inflammation, hydrogen peroxide, produced by polymorphonuclear leukocytes, provokes cell death mainly by disarranging filamentous (polymerized) actin (F-actin). To show the molecular mechanism(s) by which hydrogen peroxide could alter actin dynamics, we analyzed the ability of H2O2-treated actin samples to polymerize as well as the suitability of actin polymers (from oxidized monomers) to interact with cross-linking proteins. H2O2-treated monomeric (globular) actin (G-actin) shows an altered time course of polymerization. The increase in the lag phase and the lowering in both the polymerization rate and the polymerization extent have been evidenced. Furthermore, steady-state actin polymers, from oxidized monomers, are more fragmented than control polymers. This seems to be ascribable to the enhanced fragility of oxidized filaments rather than to the increase in the nucleation activity, which markedly falls. These facts; along with the unsuitability of actin polymers from oxidized monomers to interact with both filamin and alpha-actinin, suggest that hydrogen peroxide influences actin dynamics mainly by changing the F-actin structure. H2O2, via the oxidation of actin thiols (in particular, the sulfhydryl group of Cys-374), likely alters the actin C-terminus, influencing both subunit/subunit interactions and the spatial structure of the binding sites for cross-linking proteins in F-actin. We suggest that most of the effects of hydrogen peroxide on actin could be explained in the light of the "structural connectivity," demonstrated previously in actin.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bellomo G., Jewell S. A., Thor H., Orrenius S. Regulation of intracellular calcium compartmentation: studies with isolated hepatocytes and t-butyl hydroperoxide. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6842–6846. doi: 10.1073/pnas.79.22.6842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlier M. F., Pantaloni D., Korn E. D. Evidence for an ATP cap at the ends of actin filaments and its regulation of the F-actin steady state. J Biol Chem. 1984 Aug 25;259(16):9983–9986. [PubMed] [Google Scholar]
- Colombo R., Milzani A., Contini P., Dalle Donne I. Effects of lithium ions on actin polymerization in the presence of magnesium ions. Biochem J. 1991 Mar 1;274(Pt 2):421–425. doi: 10.1042/bj2740421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombo R., Milzani A., Donne I. D. Lithium increases actin polymerization rates by enhancing the nucleation step. J Mol Biol. 1991 Feb 5;217(3):401–404. doi: 10.1016/0022-2836(91)90742-o. [DOI] [PubMed] [Google Scholar]
- Cooper J. A., Walker S. B., Pollard T. D. Pyrene actin: documentation of the validity of a sensitive assay for actin polymerization. J Muscle Res Cell Motil. 1983 Apr;4(2):253–262. doi: 10.1007/BF00712034. [DOI] [PubMed] [Google Scholar]
- Crosbie R. H., Miller C., Cheung P., Goodnight T., Muhlrad A., Reisler E. Structural connectivity in actin: effect of C-terminal modifications on the properties of actin. Biophys J. 1994 Nov;67(5):1957–1964. doi: 10.1016/S0006-3495(94)80678-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELLMAN G. L. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959 May;82(1):70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- Egelman E. H. The structure of F-actin. J Muscle Res Cell Motil. 1985 Apr;6(2):129–151. doi: 10.1007/BF00713056. [DOI] [PubMed] [Google Scholar]
- Elzinga M., Collins J. H. The primary structure of actin from rabbit skeletal muscle. Five cyanogen bromide peptides, including the NH2 and COOH termini. J Biol Chem. 1975 Aug 10;250(15):5897–5905. [PubMed] [Google Scholar]
- Feramisco J. R., Burridge K. A rapid purification of alpha-actinin, filamin, and a 130,000-dalton protein from smooth muscle. J Biol Chem. 1980 Feb 10;255(3):1194–1199. [PubMed] [Google Scholar]
- Frieden C., Goddette D. W. Polymerization of actin and actin-like systems: evaluation of the time course of polymerization in relation to the mechanism. Biochemistry. 1983 Dec 6;22(25):5836–5843. doi: 10.1021/bi00294a023. [DOI] [PubMed] [Google Scholar]
- Gordon D. J., Yang Y. Z., Korn E. D. Polymerization of Acanthamoeba actin. Kinetics, thermodynamics, and co-polymerization with muscle actin. J Biol Chem. 1976 Dec 10;251(23):7474–7479. [PubMed] [Google Scholar]
- Hinshaw D. B., Armstrong B. C., Burger J. M., Beals T. F., Hyslop P. A. ATP and microfilaments in cellular oxidant injury. Am J Pathol. 1988 Sep;132(3):479–488. [PMC free article] [PubMed] [Google Scholar]
- Hinshaw D. B., Burger J. M., Beals T. F., Armstrong B. C., Hyslop P. A. Actin polymerization in cellular oxidant injury. Arch Biochem Biophys. 1991 Aug 1;288(2):311–316. doi: 10.1016/0003-9861(91)90200-3. [DOI] [PubMed] [Google Scholar]
- Hinshaw D. B., Sklar L. A., Bohl B., Schraufstatter I. U., Hyslop P. A., Rossi M. W., Spragg R. G., Cochrane C. G. Cytoskeletal and morphologic impact of cellular oxidant injury. Am J Pathol. 1986 Jun;123(3):454–464. [PMC free article] [PubMed] [Google Scholar]
- Holeysovsky V., Lazdunski M. The structural properties of trypsinogen and trypsin. Alkylation and oxidation of methionines. Biochim Biophys Acta. 1968 Apr 9;154(3):457–467. doi: 10.1016/0005-2795(68)90005-6. [DOI] [PubMed] [Google Scholar]
- Jewell S. A., Bellomo G., Thor H., Orrenius S., Smith M. Bleb formation in hepatocytes during drug metabolism is caused by disturbances in thiol and calcium ion homeostasis. Science. 1982 Sep 24;217(4566):1257–1259. doi: 10.1126/science.7112127. [DOI] [PubMed] [Google Scholar]
- Kabsch W., Mannherz H. G., Suck D., Pai E. F., Holmes K. C. Atomic structure of the actin:DNase I complex. Nature. 1990 Sep 6;347(6288):37–44. doi: 10.1038/347037a0. [DOI] [PubMed] [Google Scholar]
- Kawamura M., Maruyama K. Electron microscopic particle length of F-actin polymerized in vitro. J Biochem. 1970 Mar;67(3):437–457. doi: 10.1093/oxfordjournals.jbchem.a129267. [DOI] [PubMed] [Google Scholar]
- Kinosian H. J., Selden L. A., Estes J. E., Gershman L. C. Nucleotide binding to actin. Cation dependence of nucleotide dissociation and exchange rates. J Biol Chem. 1993 Apr 25;268(12):8683–8691. [PubMed] [Google Scholar]
- Knight P., Offer G. p-NN'-phenylenebismaleimide, a specific cross-linking agent for F-actin. Biochem J. 1978 Dec 1;175(3):1023–1032. doi: 10.1042/bj1751023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korn E. D. The regulation of actin and myosin by ATP. Curr Top Cell Regul. 1985;26:221–232. doi: 10.1016/b978-0-12-152826-3.50024-3. [DOI] [PubMed] [Google Scholar]
- Kouyama T., Mihashi K. Fluorimetry study of N-(1-pyrenyl)iodoacetamide-labelled F-actin. Local structural change of actin protomer both on polymerization and on binding of heavy meromyosin. Eur J Biochem. 1981;114(1):33–38. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lehrer S. S., Kerwar G. Intrinsic fluorescence of actin. Biochemistry. 1972 Mar 28;11(7):1211–1217. doi: 10.1021/bi00757a015. [DOI] [PubMed] [Google Scholar]
- Martin W. J., 2nd Neutrophils kill pulmonary endothelial cells by a hydrogen-peroxide-dependent pathway. An in vitro model of neutrophil-mediated lung injury. Am Rev Respir Dis. 1984 Aug;130(2):209–213. doi: 10.1164/arrd.1984.130.2.209. [DOI] [PubMed] [Google Scholar]
- McGough A., Way M., DeRosier D. Determination of the alpha-actinin-binding site on actin filaments by cryoelectron microscopy and image analysis. J Cell Biol. 1994 Jul;126(2):433–443. doi: 10.1083/jcb.126.2.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesland D. A., Los G., Spiele H. Cytochalasin B disrupts the association of filamentous web and plasma membrane in hepatocytes. Exp Cell Res. 1981 Oct;135(2):431–435. doi: 10.1016/0014-4827(81)90184-1. [DOI] [PubMed] [Google Scholar]
- Milligan R. A., Whittaker M., Safer D. Molecular structure of F-actin and location of surface binding sites. Nature. 1990 Nov 15;348(6298):217–221. doi: 10.1038/348217a0. [DOI] [PubMed] [Google Scholar]
- Millonig R., Salvo H., Aebi U. Probing actin polymerization by intermolecular cross-linking. J Cell Biol. 1988 Mar;106(3):785–796. doi: 10.1083/jcb.106.3.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milzani A., DalleDonne I., Colombo R. N-ethylmaleimide-modified actin filaments do not bundle in the presence of alpha-actinin. Biochem Cell Biol. 1995 Jan-Feb;73(1-2):116–122. doi: 10.1139/o95-014. [DOI] [PubMed] [Google Scholar]
- Mirabelli F., Salis A., Marinoni V., Finardi G., Bellomo G., Thor H., Orrenius S. Menadione-induced bleb formation in hepatocytes is associated with the oxidation of thiol groups in actin. Arch Biochem Biophys. 1988 Jul;264(1):261–269. doi: 10.1016/0003-9861(88)90593-0. [DOI] [PubMed] [Google Scholar]
- Mossakowska M., Moraczewska J., Khaitlina S., Strzelecka-Golaszewska H. Proteolytic removal of three C-terminal residues of actin alters the monomer-monomer interactions. Biochem J. 1993 Feb 1;289(Pt 3):897–902. doi: 10.1042/bj2890897. [DOI] [PMC free article] [PubMed] [Google Scholar]
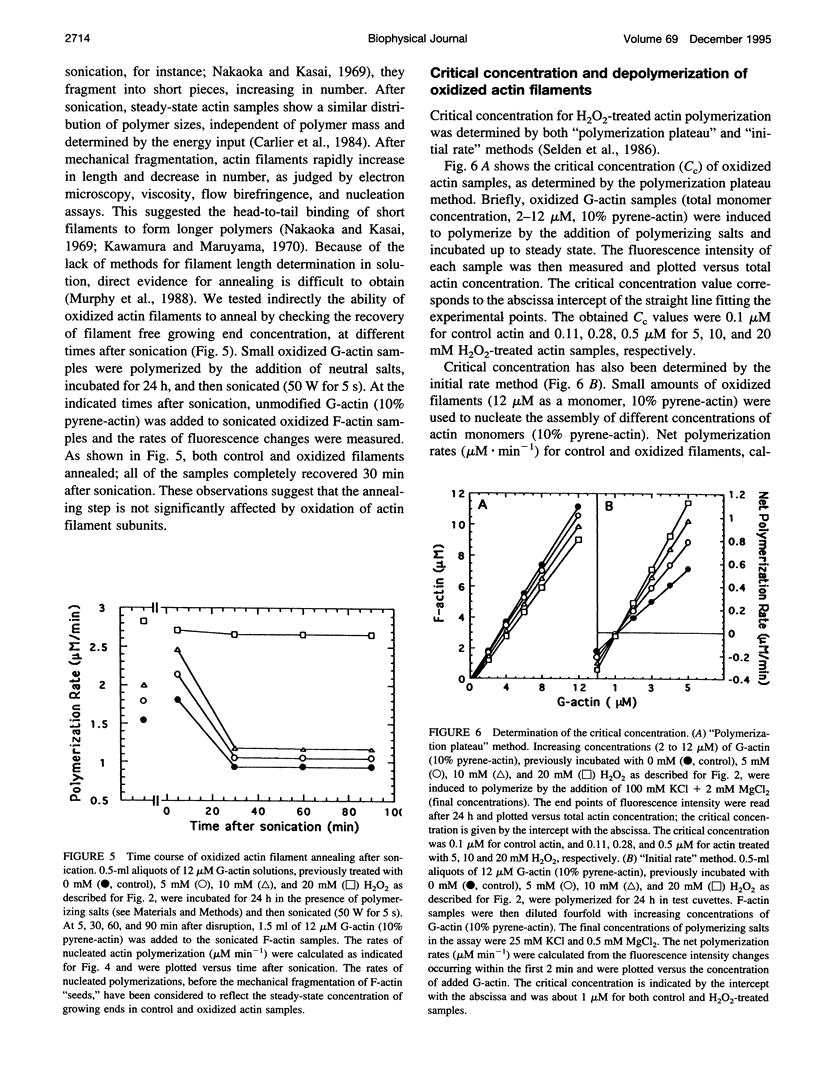
- Murphy D. B., Gray R. O., Grasser W. A., Pollard T. D. Direct demonstration of actin filament annealing in vitro. J Cell Biol. 1988 Jun;106(6):1947–1954. doi: 10.1083/jcb.106.6.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakaoka Y., Kasai M. Behaviour of sonicated actin polymers: adenosine triphosphate splitting and polymerization. J Mol Biol. 1969 Sep 14;44(2):319–332. doi: 10.1016/0022-2836(69)90178-8. [DOI] [PubMed] [Google Scholar]
- Nathan C. F., Silverstein S. C., Brukner L. H., Cohn Z. A. Extracellular cytolysis by activated macrophages and granulocytes. II. Hydrogen peroxide as a mediator of cytotoxicity. J Exp Med. 1979 Jan 1;149(1):100–113. doi: 10.1084/jem.149.1.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donoghue S. I., Miki M., dos Remedios C. G. Removing the two C-terminal residues of actin affects the filament structure. Arch Biochem Biophys. 1992 Feb 14;293(1):110–116. doi: 10.1016/0003-9861(92)90372-4. [DOI] [PubMed] [Google Scholar]
- Omann G. M., Harter J. M., Burger J. M., Hinshaw D. B. H2O2-induced increases in cellular F-actin occur without increases in actin nucleation activity. Arch Biochem Biophys. 1994 Feb 1;308(2):407–412. doi: 10.1006/abbi.1994.1057. [DOI] [PubMed] [Google Scholar]
- Pollard T. D., Cooper J. A. Methods to characterize actin filament networks. Methods Enzymol. 1982;85(Pt B):211–233. doi: 10.1016/0076-6879(82)85022-2. [DOI] [PubMed] [Google Scholar]
- Prentki M., Chaponnier C., Jeanrenaud B., Gabbiani G. Actin microfilaments, cell shape, and secretory processes in isolated rat hepatocytes. Effect of phalloidin and cytochalasin D. J Cell Biol. 1979 Jun;81(3):592–607. doi: 10.1083/jcb.81.3.592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacks T., Moldow C. F., Craddock P. R., Bowers T. K., Jacob H. S. Oxygen radicals mediate endothelial cell damage by complement-stimulated granulocytes. An in vitro model of immune vascular damage. J Clin Invest. 1978 May;61(5):1161–1167. doi: 10.1172/JCI109031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schraufstatter I. U., Hinshaw D. B., Hyslop P. A., Spragg R. G., Cochrane C. G. Oxidant injury of cells. DNA strand-breaks activate polyadenosine diphosphate-ribose polymerase and lead to depletion of nicotinamide adenine dinucleotide. J Clin Invest. 1986 Apr;77(4):1312–1320. doi: 10.1172/JCI112436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schraufstätter I. U., Hinshaw D. B., Hyslop P. A., Spragg R. G., Cochrane C. G. Glutathione cycle activity and pyridine nucleotide levels in oxidant-induced injury of cells. J Clin Invest. 1985 Sep;76(3):1131–1139. doi: 10.1172/JCI112068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selden L. A., Gershman L. C., Estes J. E. A kinetic comparison between Mg-actin and Ca-actin. J Muscle Res Cell Motil. 1986 Jun;7(3):215–224. doi: 10.1007/BF01753554. [DOI] [PubMed] [Google Scholar]
- Shizuta Y., Shizuta H., Gallo M., Davies P., Pastan I. Purification and properties of filamin, and actin binding protein from chicken gizzard. J Biol Chem. 1976 Nov 10;251(21):6562–6567. [PubMed] [Google Scholar]
- Simon R. H., Scoggin C. H., Patterson D. Hydrogen peroxide causes the fatal injury to human fibroblasts exposed to oxygen radicals. J Biol Chem. 1981 Jul 25;256(14):7181–7186. [PubMed] [Google Scholar]
- Spragg R. G., Hinshaw D. B., Hyslop P. A., Schraufstätter I. U., Cochrane C. G. Alterations in adenosine triphosphate and energy charge in cultured endothelial and P388D1 cells after oxidant injury. J Clin Invest. 1985 Oct;76(4):1471–1476. doi: 10.1172/JCI112126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spudich J. A., Watt S. The regulation of rabbit skeletal muscle contraction. I. Biochemical studies of the interaction of the tropomyosin-troponin complex with actin and the proteolytic fragments of myosin. J Biol Chem. 1971 Aug 10;246(15):4866–4871. [PubMed] [Google Scholar]
- Suzuki A., Goll D. E., Singh I., Allen R. E., Robson R. M., Stromer M. H. Some properties of purified skeletal muscle alpha-actinin. J Biol Chem. 1976 Nov 10;251(21):6860–6870. [PubMed] [Google Scholar]
- Tait J. F., Frieden C. Chemical modification of actin. Acceleration of polymerization and reduction of network formation by reaction with N-ethylmaleimide, (iodoacetamido)tetramethylrhodamine, or 7-chloro-4-nitro-2,1,3-benzoxadiazole. Biochemistry. 1982 Nov 23;21(24):6046–6053. doi: 10.1021/bi00267a004. [DOI] [PubMed] [Google Scholar]
- Tellam R., Frieden C. Cytochalasin D and platelet gelsolin accelerate actin polymer formation. A model for regulation of the extent of actin polymer formation in vivo. Biochemistry. 1982 Jun 22;21(13):3207–3214. doi: 10.1021/bi00256a027. [DOI] [PubMed] [Google Scholar]
- Wang K., Ash J. F., Singer S. J. Filamin, a new high-molecular-weight protein found in smooth muscle and non-muscle cells. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4483–4486. doi: 10.1073/pnas.72.11.4483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegner A., Savko P. Fragmentation of actin filaments. Biochemistry. 1982 Apr 13;21(8):1909–1913. doi: 10.1021/bi00537a032. [DOI] [PubMed] [Google Scholar]
- Weiss S. J., Young J., LoBuglio A. F., Slivka A., Nimeh N. F. Role of hydrogen peroxide in neutrophil-mediated destruction of cultured endothelial cells. J Clin Invest. 1981 Sep;68(3):714–721. doi: 10.1172/JCI110307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkins J. A., Lin S. A re-examination of the interaction of vinculin with actin. J Cell Biol. 1986 Mar;102(3):1085–1092. doi: 10.1083/jcb.102.3.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]